Current knowledge about the pathogenesis of periodontal diseased—obtained mainly from the results of animal experiments, analysis of periodontal histopathology, epidemiologic studies, and clinical trials—describes a complex and multifactorial etiology [1]. Generally, the extent and severity of periodontitis increases with age and relates to the control of pathogens associated with dental plaque biofilms [2]. Periodontal disease is found with high prevalence and variability, with few individuals experiencing advanced destruction [3]. Periodontal disease is characterized by an inflammatory reaction of periodontal tissues that leads to destruction of tooth-associated structures, including alveolar bone, tooth root cementum, and periodontal ligament (PDL) [4]. Current views consider a course of the disease that is chronic with brief episodes of localized exacerbation and remission [5,6]. Consequently, if the disease is left untreated, tooth loss can occur. Despite the remarkably high standards of professional periodontal care currently available, incomplete adult dentitions or edentulism are still evident in the United States and worldwide populations.
A challenge faced by periodontal therapy is the predictable regeneration of periodontal tissues lost as a consequence of disease, including alveolar bone, PDL, and cementum. Thus far, the ability to regenerate completely the damaged periodontal supporting structures has not been achieved in humans. The application of various regenerative biomaterials, such as bone autografts, allografts, cell occlusive barrier membranes used in guided tissue regeneration procedures, applications of growth factors (eg, enamel matrix proteins), or their combinations, have been pursued with varying degrees of success to regenerate lost tooth support, however [7]. Examples of currently available regenerative biomaterials are shown in Table 1 [8-37]. In summary, these therapeutic measures are shown to be limited in the predictability of healing and regenerative response in modern clinical practice, because the oral environment presents several complicating factors that border regeneration: (1) Periodontal wounds are contaminated with tooth-associated biofilms of anaerobic bacteria. (2) Transmucosal hard-soft tissue environment allows entry of pathogens into wounds. (3) Multiple junctional complexes and stromal-cellular interactions create difficulty in rebuilding tissue interfaces (eg, tooth-PDL-bone and epithelial-connective tissue-bone). (4) The effects of occlusal forces deliver intermittent loads in axial and transverse dimensions [38,39].
Table 1.
Various biomaterials available for clinical periodontal regenerative therapy
| Regenerative biomaterials | Components | References |
|---|---|---|
| Bone allografts | Demineralized freeze dried bone allograft | Gurinsky et al, 2004 [8] |
| Kimble et al, 2004 [9] | ||
| Trejo et al, 2000 [10] | ||
| Bone xenografts | Bovine mineral matrix | Hartman et al, 2004 [11] |
| Camelo et al, 2001 [12] | ||
| Mellonig, 2000 [13] | ||
| Nevins et al, 2000 [14] | ||
| Richardson et al, 1999 [15] | ||
| Bone alloplast grafts | Beta tricalcium phosphate | Palti and Hoch, 2002 [16] |
| Scher et al, 1999 [17] | ||
| Nery et al, 1992 [18] | ||
| Bioactive glass | Sculean et al, 2005 [19] | |
| Reynolds et al, 2003 [20] | ||
| Trombelli et al, 2002 [21] | ||
| Fetner et al, 1994 [22] | ||
| Nonresorbable cell occlusive barrier membranes | Polytetrafluorethylene | Trombelli et al, 2005 [23] |
| Moses et al, 2005 [24] | ||
| Murphy and Gunsolley, 2003 [25] | ||
| Needleman et al, 2002 [26] | ||
| Resorbable cell occlusive barrier membranes | Polyglycolide/polylactide (synthetic) | Minenna et al, 2005 [27] |
| Stavropoulos et al, 2004 [28] | ||
| Parashis et al, 2003 [29] | ||
| Collagen membrane (xenogen) | Sculean et al, 2005 [30] | |
| Owczarek et al, 2003 [31] | ||
| Camelo et al, 1998 [32] | ||
| Enamel proteins | Enamel matrix derivative | Rasperini et al, 2005 [33] |
| Rosing et al, 2005 [34] | ||
| Sanz et al, 2004 [35] | ||
| Francetti et al, 2004 [36] | ||
| Tonetti et al, 2002 [37] |
The role of growth factors used in periodontal regenerative medicine
After periodontal therapy (eg, deep scaling or periodontal flap surgery), a blood coagulum forms at the wound site and releases tissue growth factors locally, such as platelet-derived growth factor (PDGF) and transforming growth factor-β from degranulating platelets [40,41]. These mitogenic polypeptides attract mesenchymal cells and fibroblasts to migrate into the periodontal wound and stimulate their proliferation [42]. The continuing process of periodontal tissue repair is followed by the formation of granulation tissue as a source for future periodontal connective tissue cells, such as osteoblasts, PDL fibroblasts, and cementoblasts [43]. For alveolar bone regeneration, mesenchymal cells are induced into osteoprogenitor cells by locally expressed bone morphogenetic proteins (BMPs) [44,45].
Wound-healing approaches that use growth factors to target restoration of tooth-supporting bone, PDL, and cementum can advance greatly the field of periodontal regenerative medicine. A major focus of periodontal research has evaluated the impact of growth factor applications on periodontal tissue regeneration [39,46-48]. Articles describe various delivery systems and applications of growth factors, which are highlighted in Table 2. Advances in molecular cloning have made available unlimited quantities of recombinant growth factors for applications in tissue engineering. Recombinant growth factors known to promote skin and bone wound healing, such as PDGFs [49-53], insulin-like growth factors [46,50,54,55], fibroblast growth factors [56-60], and BMPs [61-65], have been used in preclinical and clinical trials for the treatment of large periodontal or intrabony defects and around dental implants [66,67].
Table 2.
