Abstract
The localized and temporally controlled delivery of growth factors is key to achieving optimal clinical efficacy. In sophisticated tissue engineering strategies, the biodegradable scaffold is preferred to serve as both a three-dimensional (3-D) substrate and a growth factor delivery vehicle to promote cellular activity and enhance tissue neogenesis. This study presents a novel approach to fabricate tissue engineering scaffolds capable of controlled growth factor delivery whereby growth factor containing microspheres were incorporated into 3-D scaffolds with good mechanical properties, well-interconnected macroporous and nano-fibrous structures. The microspheres were uniformly distributed throughout the nano-fibrous scaffold and their incorporation did not interfere the macro-, micro-, and nanostructures of the scaffold. The release kinetics of platelet-derived growth factor-BB (PDGF-BB) from microspheres and scaffolds was investigated using poly(lactic-co-glycolic acid) (PLGA50) microspheres with different molecular weights (6.5 and 64kDa, respectively) and microsphere-incorporated poly(l-lactic acid) (PLLA) nano-fibrous scaffolds. Incorporation of microspheres into scaffolds significantly reduced the initial burst release. Sustained release from several days to months was achieved through different microspheres in scaffolds. Released PDGF-BB was demonstrated to possess biological activity as evidenced by stimulation of human gingival fibroblast DNA synthesis in vitro. The successful generation of 3-D nano-fibrous scaffold incorporating controlled-release factors indicates significant potential for more complex tissue regeneration.
Keywords: PDGF, Factor, Controlled delivery, Nano-fiber, Scaffold, Tissue engineering, Microspheres, Matrix, Polymer
1. Introduction
A variety of bioactive factors that induce chemotaxis, proliferation, differentiation and matrix synthesis are essential in natural tissue/organ development and wound healing [1]. Owing to the rapid advances in recombinant technology and the availability of large scale manufacturing of cytokines and growth factors, many recent tissue engineering strategies have turned to the use of specific growth factors to stimulate cellular activity in vitro and for improved functional neotissue formation in vivo [2-5]. Importantly, the delivery mode appears to be critical for the application of these factors in tissue engineering [6-8].
Platelet-derived growth factor (PDGF) has been demonstrated to stimulate the proliferation and recruitment of both periodontal ligament (PDL) and bone cells in vitro. In vivo study also showed that PDGF-BB enhances periodontal regeneration in beagle dogs [9] and non-human primates [10] as well as bone defect fill in a human clinical trial [11,12]. Due to its capability in promoting wound healing [13] through enhancing the formation of granulation tissues, recombinant human PDGF-BB has been approved by US Food and Drug Administration (FDA) for use in diabetic foot ulcers [14]. Despite of its superior functions in enhancing tissue regeneration, high or repeated doses of PDGF need to be frequently administrated to maintain sufficient therapeutic concentration with desired duration at soft tissue defects giving that intravenously injected PDGF is rapidly cleared by the circulation (half-life less than 2min in vivo) [15,16]. Gene therapy has been used to deliver PDGF to soft tissue and periodontal wounds, however, safety concerns exist with the use of viral vectors [17-19]. Localized delivery in a controlled fashion of this potent factor may achieve the optimal function.
Biodegradable polyesters such as poly(lactide) (PLA) and poly(lactide-co-glycolide) (PLGA) have been widely used for the controlled delivery of polypeptides and proteins in the format of microspheres or nanospheres [20-25]. The release kinetics can be modulated by adjusting the factor loading, polymer molecular weight, lactide / glycolide ratio in the copolymer, and formulation methods [21,23,26,27]. Notably, the biological activity of the released factors can be largely maintained during the encapsulation process and upon release [5,21]. However, in terms of tissue engineering applications, it is preferable that a tissue engineering construct serves as both a factor delivery carrier and as a 3-dimensional (3-D) scaffold for cellular activities. These two components can coordinately enhance tissue regeneration. Simple adsorption of growth factors into natural or synthetic polymeric matrices allows local delivery, but the temporal control over release kinetics is limited [28-30]. Other approaches including emulsion incorporation and gas foaming incorporation may achieve some slow release of growth factors. However, the architecture and mechanical properties of the scaffolds, and the control over the release kinetics were limited [31-33].
Previously, our laboratory has developed a new technology to fabricate biodegradable PLLA nano-fibrous scaffold with well-interconnected macropores and good mechanical properties [34,35]. Due to its structural similarity to collagen (which is a major extracellular component of bone, cementum, and periodontal ligament (PDL)), the nano-fibrous structure has been demonstrated to improve cell attachment [36] and possibly to stimulate cell proliferation and differentiation as well [37]. In this paper, we incorporate the growth factor (PDGF-BB) containing microspheres into the nano-fibrous scaffold to develop a microsphere-scaffold system with the capacity of releasing bioactive PDGF-BB in a well-controlled manner. The demonstrated bioactivity resulted from locally delivered PDGF-BB, along with the advanced nano-fibrous scaffold, would make the microsphere-scaffold system a superior candidate for periodontal tissue regeneration.
2. Materials and methods
2.1. Materials
Poly(lactic-co-glycolic acid) (PLGA) with LA/GA ratio of 50:50 (Medisorb®, PLGA50-6.5K, Mw=6.5kDa; PLGA50-64K, Mw=64kDa) was purchased from Alkermes Inc. (Wilmington, OH). Poly(l-lactic acid) (PLLA) with inherent viscosity of 1.6dl/g was purchased from Boehringer Ingelheim (Ingelheim, Germany). Recombinant human platelet-derived growth factor (rhPDGF-BB) was kindly provided by BioMimetic Pharmaceutics (Franklin, TN). I125-PDGF-BB and [methyl-3H]thymidine were purchased from Amersham Biosciences Corp. (Piscataway, NJ). Dulbecco's Modified Eagle Medium (DMEM) and antibiotics were from Invitrogen Corp. (Carlsbad, CA). Other chemicals used were: poly(vinyl alcohol) (PVA) (88mol% hydrolyzed, MW=25,000) obtained from Polysciences Inc. (Warrington, PA); fluorescein isothiocyanate bovine serum albumin (FITC-BSA, 67kDa) from Sigma; dichloromethane, sodium dodecyl sulfate (SDS), sodium acetate and acetic acid from Aldrich Chemical Company (Milwaukee, WI).