Effects of growth factors used for periodontal tissue engineering
| Growth factor | Effects |
|---|---|
| Platelet-derived growth factor | Migration, proliferation, and noncollagenous matrix synthesis of mesenchymal cells |
| Insulin-like growth factor-1 | Cell migration, proliferation, differentiation, and matrix synthesis |
| Fibroblast growth factor-2 | Proliferation and attachment of endothelial cells and PDL cells |
| Transforming growth factor-beta | Proliferation of cementoblasts and PDL fibroblasts |
| Bone morphogenetic protein | Differentiation of osteoblasts, differentiation of PDL cells into osteoblasts |
| Enamel matrix derivative | Proliferation, protein synthesis, and mineral nodule formation in PDL cells, osteoblasts, and cementoblasts |
Biologic effects of growth factors: platelet-derived growth factors
PDGF is a member of a multifunctional polypeptide family that binds to two cell membrane tyrosine kinase receptors (PDGF-Rα and PDGF-Rβ) and subsequently exerts its biologic effects on cell proliferation, migration, extracellular matrix synthesis, and anti-apoptosis [68-71]. PDGF-α and -β receptors are expressed in regenerating periodontal soft and hard tissues [72]. PDGF also initiates tooth-supporting PDL cell chemotaxis [73], mitogenesis [74], matrix synthesis [75], and attachment to tooth dentinal surfaces [76]. More importantly, in vivo application of PDGF alone or in combination with insulin-like growth factor-1 results in partial repair of periodontal tissues [49,50,55,77,78]. PDGF has been shown to have a significant regenerative impact on PDL cells and osteoblasts [42,52,74,79].
Biologic effects of growth factors: bone morphogenetic proteins
BMPs are multifunctional polypeptides that belong to the transforming growth factor-β superfamily of proteins [80]. The human genome encodes at least 20 BMPs [81]. BMPs bind to type I and II receptors that function as serine-threonine kinases. The type I receptor protein kinase phosphorylates intracellular signaling substrates called Smads (Sma gene in C elegans and Mad gene in Drosophila). The phosphorylated BMP-signaling Smads enter the nucleus and initiate the production of bone matrix proteins leading to bone morphogenesis. The most remarkable feature of BMPs is the ability to induce ectopic bone formation [82]. BMPs not only are powerful regulators of cartilage and bone formation during embryonic development and regeneration in postnatal life but also participate in the development and repair of other organs, such as the brain, kidney, and nerves [83]. Studies have demonstrated the expression of BMPs during tooth development and periodontal repair, including alveolar bone [84,85]. Investigations in animal models have shown the potential repair of alveolar bony defects using recombinant human BMP-12 (rhBMP-12) [63] or rhBMP-2 [86,87]. In a recent clinical trial by Fiorellini and colleagues [88], rhBMP-2 delivered by a bioabsorbable collagen sponge revealed significant bone formation in a human buccal wall defect model after tooth extraction when compared with collagen sponge alone. BMP-7, also known as osteogenic protein-1, stimulates bone regeneration around teeth and endosseous dental implants and in maxillary sinus floor augmentation procedures [56,89,90].
Clinical applications of growth factors for use in periodontal regeneration
In general, the impact of a topical delivery of growth factors to periodontal wounds has shown to be promising yet insufficient for the promotion of predictable periodontal tissue engineering [48,51]. Growth factor proteins, once delivered to the target site, tend to suffer from instability and quick dilution, presumably because of proteolytic breakdown, receptor-mediated endocytosis, and solubility of the delivery vehicle [39]. Because their half-lives are significantly reduced, the period of exposure may not be sufficient to act on osteoblasts, cementoblasts, or PDL cells. Different methods of growth factor delivery must be considered [91].
Investigations for periodontal bioengineering have examined various methods of combining delivery vehicles (eg, scaffolds) with growth factors to target the defect site to optimize bioavailability [92]. The scaffolds are designed to optimize the dosage of the growth factor and control its release pattern, which may be pulsatile, constant, or time programmed [93]. The kinetics of the release and the duration of the exposure of the growth factor also may be controlled [94].
A new polymeric system was reported in an animal study by Richardson and colleagues [95] that enabled the tissue-specific delivery of two or more growth factors, with a controlled dose and rate of delivery. The dual delivery of vascular endothelial growth factor (VEGF) together with PDGF from a single, structural polymer scaffold results in the rapid formation of a mature vascular network.
Gene therapy methods investigated for periodontal tissue regeneration
The single administration of purified tissue growth factors has not been shown to be clinically effective in supporting the horizontal regeneration of periodontal tissue breakdown. This may be caused by insufficient capabilities to maintain therapeutic protein levels at the wound site and the three-dimensional architecture of the defects. Gene transfer methods may circumvent many of the limitations with protein delivery to soft tissue wounds [96,97]. The application of growth factors [45,98-100] or soluble forms of cytokine receptors [101] by gene transfer provides a greater sustainability than that of single protein application. Gene therapy may achieve greater bioavailability of growth factors within periodontal wounds, which may provide greater regenerative potential (Fig. 1).
Fig. 1.
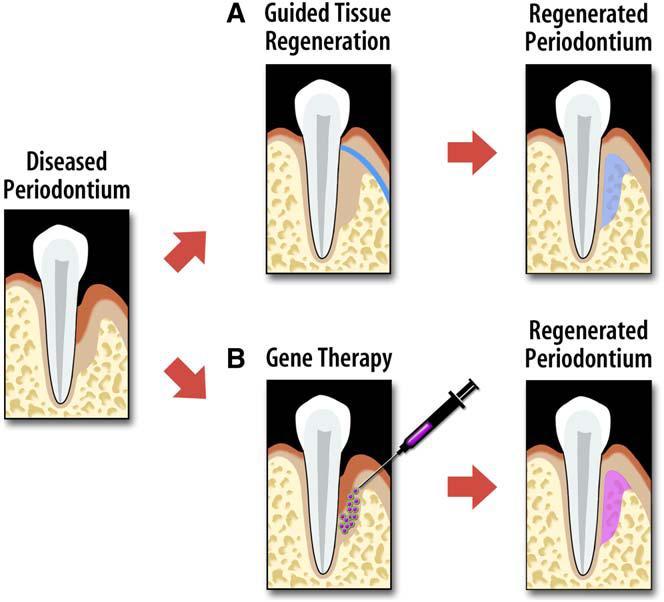
Approaches for regenerating tooth-supporting structures. (A) Guided tissue regeneration uses a cell occlusive barrier membrane to restore periodontal tissues. (B) Alternatively, an example of gene therapy uses vector-encoding growth factors aimed at stimulating the regeneration of host cells derived from the periodontium.
Gene delivery methods
In general, gene therapy involves the transfer of genetic information to target cells, which enables them to synthesize a protein of interest to treat disease [102-104]. The technology can be used to treat disorders that result from single point mutations [105] or to increase protein production [106]. The preferred strategy for gene transfer depends on the required duration of protein release and the morphology of the target site. Gene transfer is accomplished through the use of viral and nonviral vectors. Examples of viral vectors are retroviruses, adenoviruses (Ad) and adeno-associated viruses (AAV), and nonviral vectors include plasmids and DNA polymer complexes (Table 3) [107].
Table 3.
Viral and nonviral gene therapy vectors used in tissue engineering
| Vector | Type | Advantages/disadvantages |
|---|---|---|
| Retrovirus | Viral | Advantages: |
| Nonimmunogenic | ||
| Disadvantages: | ||
| Infects only dividing cells | ||
| Insertional mutagenesis | ||
| Adenovirus | Viral | Advantages: |
| Infects dividing and nondividing cells | ||
| Does not integrate into target cell genome | ||
| Disadvantages: | ||
| Potentially immunogenic | ||
| Adeno-associated virus | Viral | Advantages: |
| Infects dividing and nondividing cells | ||
| Low immunogenicity | ||
| Nonpathogenic in human | ||
| Disadvantages: | ||
| Difficult to produce at high titers | ||
| Small transgenes | ||
| Plasmid | Nonviral | Advantages: |
| Nonimmunogenic | ||
| Nonpathogenic | ||
| Disadvantages: | ||
| Low transduction efficiency | ||
| DNA polymer complexes | Nonviral | Advantages: |
| Infects dividing and nondividing cells | ||
| Cell-specific targeting | ||
| Disadvantages: | ||
| Low transduction efficiency |
Retroviruses introduce RNA together with two enzymes, called reverse transcriptase and integrase, into the target cell. Initially, the reverse transcriptase enables the production of a DNA copy from the retrovirus RNA molecule. Subsequently, the integrase adds the DNA copy into the target cell DNA. When the genetically altered host cell divides later, its descendants contain the modified DNA. Because the integrase enzyme may insert the DNA copy into an arbitrary position of the target cell DNA, gene disruption and uncontrolled cell division (ie, cancer) may occur [107].
Ad contains DNA, which is introduced into the target cell and subsequently transferred into its nucleus. In contrast to the fate of the retrovirus DNA copy, the Ad-DNA is not incorporated into the host cell's genetic material. Consequently, when the Ad-infected target cell divides later, its descendants are not genetically altered, nor do they contain the Ad-DNA genetic material [108]. AAV derive from the parvovirus family and are small viruses with a single-stranded DNA genome that causes no known human diseases [107-109]. The AAV infects dividing and nondividing cells by integrating its genetic material on chromosomes of the target cell. Types of recombinant AAV have been developed either to remain extrachromosomal or integrate into nonspecific chromosomal sites. Research has demonstrated that the AAV can be used to correct genetic defects in animals [107,110]. One disadvantage of the AAV is that it is small and possesses the capacity to carry no more than usually two genes [109].
Because nonviral alternatives do not have the drawbacks of undesired host immune reactions or potential tumorigenesis, they likely will be given more consideration in the future. Plasmids and DNA polymer complexes carry the genetic information in the form of DNA to express a foreign protein. Design features of nonviral delivery of DNA match various requirements, such as chromosomal integration or the ability to alter gene expression [107].
Gene therapy for periodontal tissue engineering
Various gene delivery methods are available to administer growth factors to periodontal defects and offer great flexibility for tissue engineering (Fig. 2). The delivery method can be tailored to the specific characteristics of the wound site. For example, a horizontal one- or two-walled defect may require the use of a supportive carrier, such as a scaffold. Other defect sites may be conducive to the use of an Ad vector embedded in a collagen matrix.
Fig. 2.
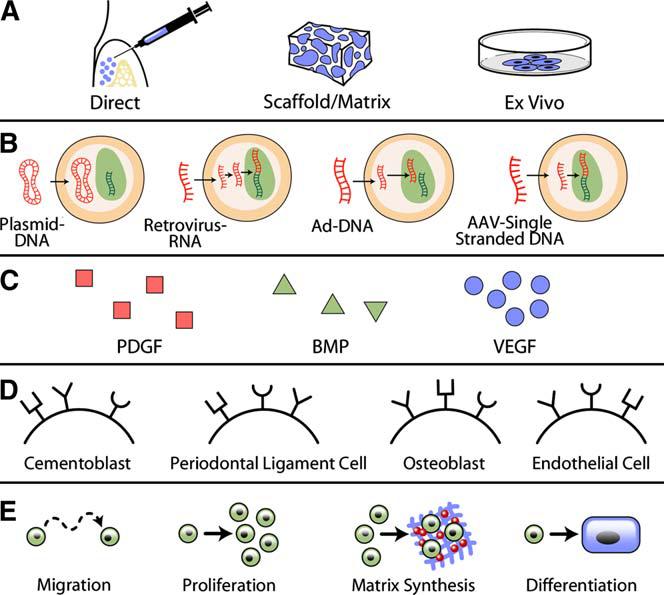
The current paradigm of gene therapy used in periodontal tissue engineering. Approaches consider (A) methods of delivery, (B) gene therapy vector, (C) tissue growth factor, (D) cellular target receptors, and (E) local effect. The choice of delivery method, DNA vector, and growth factor should maximize expected effect, minimize patient risk, and reflect the characteristics of the wound site.
More important from a clinical point of view is the risk associated with the use of gene therapy in periodontal tissue engineering [111]. As with maximizing growth factor sustainability and accounting for specific characteristics of the wound site, the DNA vector and delivery method must be considered when assessing patient safety. In summary, studies that examine the use of specific delivery methods and DNA vectors in periodontal tissue engineering reflect the aim to maximize the duration of growth factor expression, optimize delivery method to periodontal defect, and minimize patient risk.