2.2. PLGA50 microsphere (MS) preparation
PLGA microspheres were fabricated using an established double emulsion technique [21]. Briefly, 100μl FITC-BSA (3mg/ml) aqueous solution or PDGF-BB buffer solution (PDGF-BB in 20mM sodium acetate buffer with pH=6.3, varying in concentration of 0, 10, 100, 300, 600 and 1000, 3000μg/ml) was emulsified into 1ml of 10% PLGA/dichloromethane (DCM) solution, using a probe sonicator at 15W (Virsonic 100, Cardiner, NY) for 10s over an ice bath to form the primary water-in-oil (w/o) emulsion. The w/o emulsion was mixed with 20ml 1% PVA aqueous solution under sonication to form a water-in-oil-in-water (w/o/w) double emulsion. The solution was then stirred magnetically at room temperature for at least 3h to evaporate dichloromethane and centrifuged to collect solid microspheres. The resultant microspheres were washed with distilled water twice, freeze-dried and stored under vacuum. I125-PDGF-BB was added during the preparation of PDGF-BB encapsulated microspheres for the purpose of encapsulation efficiency measurement and study of release kinetics. The morphology of microspheres was examined using Scanning Electric Microscopy (SEM, Philips XL30 FEG).
2.3. Fabrication of PLLA nano-fibrous scaffolds
PLLA macroporous nano-fibrous scaffolds were fabricated by the combination of phase separation and sugar-leaching techniques [35]. Briefly, six hundred μl of 10% PLLA/THF solution was cast into an assembled sugar template (formed from bound sugar spheres 250–425μm in diameter) under mild vacuum. The polymer/sugar composite was phase separated at −20°C overnight and then immersed into cyclohexane to exchange THF for 2days. The resulting composites were freeze-dried and the sugar spheres were leached out in distilled water and freeze-dried again to obtain highly porous scaffolds. Scaffolds were cut into circular disks with dimensions of 7.2mm in diameter and 2mm in thickness. The average weight of the porous scaffold was 2.5–3.0mg.
2.4. Incorporation of PLGA microspheres into PLLA nano-fibrous scaffolds
PLGA microspheres with FITC-BSA or PDGF-BB were incorporated into PLLA nano-fibrous scaffolds using a post-seeding method. Briefly, dry PLGA microspheres were suspended in hexane with a concentration of 5mg MS/ml. Eighty microliters of the suspension were seeded onto each scaffold and the scaffold was left in air for 30min to evaporate the solvent. After that, another 80μl of suspension was dropped onto the other side of the scaffold. The whole procedure was repeated twice, resulting in about 1.6mg of microspheres incorporated into each scaffold. The scaffold was then subjected to a mixed solvent of hexane /THF (volume ratio of 90/10) to immobilize the microspheres on the scaffold and was vacuum-dried for 3days to remove the solvent. Controls used were scaffolds seeded with microspheres without any growth factors. The total amount of microspheres in a scaffold can be varied by changing the concentration of microsphere suspension and seeding times. The morphology and distribution of microspheres in scaffold were examined using SEM and laser scanning confocal microscopy (LSCM, Bio-Rad, exciting wavelength 488nm for FITC-BSA).
2.5. Encapsulation efficiency
The encapsulation efficiency (EE) of PDGF-BB in the microspheres was calculated as follows:
where Mactual is the actual PDGF-BB amount in 10mg MS and Mtheoretical, the theoretical loading amount of PDGF-BB encapsulated in 10mg MS. The actual quantity of PDGF-BB was determined by comparing the radioactivity of the sample to that of a known amount of PDGF-BB solution using I125-PDGF-BB as a tracer. The radioactivity of the samples was analyzed using a gamma counter (Gamma 5500, Beckman).
2.6. In vitro release study
In vitro PDGF-BB release profiles from PLGA microspheres and microsphere-incorporated PLLA scaffolds were determined as follows. Ten milligrams of microspheres or one MS-scaffold were suspended in 1ml phosphate buffered saline (PBS, 10mM, pH=7.4) or PBS/SDS (PBS with 5mM SDS). The microsphere suspension was incubated at 37°C with orbital shaking at 60rpm. At designated time points, samples were centrifuged to collect supernatant replaced with equal amounts of fresh medium. The radioactivity of collected supernatant was analyzed using a gamma counter and converted to calculate the quantity of the released PDGF-BB. Blank microspheres (without any protein) were used as control.
2.7. PDGF-BB bioactivity assay
The bioactivity of the released PDGF-BB was determined through human gingival fibroblast (HGF) DNA synthesis as measured by [3H]thymidine incorporation. Human gingival fibroblasts (HGF, passage 8 derived from a healthy patient) were plated at 2×104 cells/well in 24-well plates. These cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100μg/ml streptomycin, and 2mM glutamine for 24h, and then the medium was changed to the serum-free DMEM. After 24h of culture, the cells were treated with the mixture of the supernatant containing released PDGF-BB (from microspheres) and serum-free DMEM at the ratio of 1:4 or 3:7 in different groups. In addition, 2×105 cpm (count per minute) [methyl-3H]thymidine was added to each well. Following a 5-day culture period without medium change, the medium was removed and each well was washed twice with cold PBS. The DNA in each well was precipitated with 5% cold trichloroacetic acid at 4°C for 2h, solubilized with 1% SDS solution at 55°C for 2h, followed by counting the radioactivity of [methyl-3H] thymidine in the solution with a scintillation counter. PDGF-BB was shown to increase HGF proliferation with the increase of concentration within 0–100ng/ml range. Therefore, the supernatant from the microspheres without incorporation of PDGFBB was used as negative control while PDGF-BB solution with a concentration of 60ng/ml served as positive control. A two-tail Student's t test was used to determine the significant differences between sample groups.