Recently, a combination of an AAV-delivered angiogenic molecule such as VEGF, BMP signaling receptor (caALK2), and RANKL (receptor activator of nuclear factor kappa B ligand) were demonstrated to promote bone allograft turnover and osteogenesis as a mode to enrich human bone allografts [112]. To date, combinations of VEGF/BMP [113] and PDGF/VEGF [95] have been performed with highly positive synergistic responses in bone repair. Promising preliminary results from preclinical studies reveal that host modulation achieved through gene delivery of soluble proteins, such as tumor necrosis factor receptor-1, reduces tumor necrosis factor activity and inhibits alveolar bone loss [101]. These results are comparable to the findings in the research on rheumatoid arthritis, in which pathogenesis, including high tumor necrosis factor activity and pathways for bone resorption, is similar [114].
Preclinical studies that evaluate growth factor gene therapy for tissue engineering
To overcome the short half-lives of growth factor peptides in vivo, gene therapy that uses a vector that encodes the growth factor is utilized to stimulate tissue regeneration. So far, two main strategies of gene vector delivery have been applied to periodontal tissue engineering. Gene vectors can be introduced directly to the target site (in vivo technique) [99], or selected cells can be harvested, expanded, genetically transduced, and then reimplanted (ex vivo technique) (Fig. 3) [98]. In vivo gene transfer involves the insertion of the gene of interest directly into the body anticipating the genetic modification of the target cell. Ex vivo gene transfer includes the incorporation of genetic material into cells exposed from a tissue biopsy with subsequent reimplantation into the recipient.
Fig. 3.
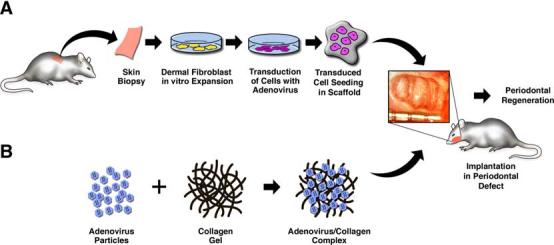
Gene delivery approaches for periodontal tissue engineering. (A) Ex vivo gene delivery involves the harvesting of tissue biopsies, expansion of cell populations, genetic manipulations of cells, and subsequent transplantation to periodontal osseous defects. (B) The in vivo gene transfer approach involves the direct delivery of growth factor transgenes to the periodontal osseous defects.
Platelet-derived growth factor gene delivery
The application of PDGF-gene transfer strategies to tissue engineering originally was generated to improve healing in soft tissue wounds, such as skin lesions [115]. Plasmid [116] and Ad/PDGF gene delivery [117] have been evaluated in preclinical and human trials. The latter approach has been able to exhibit more safety favorable for clinical use, however. [111].
Early studies in dental applications using recombinant adenoviral vectors that encode PDGF demonstrated the ability of these vector constructs to transduce potently the cells isolated from the periodontium (eg, osteoblasts, cementoblasts, PDL cells, and gingival fibroblasts) [118,119]. These studies revealed the extensive and prolonged transduction of periodontal-derived cells. Chen and Giannobile [120] were able to demonstrate the prolonged effects of Ad delivery of PDGF for the better understanding of sustained PDGF signaling. An ex vivo investigation by Anusaksathien and colleagues [121] showed that the expression of PDGF genes was prolonged for up to 10 days in gingival wounds. Ad encoding PDGF-β transduced gingival fibroblasts and enhanced defect fill by induction of human gingival fibroblast migration and proliferation. On the other hand, continuous exposure of cementoblasts to PDGF-α had an inhibitory effect on cementum mineralization, possibly via the upregulation of osteopontin and subsequent enhancement of multinucleated giant cells in cementum-engineered scaffolds. Ad/PDGF-1308 (a dominant-negative mutant of PDGF) inhibited mineralization of tissue-engineered cementum possibly because of downregulation of bone sialoprotein and osteocalcin with a persistence of stimulation of multinucleated giant cells. These findings suggested that continuous exogenous delivery of PDGF-α may delay mineral formation induced by cementoblasts, whereas PDGF clearly is required for mineral neogenesis [122].
Jin and colleagues [99] demonstrated that direct in vivo gene transfer of PDGF-B stimulated tissue regeneration in large periodontal defects. Descriptive histology and histomorphometry revealed that human PDGF-B gene delivery promotes the regeneration of cementum and alveolar bone, whereas PDGF-1308, a dominant-negative mutant of PDGF-A, has minimal effects on periodontal tissue regeneration (Fig. 4).
Fig. 4.
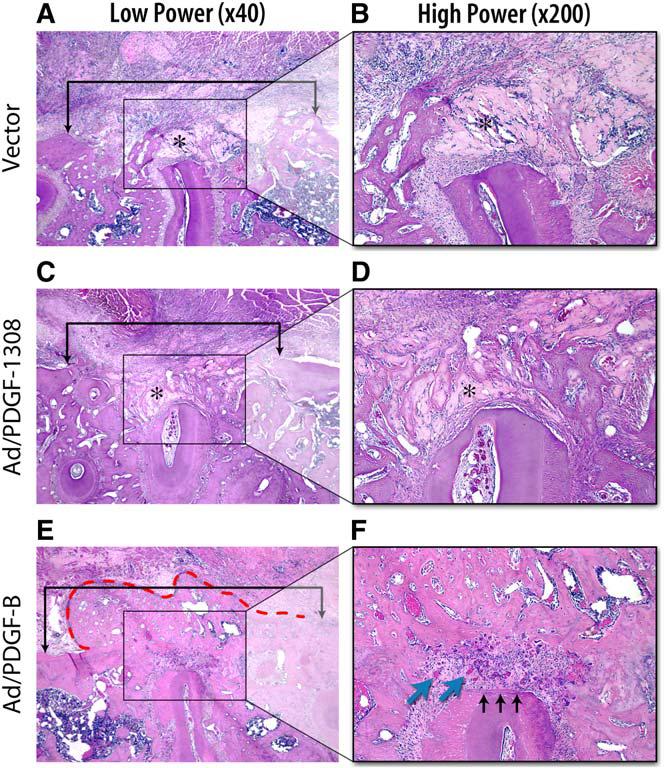
In vivo gene transfer of PDGF-B stimulates periodontal tissue regeneration. Histologic microphotographs of periodontal alveolar bone defects treated for 14 days after gene delivery of Ad/PDGF-B, Ad/PDGF-1308, or vector alone. (A, C, E, original magnification ×40; B, D, F, original magnification ×200.) Brackets in the low-power (×40) slides indicate alveolar bone wound edges, with no significant differences between the sizes of the defects based on histomorphometric analyses. Limited alveolar bone formation occurred in the Ad/PDGF-1308 and vector alone defects, whereas significant bone bridging was noted most extensively in sites treated with Ad/PDGF-B (red dashed line). (F) A thin layer of newly formed cementum (black arrows) was observed only in the Ad/PDGF-B–treated defects. More vascularization (blue arrows) was seen in the periodontal ligament region of the Ad/PDGF-B–treated lesions. Asterisks indicate the collagen carrier. (Adapted from Jin Q, Anusaksathien O, Webb SA, et al. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther 2004;9(4):522; with permission.)