3. Results
3.1. Characterization of microspheres, nano-fibrous scaffolds and microsphere-incorporated scaffolds
PDGF-BB encapsulated PLGA microspheres were fabricated utilizing a double emulsion technique. Two different PLGA formulations, PLGA50-6.5K and PLGA50-64K, with the same lactide / glycolide ratio of 50/50 but different molecular weights (6.5 and 64kDa, respectively) were used. The microspheres were spherical with smooth and non-porous surfaces (Fig. 1 (A)). The average diameter of the microspheres was less than 1μm based on SEM observation. High PDGF-BB encapsulation efficiency (77%∼93%) was achieved regardless of varying loading amount of PDGF-BB from 10 to 3000ng/mg MS (Fig. 1 (B)). The encapsulation efficiency was not significantly different between the different dose levels. The encapsulation efficiency for FITC-BSA was 84%. Based on these results, the encapsulation efficiency was not correlated to the molecular weight and concentration of the targeting protein encapsulated.
Fig. 1.
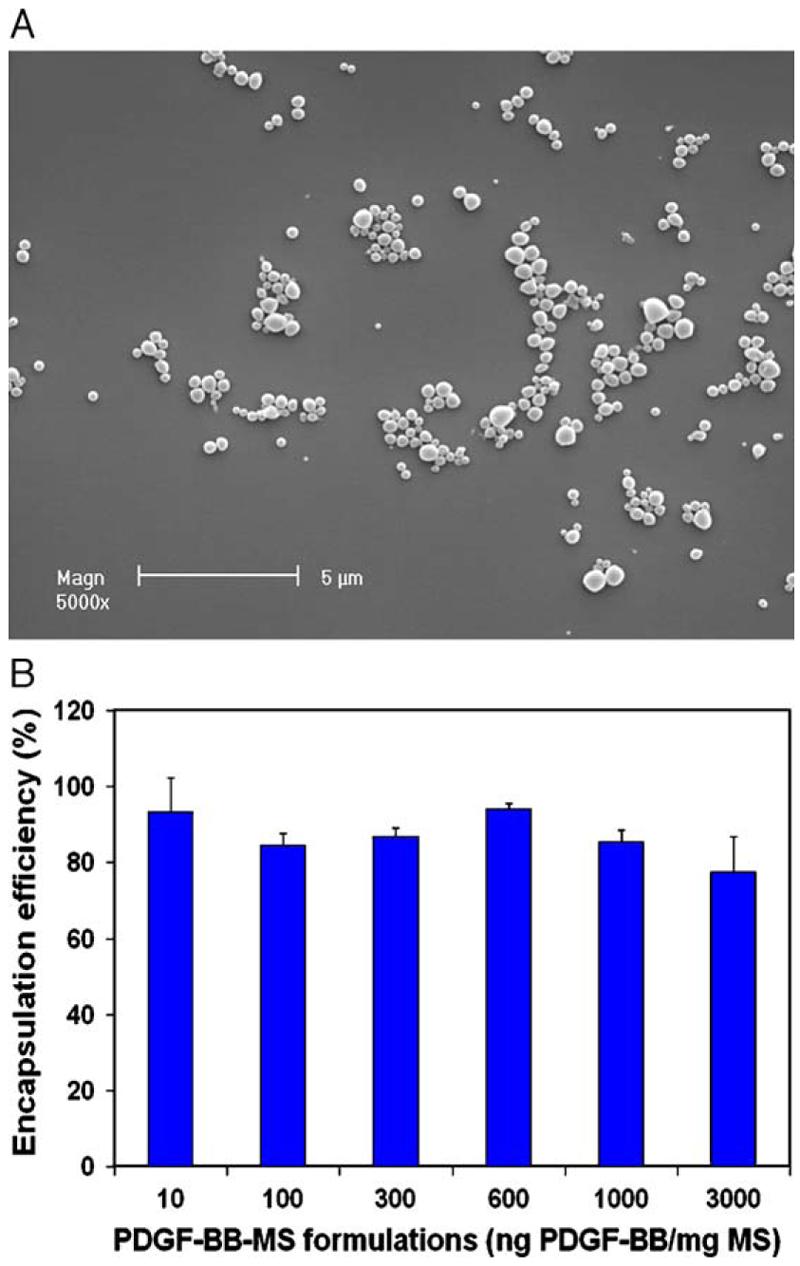
Characterization of PLGA50-6.5K microspheres (MS). (A) Scanning electron micrograph of FITC-BSA containing PLGA50-6.5K microspheres; (B) encapsulation efficiency of PDGF-BB in PLGA50-6.5K microspheres with varying loading amount from 10 to 3000ng/mg MS.
Using a new fabrication technique, we have developed macroporous PLLA nano-fibrous scaffolds with high porosity of 98%. The scaffolds are characterized of multi-level porous structures with regular spherical macropores 250–425μm in diameter, micro-interpore openings of ∼100μm, and nano-fibers in diameter of 50–500nm (similar size range to native collagen fibers) (Fig. 2). The macropores were well connected and the well-interconnected multi-level pore structures (from macro-, micro- to nanometers) not only were potentially important for the cellular activity within the scaffold, but also made subsequent incorporation of microspheres or nanospheres feasible and efficient.
Fig. 2.