Bone morphogenetic protein gene delivery
An experimental study in rodents by Lieberman and colleagues [123] demonstrated gene therapy for bone regeneration, with results revealing that the transduction of bone marrow stromal cells with rhBMP-2 lead to bone formation within an experimental defect comparable to skeletal bone. Another group was similarly able to regenerate skeletal bone by directly administering Ad5/BMP-2 into a bony segmental defect in rabbits [124]. Additional advances in the area of orthopedic gene therapy using viral delivery of BMP-2 have provided further evidence for the ability of in vivo and ex vivo bone engineering [125-128]. Franceschi and colleagues [100] investigated in vitro and in vivo Ad gene transfer of BMP-7 for bone formation. Ad-transduced nonosteogenic cells also were found to differentiate into bone-forming cells and produce BMP-7 [45] or BMP-2 [125] in vitro and in vivo. In another study by Huang and colleagues [129], plasmid DNA encoding for BMP-4 administered with a scaffold delivery system was found to enhance bone formation when compared with blank scaffolds.
In an early approach to regenerate alveolar bone in an animal model, the ex vivo delivery of Ad-encoding murine BMP-7 was found to promote periodontal tissue regeneration in large mandibular periodontal bone defects [98]. BMP-7 gene transfer not only enhanced alveolar bone repair but also stimulated cementogenesis and PDL fiber formation. Of interest, the alveolar bone formation was found to occur via a cartilage intermediate. When genes that encoded the BMP antagonist noggin were delivered, inhibition of periodontal tissue formation resulted [130]. A recent study by Dunn and colleagues [131] showed that direct in vivo gene delivery of Ad/BMP-7 in a collagen gel carrier promoted successful regeneration of alveolar bone defects around dental implants. These experiments provide promising evidence that shows the feasibility of in vivo and ex vivo gene therapy for periodontal tissue regeneration and peri-implant osseointegration.
Future perspectives: targeted gene therapy in vivo
Major advances have been made over the past decade in the reconstruction of complex periodontal and alveolar bone wounds that have resulted from disease or injury. Developments in scaffolding matrices for cell, protein, and gene delivery have demonstrated significant potential to provide “smart” biomaterials that can interact with the matrix, cells, and bioactive factors. The targeting of signaling molecules or growth factors (via proteins or genes) to periodontia has led to significant new knowledge generation using factors that promote cell replication, differentiation, matrix biosynthesis, and angiogenesis. A major challenge that has been less studied is the modulation of the exuberant host response to microbial contamination that plagues the periodontal wound microenvironment. For improvements in the outcomes in periodontal regenerative medicine, scientists must examine dual delivery of host modifiers or anti-infective agents to optimize the results of therapy. Further advancements in the field will continue to rely heavily on multidisciplinary approaches that combine engineering, dentistry, medicine, and infectious disease specialists in repairing the complex periodontal wound environment.
Summary
This article highlights the active developments in the field of periodontal regenerative medicine. Significant advancements have been made within the areas of scaffold design to promote targeted delivery of cells, genes, and proteins to chronic periodontal wounds. Results from preclinical and early clinical studies are presented, with special emphasis on the use of growth factors to promote periodontal and peri-implant bone repair.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research grants RO1-DE13397, R21-DE 016619, and RO1-DE 015384 (to W.V. Giannobile).
References
- 1.Page RC, Offenbacher S, Schroeder HE, et al. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. J Periodontol Res 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams RC. Periodontal disease. N Engl J Med. 1990;322(6):373–82. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 3.Brown LJ, Loe H. Prevalence, extent, severity and progression of periodontal disease. Periodontology 2000. 1993;2:57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 4.Kocher T, Konig J, Dzierzon U, et al. Disease progression in periodontally treated and untreated patients: a retrospective study. J Clin Periodontol. 2000;27(11):866–72. doi: 10.1034/j.1600-051x.2000.027011866.x. [DOI] [PubMed] [Google Scholar]
- 5.Heitz-Mayfield LJ, Schatzle M, Loe H, et al. Clinical course of chronic periodontitis. II. Incidence, characteristics and time of occurrence of the initial periodontal lesion. J Clin Periodontol. 2003;30(10):902–8. doi: 10.1034/j.1600-051x.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 6.Schatzle M, Loe H, Lang NP, et al. Clinical course of chronic periodontitis. III. Patterns, variations and risks of attachment loss. J Clin Periodontol. 2003;30(10):909–18. doi: 10.1034/j.1600-051x.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Taba M, Jr, Jin Q, Sugai JV, et al. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8(4):292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurinsky BS, Mills MP, Mellonig JT. Clinical evaluation of demineralized freeze-dried bone allograft and enamel matrix derivative versus enamel matrix derivative alone for the treatment of periodontal osseous defects in humans. J Periodontol. 2004;75(10):1309–18. doi: 10.1902/jop.2004.75.10.1309. [DOI] [PubMed] [Google Scholar]
- 9.Kimble KM, Eber RM, Soehren S, et al. Treatment of gingival recession using a collagen membrane with or without the use of demineralized freeze-dried bone allograft for space maintenance. J Periodontol. 2004;75(2):210–20. doi: 10.1902/jop.2004.75.2.210. [DOI] [PubMed] [Google Scholar]
- 10.Trejo PM, Weltman R, Caffesse R. Treatment of intraosseous defects with bioabsorbable barriers alone or in combination with decalcified freeze-dried bone allograft: a randomized clinical trial. J Periodontol. 2000;71(12):1852–61. doi: 10.1902/jop.2000.71.12.1852. [DOI] [PubMed] [Google Scholar]
- 11.Hartman GA, Arnold RM, Mills MP, et al. Clinical and histologic evaluation of anorganic bovine bone collagen with or without a collagen barrier. Int J Periodontics Restorative Dent. 2004;24(2):127–35. [PubMed] [Google Scholar]
- 12.Camelo M, Nevins ML, Lynch SE, et al. Periodontal regeneration with an autogenous bone-Bio-Oss composite graft and a Bio-Gide membrane. Int J Periodontics Restorative Dent. 2001;21(2):109–19. [PubMed] [Google Scholar]