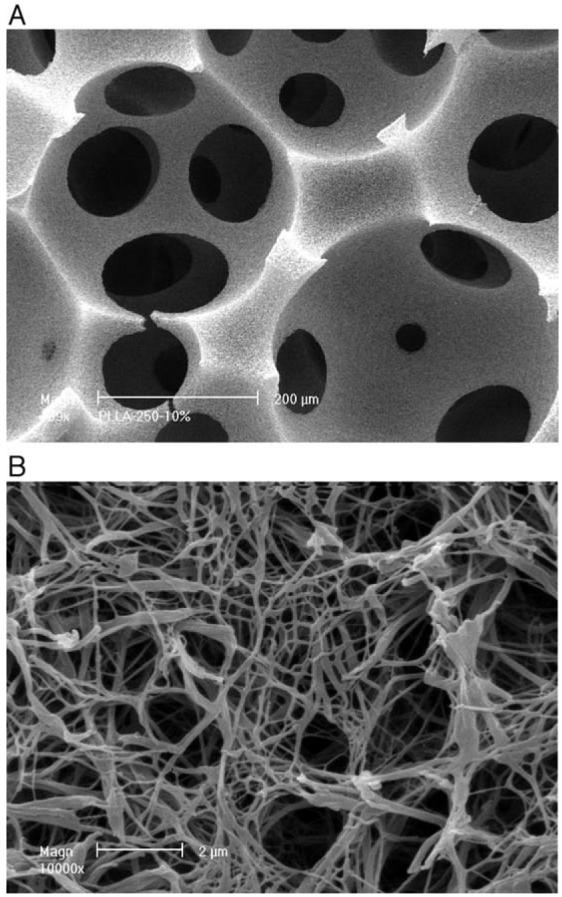
Scanning electron micrographs of PLLA nano-fibrous scaffolds before microsphere incorporation. (A) Low magnification at 200×; and (B) high magnification at 10000×.
Fig. 3 shows the cross section of a nano-fibrous PLLA scaffold after post-seeding of PLGA50-6.5K microspheres. Although the weight percentage of microspheres in the scaffolds reached 35%, the macropores and interpore openings of the scaffolds were well-maintained and the microspheres distributed uniformly throughout the nano-fibrous pore walls. The uniform distribution of FITC-BSA-microspheres throughout the scaffold was also confirmed by LSCM (Fig. 4).
Fig. 3.
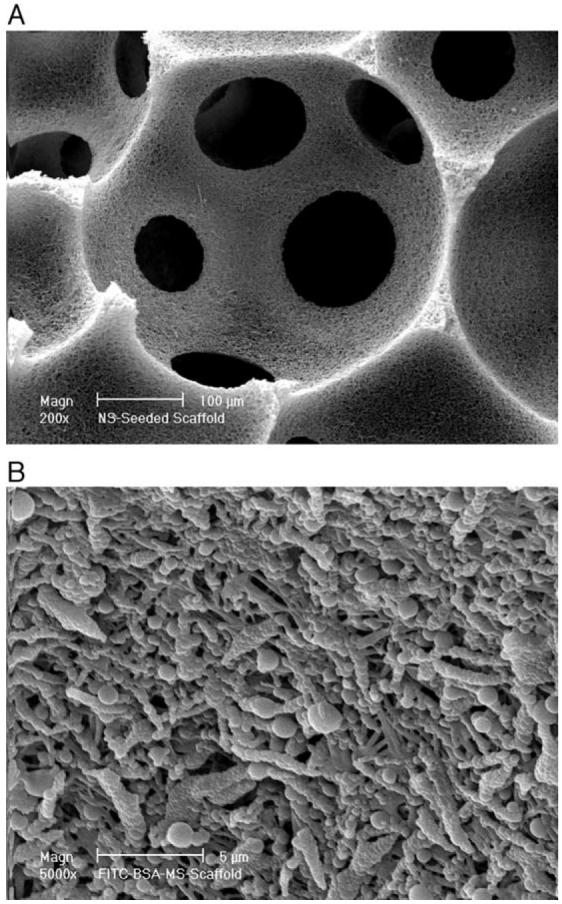
Scanning electron micrographs of PLLA nano-fibrous scaffolds after PLGA50-6.5K microsphere incorporation using post-seeding method. (A) Low magnification at 200×; and (B) high magnification at 5000×.
Fig. 4.
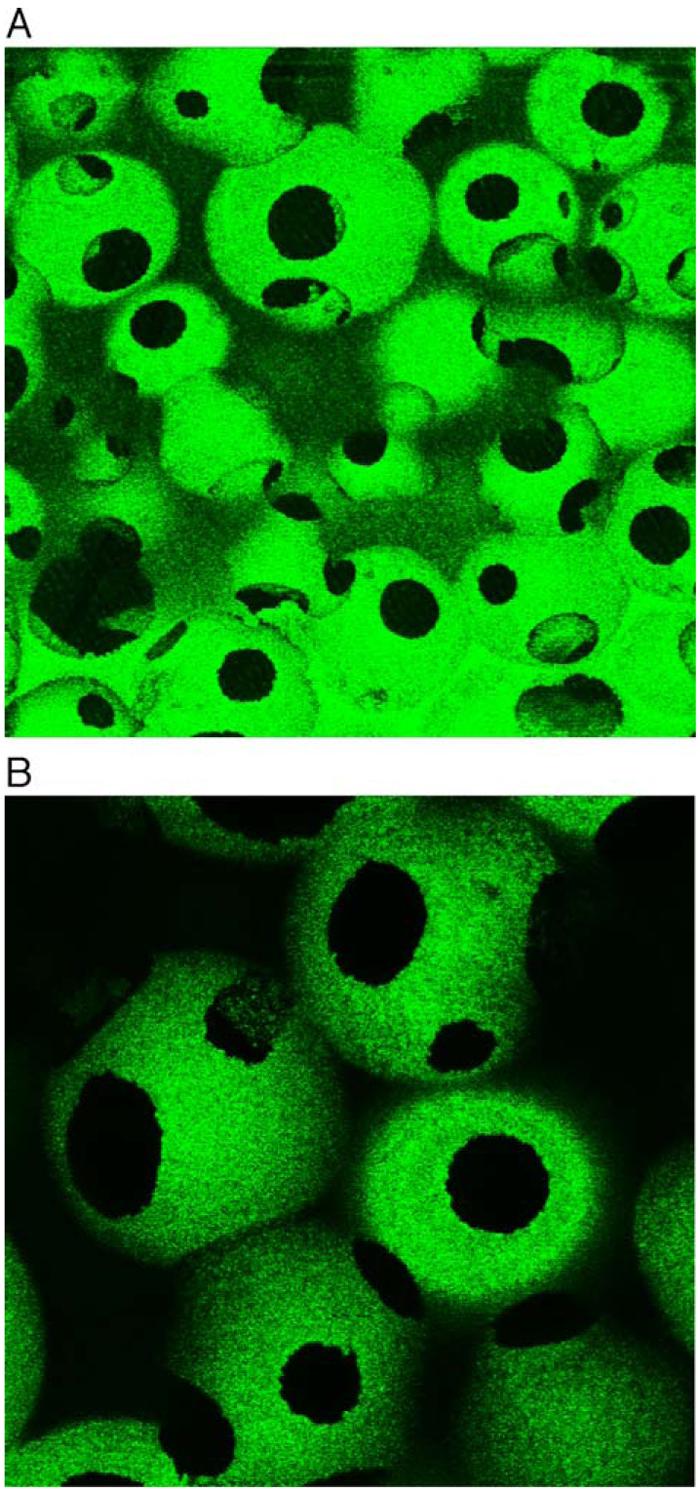
Laser scanning confocal microscopy (LSCM) of cross section through PLLA nano-fibrous scaffold containing FITC-BSA microspheres incorporated, revealing uniform microsphere distribution throughout the scaffold. Original magnification is (A) 100×; and (B) 200×.
3.2. In vitro PDGF-BB release kinetics
Cumulative PDGF-BB release from PLGA50-6.5K microspheres in PBS with different loadings is shown in Fig. 5(A). All sample groups had low burst release less than 12% after the first day. Subsequent to that, the release profiles were fairly constant with a sustained release of 0.75–0.92% per day for up to 42days. Depending on the loading amount of PDGF-BB in microspheres, the released amount of PDGF-BB could vary from 0.7 to 65ng/day. However, about 50% of PDGF-BB remained in the polymer residues for all sample groups despite the fact that 92% weight loss of PLGA50-6.5K microspheres was observed after 6-week incubation in PBS (degradation data not shown). The PDGF-BB release profile was very different from the degradation rate of microspheres. To address concerns with adherence of PDGF-BB to sample collection vessels or possible polymer degradation residues, further release experiments were conducted in PBS with 5mM SDS which is a strong surfactant to reduce the adsorption. The release profiles were dramatically different from those in PBS. Almost all PDGF-BB was released within 1week (Fig. 5(B)). The results demonstrated that a significant amount of released PDGF-BB was adsorbed on the surface of degraded microspheres or test tubes.
Fig. 5.
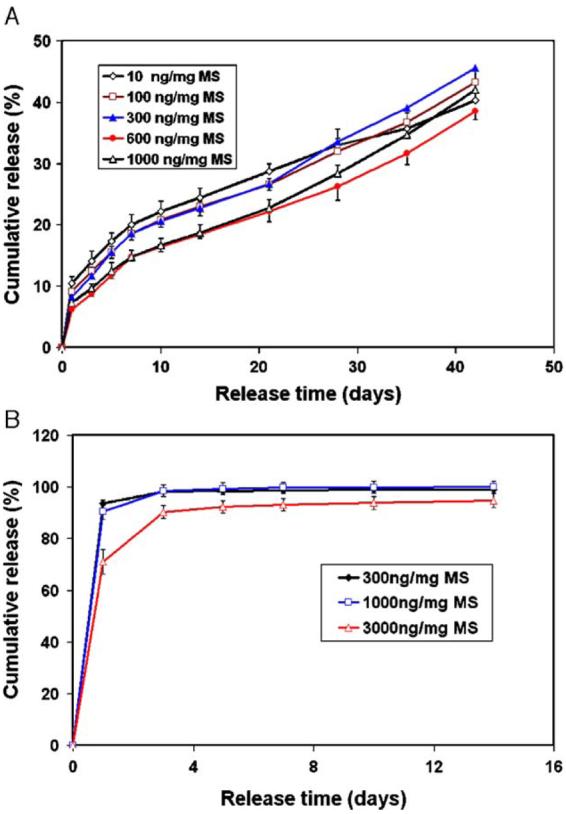
In vitro release kinetics of PDGF-BB from microspheres. (A) In 10mM PBS with varying PDGF-BB loadings of 10, 100, 300, 600, 1000ng/mg MS; and (B) in PBS/SDS (5mM) with varying PDGF-BB loadings of 300, 1000, and 3000ng/mg MS. Each data point represents an average±standard deviation (n=3). The experiment was repeated twice.
To study the release kinetics of growth factors from scaffold, PLGA50-6.5K (1000, 3000ng/mg MS) and PLGA50-64K (3000ng/mg MS) microspheres were incorporated into PLLA nano-fibrous scaffold using a newly developed post-seeding method. The release from the scaffolds showed a significantly decreased initial burst release when compared to the release from the microspheres alone (Fig. 6). The PLGA50-6.5K-MS-scaffold presented a fast release of 54% in first 3 days followed by a relative slow release of 40% in the following 40 days. The release pattern of PDGF-BB from PLGA50-64K-MS-scaffold was similar to that of release directly from microspheres, both showing an accelerated release at day 35 (Fig. 6(B)). However, very little initial burst release (only 3%) was observed for PDGF-BB release from PLGA50-64K microsphere-incorporated scaffolds.
Fig. 6.