- 13.Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20(1):19–29. [PubMed] [Google Scholar]
- 14.Nevins ML, Camelo M, Nevins M, et al. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20(5):458–67. [PubMed] [Google Scholar]
- 15.Richardson CR, Mellonig JT, Brunsvold MA, et al. Clinical evaluation of Bio-Oss: a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26(7):421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 16.Palti A, Hoch T. A concept for the treatment of various dental bone defects. Implant Dent. 2002;11(1):73–8. doi: 10.1097/00008505-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Scher EL, Day RB, Speight PM. New bone formation after a sinus lift procedure using demineralized freeze-dried bone and tricalcium phosphate. Implant Dent. 1999;8(1):49–53. doi: 10.1097/00008505-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nery EB, LeGeros RZ, Lynch KL, et al. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992;63(9):729–35. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 19.Sculean A, Pietruska M, Schwarz F, et al. Healing of human intrabony defects following regenerative periodontal therapy with an enamel matrix protein derivative alone or combined with a bioactive glass: a controlled clinical study. J Clin Periodontol. 2005;32(1):111–7. doi: 10.1111/j.1600-051X.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, et al. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects: a systematic review. Ann Periodontol. 2003;8(1):227–65. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 21.Trombelli L, Heitz-Mayfield LJ, Needleman I, et al. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29(Suppl 3):117–35. doi: 10.1034/j.1600-051x.29.s3.7.x. discussion 60–2. [DOI] [PubMed] [Google Scholar]
- 22.Fetner AE, Hartigan MS, Low SB. Periodontal repair using PerioGlas in nonhuman primates: clinical and histologic observations. Compendium. 1994;15(7):932. [PubMed] [Google Scholar]
- 23.Trombelli L, Minenna L, Farina R, et al. Guided tissue regeneration in human gingival recessions: a 10-year follow-up study. J Clin Periodontol. 2005;32(1):16–20. doi: 10.1111/j.0303-6979.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 24.Moses O, Pitaru S, Artzi Z, et al. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005;16(2):210–9. doi: 10.1111/j.1600-0501.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 25.Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects: a systematic review. Ann Periodontol. 2003;8(1):266–302. doi: 10.1902/annals.2003.8.1.266. [DOI] [PubMed] [Google Scholar]
- 26.Needleman I, Tucker R, Giedrys-Leeper E, et al. A systematic review of guided tissue regeneration for periodontal infrabony defects. J Periodontal Res. 2002;37(5):380–8. doi: 10.1034/j.1600-0765.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 27.Minenna L, Herrero F, Sanz M, et al. Adjunctive effect of a polylactide/polyglycolide co-polymer in the treatment of deep periodontal intra-osseous defects: a randomized clinical trial. J Clin Periodontol. 2005;32(5):456–61. doi: 10.1111/j.1600-051X.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 28.Stavropoulos A, Sculean A, Karring T. GTR treatment of intrabony defects with PLA/PGA copolymer or collagen bioresorbable membranes in combination with deproteinized bovine bone (Bio-Oss) Clin Oral Investig. 2004;8(4):226–32. doi: 10.1007/s00784-004-0277-0. [DOI] [PubMed] [Google Scholar]
- 29.Parashis A, Andronikaki-Faldami A, Tsiklakis K. Clinical and radiographic comparison of three regenerative procedures in the treatment of intrabony defects. Int J Periodontics Restorative Dent. 2004;24(1):81–90. [PubMed] [Google Scholar]
- 30.Sculean A, Chiantella GC, Windisch P, et al. Healing of intra-bony defects following treatment with a composite bovine-derived xenograft (Bio-Oss Collagen) in combination with a collagen membrane (Bio-Gide PERIO) J Clin Periodontol. 2005;32(7):720–4. doi: 10.1111/j.1600-051X.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Owczarek B, Kiernicka M, Galkowska E, et al. The application of Bio-Oss and Bio-Gide as implant materials in the complex treatment of aggressive periodontitis. Ann Univ Mariae Curie Sklodowska Med. 2003;58(1):392–6. [PubMed] [Google Scholar]
- 32.Camelo M, Nevins ML, Schenk RK, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998;18(4):321–31. [PubMed] [Google Scholar]
- 33.Rasperini G, Silvestri M, Ricci G. Long-term clinical observation of treatment of infrabony defects with enamel matrix derivative (Emdogain): surgical reentry. Int J Periodontics Restorative Dent. 2005;25(2):121–7. [PubMed] [Google Scholar]
- 34.Rosing CK, Aass AM, Mavropoulos A, et al. Clinical and radiographic effects of enamel matrix derivative in the treatment of intrabony periodontal defects: a 12-month longitudinal placebo-controlled clinical trial in adult periodontitis patients. J Periodontol. 2005;76(1):129–33. doi: 10.1902/jop.2005.76.1.129. [DOI] [PubMed] [Google Scholar]
- 35.Sanz M, Tonetti MS, Zabalegui I, et al. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: results from a multicenter practice-based clinical trial. J Periodontol. 2004;75(5):726–33. doi: 10.1902/jop.2004.75.5.726. [DOI] [PubMed] [Google Scholar]
- 36.Francetti L, Del Fabbro M, Basso M, et al. Enamel matrix proteins in the treatment of intra-bony defects: a prospective 24-month clinical trial. J Clin Periodontol. 2004;31(1):52–9. doi: 10.1111/j.0303-6979.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 37.Tonetti MS, Lang NP, Cortellini P, et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defects. J Clin Periodontol. 2002;29(4):317–25. doi: 10.1034/j.1600-051x.2002.290407.x. [DOI] [PubMed] [Google Scholar]
- 38.McCulloch CA. Basic considerations in periodontal wound healing to achieve regeneration. J Periodontol. 2000;1:16–25. [PubMed] [Google Scholar]
- 39.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3(2):129–39. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 40.Tozum TF, Demiralp B. Platelet-rich plasma: a promising innovation in dentistry. J Can Dent Assoc. 2003;69(10):664. [PubMed] [Google Scholar]
- 41.Okuda K, Kawase T, Momose M, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849–57. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 42.Marcopoulou CE, Vavouraki HN, Dereka XE, et al. Proliferative effect of growth factors TGF-beta1, PDGF-BB and rhBMP-2 on human gingival fibroblasts and periodontal ligament cells. J Int Acad Periodontol. 2003;5(3):63–70. [PubMed] [Google Scholar]
- 43.Bosshardt DD. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res. 2005;84(5):390–406. doi: 10.1177/154405910508400501. [DOI] [PubMed] [Google Scholar]
- 44.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45(2):57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 45.Krebsbach PH, Gu K, Franceschi RT, et al. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11(8):1201–10. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 46.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19(1 Suppl):23S–37. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 47.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21(9):1025–32. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 48.Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. J Periodontol 2000. 1999;19:40–58. doi: 10.1111/j.1600-0757.1999.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 49.Rutherford RB, Niekrash CE, Kennedy JE, et al. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J Periodontal Res. 1992;27(4 Pt 1):285–90. doi: 10.1111/j.1600-0765.1992.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 50.Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol. 1994;65(12):1158–68. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- 51.Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGFBB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213–25. [PubMed] [Google Scholar]
- 52.Ojima Y, Mizuno M, Kuboki Y, et al. In vitro effect of platelet-derived growth factor-BB on collagen synthesis and proliferation of human periodontal ligament cells. Oral Dis. 2003;9(3):144–51. doi: 10.1034/j.1601-0825.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 53.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor (rhPDGFBB) stimulates bone fill and rate of attachment level gain: results of a large multi-center randomized controlled trial. J Periodontol. 2005;76(12):2205–15. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 54.Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68(12):1186–93. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 55.Lynch SE, de Castilla GR, Williams RC, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62(7):458–67. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 56.Giannobile WV, Ryan S, Shih MS, et al. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol. 1998;69(2):129–37. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 57.Murakami S, Takayama S, Kitamura M, et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003;38(1):97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 58.Sigurdsson TJ, Lee MB, Kubota K, et al. Periodontal repair in dogs: recombinant human bone morphogenetic protein-2 significantly enhances periodontal regeneration. J Periodontol. 1995;66(2):131–8. doi: 10.1902/jop.1995.66.2.131. [DOI] [PubMed] [Google Scholar]
- 59.Takayama S, Murakami S, Shimabukuro Y, et al. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001;80(12):2075–9. doi: 10.1177/00220345010800121001. [DOI] [PubMed] [Google Scholar]
- 60.Terranova VP, Odziemiec C, Tweden KS, et al. Repopulation of dentin surfaces by periodontal ligament cells and endothelial cells: effect of basic fibroblast growth factor. J Periodontol. 1989;60(6):293–301. doi: 10.1902/jop.1989.60.6.293. [DOI] [PubMed] [Google Scholar]
- 61.Huang KK, Shen C, Chiang CY, et al. Effects of bone morphogenetic protein-6 on periodontal wound healing in a fenestration defect of rats. J Periodontal Res. 2005;40(1):1–10. doi: 10.1111/j.1600-0765.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 62.Wikesjo UM, Qahash M, Thomson RC, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res. 2004;15(2):194–204. doi: 10.1111/j.1600-0501.2004.00971.x. [DOI] [PubMed] [Google Scholar]
- 63.Wikesjo UM, Sorensen RG, Kinoshita A, et al. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 2004;31(8):662–70. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 64.Sorensen RG, Wikesjo UM, Kinoshita A, et al. Periodontal repair in dogs: evaluation of a bioresorbable calcium phosphate cement (Ceredex) as a carrier for rhBMP-2. J Clin Periodontol. 2004;31(9):796–804. doi: 10.1111/j.1600-051X.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 65.Gao Y, Yang L, Fang YR, et al. The inductive effect of bone morphogenetic protein (BMP) on human periodontal fibroblast-like cells in vitro. J Osaka Dent Univ. 1995;29(1):9–17. [PubMed] [Google Scholar]
- 66.Jung RE, Glauser R, Scharer P, et al. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14(5):556–68. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 67.Meraw SJ, Reeve CM, Lohse CM, et al. Treatment of peri-implant defects with combination growth factor cement. J Periodontol. 2000;71(1):8–13. doi: 10.1902/jop.2000.71.1.8. [DOI] [PubMed] [Google Scholar]
- 68.Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989;258(3):919–22. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan DR, Chao FC, Stiles CD, et al. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53(6):1043–52. [PubMed] [Google Scholar]
- 70.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16(3):201–16. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 71.Seppa H, Grotendorst G, Seppa S, et al. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92(2):584–8. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parkar MH, Kuru L, Giouzeli M, et al. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch Oral Biol. 2001;46(3):275–84. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J Dent Res. 1996;75(4):986–92. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- 74.Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64(2):142–8. doi: 10.1902/jop.1993.64.2.142. [DOI] [PubMed] [Google Scholar]