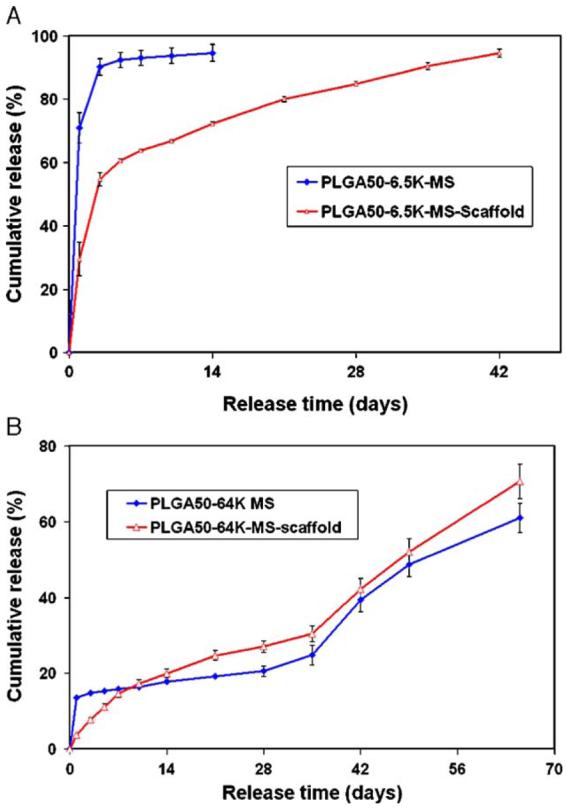
In vitro release kinetics of PDGF-BB from microsphere-incorporated PLLA scaffolds in PBS/SDS. (A) PLGA50-6.5K-MS-scaffold; and (B) PLGA50-64K-MS-scaffold. Each data point represents an average±standard deviation (n=3). The experiment was repeated twice.
3.3. In vitro bioactivity via [3H]thymidine uptake
In vitro bioactivity was tested for PDGF-BB samples released from PLGA50-6.5K microspheres (in PBS with 0.1% BSA) at time periods of day 1, day 7–10, and day 10–14. All tested samples were diluted to the same PDGF-BB concentration of 60ng/ml (determined using I125 radioactivity measurement) as positive controls (PDGF-BB solution). Results showed that the released PDGF-BB was biologically active compared to negative controls devoid of any PDGF-BB (p<0.05) (Table 1). No significant loss of biological activity was observed for day 1 samples (p=0.67) as compared to positive controls.
Table 1.
Bioactivity of released PDGF-BB from MS by [3H]thymidine incorporation
| No rhPDGF- BB |
rhPDGF- BB (60ng/ ml) |
Release samples from MS (60ng/ml) |
|||
|---|---|---|---|---|---|
| Day 1 | Day 3–7 | Day 10–14 | |||
| H3 (cpm) | 38.93± 16.94 |
186.75± 20.44 |
179.57± 24.1 a,b |
83.33± 2.2 a,c |
121.8±27.0 a,c |
p<0.05 vs. no rhPDGF-BB (0ng/ml).
Not statistically significant vs. rhPDGF-BB solution (60ng/ml).
p<0.05 vs. rhPDGF-BB solution (60ng/ml).
4. Discussion
One of the challenges in tissue engineering is to induce rapid cell penetration and vascular invasion throughout 3-dimensional scaffolds to support tissue neogenesis and function. We report here an engineered scaffold system that can potentially solve the problems of cell penetration and vascularization. First, a scaffold with good mechanical properties, well-interconnected macropores, high surface area/volume ratio and nano-fibrous networks was developed. The highly porous nano-fibrous structure, upon implantation, not only offers a beneficial 3-D substrate to promote cell attachment [36], migration, replication, differentiation as well as matrix deposition, but also facilitates mass transfer between cells within the scaffold and the surrounding host tissues. Second, we have successfully incorporated PDGF-BB, an angiogenic co-factor [32,38] into the scaffolds without interfering with the macro-and microstructures of the scaffold. Bioactive PDGF-BB was released from the scaffolds in a well-controlled fashion. The release amounts of PDGF-BB from scaffolds can be modulated either by the encapsulated amount of PDGF-BB in microspheres or the amount of microspheres incorporated into the scaffolds. It can be anticipated that the local and controlled delivery of biological active PDGF-BB would be potent to further regulate cellular activity in the scaffold. To develop such a microsphere-scaffold system with microspheres uniformly distributed throughout the scaffold, both the well-interconnected porous structure and the suitable small size of microspheres are essential.
Incorporation of PDGF-BB containing microspheres into the 3-D scaffold significantly reduced the initial burst release and resulted in a sustained release of PDGF-BB from the scaffolds. However, the release of PDGF-BB from the scaffolds was mainly controlled by the degradation of the incorporated microspheres. One important reason for the consideration of PLGA microspheres as a protein delivery system is its excellent ability to modulate the release kinetics by adjusting the molecular weight and composition of the copolymer [21,39]. Therefore, the successful incorporation of protein containing PLGA microspheres into PLLA scaffold offers an excellent opportunity to control the release kinetics of proteins from scaffolding matrices. In this study, we are able to achieve different release profiles from scaffold by adjusting the molecular weights of the PLGA50 microspheres incorporated in the scaffolds. A much faster release was observed for scaffold incorporated with PLGA50-6.5K microspheres than that with PLGA50-64K microspheres. Microsphere incorporation also protects bioactive growth factors from denaturing as compared to direct adsorption of growth factors onto the biodegradable scaffolds/implants, which results in complete degradation of growth factors such as rhVEGF, BMP-4 and bFGF during a very short release time of 3days [40]. Our in vitro bioactivity assay indicated that no significant bioactivity loss was found for PDGF-BB during encapsulation into PLGA microspheres using double emulsion technique and upon initial in vitro release in PBS. Encapsulation of proteins into microspheres which are then incorporated into the scaffolds, therefore, is a promising strategy to achieve controlled release of growth factors from scaffold and to maintain their biological activity.
The adsorption of PDGF-BB during release phase of the study played an important role in determining the release profiles (Fig. 5). The released PDGF-BB can either non-specifically adsorb onto the polypropane (PP) test tubes or onto the surface of the degraded PLGA microsphere residues. Furthermore, because PDGF-BB is a cationic protein with an isoelectric point of 9.8 and while degraded PLGA50 carried a large number of anions (−COO− groups), there is strong static ionic interaction between PDGF-BB and degraded PLGA50 residues in addition to non-specific adsorption, which would substantially affect the release rate. The addition of 0.1% BSA or 0.1% BSA/Tween80 in PBS solved the problem of adsorption onto the test tubes and greater than 95% of the proteins remained in the solution (data not shown). However, the release rate was still much slower than the degradation rate of the polymer, even though there was a modest increase in initial burst release (Fig. 7(A)). Therefore, the adsorption onto the degraded microsphere residues was the determinant factor to the PDGF-BB release kinetics due to the large surface area of microspheres (due to small size) and the static ionic interaction between degraded polymer and PDGF-BB. The application of sodium dodecyl sulfate (SDS), a much stronger anionic surfactant, was able to dissociate both the non-specific and ionic interactions between PDGF-BB and degraded polymer microspheres and largely recover the release of PDGF-BB delivered from the microspheres (Fig. 7(A)) and microsphere-incorporated scaffolds (Fig. 7(B)). The adsorption may also contribute to the reduced burst release from nano-fibrous scaffolds because nano-fibrous scaffolds have very high surface areas (∼100m2/g). The significant effect of adsorption on in vitro protein release was also observed for other proteins encapsulated in microspheres [41,42]. Surfactants such as SDS were also used to reduce adsorption and improve release kinetics. SDS, while can be used to more accurately determine in vitro release profiles, may not be a good choice in a bioactivity assay because it can cause protein denaturing. However, in vivo adsorption might be low or non-existent because many other proteins present in the body fluid may act as surfactants and/or compete for adsorption. Ions from a salt solution (sodium salicylate) have been reported to increase the solubility of poorly water-soluble drugs [43]. The ions present in the body fluid may also change the ionic interactions between growth factors and degraded polymer residues. Therefore, a combination of plasma proteins and ions may provide surfactant-like function in vivo similar to SDS in vitro to reduce adsorption without denaturing the bioactive proteins.
Fig. 7.
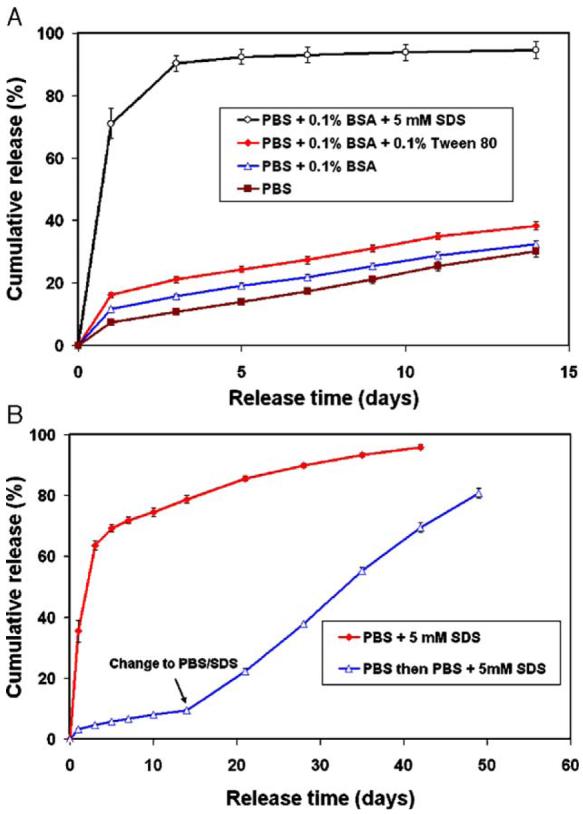
The effect of release medium on the release profiles of PDGF-BB from (A) PLGA50-6.5K microspheres and (B) PLGA50-6.5K microsphere-incorporated scaffolds. Each data point represents an average±standard deviation (n=3).
5. Conclusions
A microsphere-based scaffold release system has been developed. PDGF-BB encapsulated PLGA microspheres were incorporated into a biodegradable PLLA scaffold with well-interconnected macroporous and nano-fibrous structures. The MS-scaffold system is capable of releasing bioactive PDGF-BB in a temporally controlled fashion with prolonged duration. Release kinetics from the scaffolds were found to be governed by degradation of incorporated microspheres and the incorporation into scaffolds significantly reduced the burst effect. The MS-scaffold system can be used to deliver both cells and bioactive factors for a variety of tissue engineering applications.
Acknowledgements
This study was supported by NIH/NIDCR grants DE15384 (PXM), DE14755 (PXM), DE13397 (WVG). The authors appreciate the gift of rhPDGF-BB from Dr. Samuel Lynch.
References
- 1.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19(1):S23–S37. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med. J. 2000;41(6):704–719. doi: 10.3349/ymj.2000.41.6.704. [DOI] [PubMed] [Google Scholar]
- 3.Woo BH, Fink BF, Page R, Schrier JA, Jo YW, Jiang G, DeLuca M, Vasconez HC, DeLuca PP. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm. Res. 2001;18(12):1747–1753. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Nam SH, Im SY, Park YJ, Lee YM, Seol YJ, Chung CP, Lee SJ. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J. Control. Release. 2002;78(1–3):187–197. doi: 10.1016/s0168-3659(01)00498-9. [DOI] [PubMed] [Google Scholar]
- 5.Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, Hedberg EL, Mikos AG. Growth factor-loaded scaffolds for bone engineering. J. Control. Release. 2005;101(1–3):127–136. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 1999;5(12):1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 7.Khouri RK, Koudsi B, Deune EG, Hong SP, Ozbek MR, Serdar CM, Song SZ, Pierce GF. Tissue generation with growth-factors. Surgery. 1993;114(2):374–380. [PubMed] [Google Scholar]
- 8.Morley P, Whitfield JF, Willick GE. Parathyroid hormone: an anabolic treatment for osteoporosis. Curr. Pharm. Des. 2001;7(8):671–687. doi: 10.2174/1381612013397780. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SE, Decastilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, Antoniades HN. The effects of short-term application of a combination of platelet-derived and insulin-like growth-factors on periodontal wound-healing. J. Periodontol. 1991;62(7):458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 10.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, Dandrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal Res. 1996;31(5):301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997;68(12):1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 12.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, Paquette DW, Han TJ, Reddy MS, Lavin PT, Genco RJ, Lynch SE. Platelet-derived growth factor (rhPDGF-BB) stimulate bone fill and rate of attachment level gain: results of a large multi-center randomized controlled trial. J. Periodontol. 2005;76(12):2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 13.LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am. J. Surg. 1998;176(2A):48S–54S. doi: 10.1016/s0002-9610(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 14.Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am. J. Surg. 1998;176(2A):80S–82S. doi: 10.1016/s0002-9610(98)00186-x. [DOI] [PubMed] [Google Scholar]
- 15.Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth-factor in vivo — levels, activity, and rate of clearance. Blood. 1984;64(2):458–469. [PubMed] [Google Scholar]
- 16.Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, Cho MI. Periodontal regeneration in class-III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth-factor. J. Periodontol. 1995;66(6):462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 17.Jin QM, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004;9(4):519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9(4):745–756. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doukas J, Chandler LA, Gonzalez AM, Gu DL, Hoganson DK, Ma CL, Nguyen T, Printz MA, Nesbit M, Herlyn M, Crombleholme TM, Aukerman SL, Sosnowski BA, Pierce GF. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum. Gene Ther. 2001;12(7):783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 20.Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 21.Wei GB, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25(2):345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 22.Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, Shebuski RJ, Levy RJ. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Control. Release. 1997;43(2–3):197–212. [Google Scholar]
- 23.Yeo Y, Park K. A new microencapsulation method using an ultrasonic atomizer based on interfacial solvent exchange. J. Control. Release. 2004;100(3):379–388. doi: 10.1016/j.jconrel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim MA, Ismail A, Fetouh MI, Gopferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. J. Control. Release. 2005;106(3):241–252. doi: 10.1016/j.jconrel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Giovagnoli S, Luca G, Casaburi I, Blasi P, Macchiarulo G, Ricci M, Calvitti M, Basta G, Calafiore R, Rossi C. Long-term delivery of super-oxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: in vitro effects on isolated neonatal porcine pancreatic cell clusters. J. Control. Release. 2005;107(1):65–77. doi: 10.1016/j.jconrel.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22(3):231–241. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 27.Dorati R, Genta I, Montanari L, Cilurzo F, Buttafava A, Faucitano A, Conti B. The effect of gamma-irradiation on PLGA/PEG microspheres containing ovalbumin. J. Control. Release. 2005;107(1):78–90. doi: 10.1016/j.jconrel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor beta 1 released from a biodegradable hydrogel. J. Control. Release. 2000;64(1–3):133–142. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto S, Takaoka K, Okada T, Yoshikawa H, Hashimoto J, Suzuki S, Ono K. Evaluation of polylactic acid homopolymers as carriers for bone morphogenetic protein. Clin. Orthop. Relat. Res. 1992;278:274–285. [PubMed] [Google Scholar]
- 30.Tamura S, Kataoka H, Matsui Y, Shionoya Y, Ohno K, Michi KI, Takahashi K, Yamaguchi A. The effects of transplantation of osteoblastic cells with bone morphogenetic protein (BMP)/carrier complex on bone repair. Bone. 2001;29(2):169–175. doi: 10.1016/s8756-3282(01)00498-7. [DOI] [PubMed] [Google Scholar]
- 31.Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21(24):2545–2551. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 32.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 33.Sohier J, Haan RE, de Groot K, Bezemer JM. A novel method to obtain protein release from porous polymer scaffolds: emulsion coating. J. Control. Release. 2003;87(1–3):57–68. doi: 10.1016/s0168-3659(02)00350-4. [DOI] [PubMed] [Google Scholar]
- 34.Chen VJ, Ma PX. Nano-fibrous poly(l-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 35.Wei GB, Ma PX. Macro-porous and nano-fibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J. Biomed. Mater. Res. doi: 10.1002/jbm.a.30704. in press. [DOI] [PubMed] [Google Scholar]
- 36.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 37.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25(5):877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 38.Hao X, Mansson-Broberg A, Gustafsson T, Grinnemo KH, Blomberg P, Siddiqui AJ, Wardell E, Sylven C. Angiogenic effects of dual gene transfer of bFGF and PDGF-BB after myocardial infarction. Biochem. Biophys. Res. Commun. 2004;315(4):1058–1063. doi: 10.1016/j.bbrc.2004.01.165. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic glycolic acid) micro-spheres. Pharm. Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. J. Biomed. Mater. Res. 2002;59(3):422–428. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 41.Crotts G, Park TG. Stability and release of bovine serum albumin encapsulated within poly(d,l-lactide-co-glycolide) microparticles. J. Control. Release. 1997;44(2–3):123–134. [Google Scholar]
- 42.Crotts G, Sah H, Park TG. Adsorption determines in-vitro protein release rate from biodegradable microspheres: quantitative analysis of surface area during degradation. J. Control. Release. 1997;47(1):101–111. [Google Scholar]
- 43.Cho YW, Lee J, Lee SC, Huh KM, Park K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J. Control. Release. 2004;97(2):249–257. doi: 10.1016/j.jconrel.2004.03.013. [DOI] [PubMed] [Google Scholar]