- 75.Haase HR, Clarkson RW, Waters MJ, et al. Growth factor modulation of mitogenic responses and proteoglycan synthesis by human periodontal fibroblasts. J Cell Physiol. 1998;174(3):353–61. doi: 10.1002/(SICI)1097-4652(199803)174:3<353::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 76.Zaman KU, Sugaya T, Kato H. Effect of recombinant human platelet-derived growth factor-BB and bone morphogenetic protein-2 application to demineralized dentin on early periodontal ligament cell response. J Periodontal Res. 1999;34(5):244–50. doi: 10.1111/j.1600-0765.1999.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 77.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31(5):301–12. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 78.Lynch SE, Williams RC, Polson AM, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16(8):545–8. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 79.Matsuda N, Lin WL, Kumar NM, et al. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63(6):515–25. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 80.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 81.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 82.Urist MR. Bone: formation by autoinduction. Science. 1965;150(698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 83.Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83A(Suppl 1):S1–6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 84.Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (BMPs) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210(4):383–96. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 85.Amar S, Chung KM, Nam SH, et al. Markers of bone and cementum formation accumulate in tissues regenerated in periodontal defects treated with expanded polytetrafluoroethylene membranes. J Periodontal Res. 1997;32(1 Pt 2):148–58. doi: 10.1111/j.1600-0765.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 86.Lutolf MP, Weber FE, Schmoekel HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–8. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 87.Wikesjo UM, Xiropaidis AV, Thomson RC, et al. Periodontal repair in dogs: rhBMP-2 significantly enhances bone formation under provisions for guided tissue regeneration. J Clin Periodontol. 2003;30(8):705–14. doi: 10.1034/j.1600-051x.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 88.Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76(4):605–13. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- 89.Rutherford RB, Sampath TK, Rueger DC, et al. Use of bovine osteogenic protein to promote rapid osseointegration of endosseous dental implants. Int J Oral Maxillofac Implants. 1992;7(3):297–301. [PubMed] [Google Scholar]
- 90.van den Bergh JP, ten Bruggenkate CM, Groeneveld HH, et al. Recombinant human bone morphogenetic protein-7 in maxillary sinus floor elevation surgery in 3 patients compared to autogenous bone grafts: a clinical pilot study. J Clin Periodontol. 2000;27(9):627–36. doi: 10.1034/j.1600-051x.2000.027009627.x. [DOI] [PubMed] [Google Scholar]
- 91.Anusaksathien O, Jin Q, Ma PX, et al. Scaffolding in periodontal engineering. In: Ma PX, Eliseeff J, editors. Scaffolding in tissue engineering. CRC Press; Boca Raton (FL): 2005. pp. 427–44. [Google Scholar]
- 92.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 93.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17(5):497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 94.Hutmacher DW, Teoh SH, Zein I, et al. Tissue engineering research: the engineer's role. Med Device Technol. 2000;11(1):33–9. [PubMed] [Google Scholar]
- 95.Richardson TP, Peters MC, Ennett AB, et al. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 96.Baum BJ, Goldsmith CM, Kok MR, et al. Advances in vector-mediated gene transfer. Immunol Lett. 2003;90(2–3):145–9. doi: 10.1016/j.imlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Giannobile WV. What does the future hold for periodontal tissue engineering? Int J Periodontics Restorative Dent. 2002;22(1):6–7. [PubMed] [Google Scholar]
- 98.Jin QM, Anusaksathien O, Webb SA, et al. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74(2):202–13. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin Q, Anusaksathien O, Webb SA, et al. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9(4):519–26. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franceschi RT, Wang D, Krebsbach PH, et al. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78(3):476–86. doi: 10.1002/1097-4644(20000901)78:3<476::aid-jcb12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 101.Taba M, Jr, Huffer HH, Shelburne CE, et al. Gene delivery of TNFR:Fc by adeno-associated virus vector blocks progression of periodontitis [abstract 677]. Programs and abstracts of the American Society of Gene Therapy's Eighth Annual Meeting; American Society of Gene Therapy; Saint Louis (MO). 2005. p. 262. [Google Scholar]
- 102.Friedmann T. The maturation of human gene therapy. Acta Paediatr. 1996;85(11):1261–5. doi: 10.1111/j.1651-2227.1996.tb13908.x. [DOI] [PubMed] [Google Scholar]
- 103.Baum BJ, Kok M, Tran SD, et al. The impact of gene therapy on dentistry: a revisiting after six years. J Am Dent Assoc. 2002;133(1):35–44. doi: 10.14219/jada.archive.2002.0019. [DOI] [PubMed] [Google Scholar]
- 104.Baum BJ, O'Connell BC. The impact of gene therapy on dentistry. J Am Dent Assoc. 1995;126(2):179–89. doi: 10.14219/jada.archive.1995.0143. [DOI] [PubMed] [Google Scholar]
- 105.Parekh-Olmedo H, Ferrara L, Brachman E, et al. Gene therapy progress and prospects: targeted gene repair. Gene Ther. 2005;12(8):639–46. doi: 10.1038/sj.gt.3302511. [DOI] [PubMed] [Google Scholar]
- 106.Muramatsu S, Tsukada H, Nakano I, et al. Gene therapy for Parkinson's disease using recombinant adeno-associated viral vectors. Expert Opin Biol Ther. 2005;5(5):663–71. doi: 10.1517/14712598.5.5.663. [DOI] [PubMed] [Google Scholar]
- 107.Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10(1–2):295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 108.Worgall S. A realistic chance for gene therapy in the near future. Pediatr Nephrol. 2005;20(2):118–24. doi: 10.1007/s00467-004-1680-0. [DOI] [PubMed] [Google Scholar]
- 109.Zolotukhin S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 2005;16(5):551–7. doi: 10.1089/hum.2005.16.551. [DOI] [PubMed] [Google Scholar]
- 110.Sirninger J, Muller C, Braag S, et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther. 2004;15(9):832–41. doi: 10.1089/hum.2004.15.832. [DOI] [PubMed] [Google Scholar]
- 111.Gu DL, Nguyen T, Gonzalez AM, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9(5):699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 112.Ito H, Koefoed M, Tiyapatanaputi P, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramamurthy NS, Greenwald RA, Celiker MY, et al. Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metalloproteinases. J Periodontol. 2005;76(2):229–33. doi: 10.1902/jop.2005.76.2.229. [DOI] [PubMed] [Google Scholar]
- 115.Crombleholme TM. Adenoviral-mediated gene transfer in wound healing. Wound Repair Regen. 2000;8(6):460–72. doi: 10.1046/j.1524-475x.2000.00460.x. [DOI] [PubMed] [Google Scholar]
- 116.Hijjawi J, Mogford JE, Chandler LA, et al. Platelet-derived growth factor B, but not fibroblast growth factor 2, plasmid DNA improves survival of ischemic mucocutaneous flaps. Arch Surg. 2004;139(2):142–7. doi: 10.1001/archsurg.139.2.142. [DOI] [PubMed] [Google Scholar]
- 117.Printz MA, Gonzalez AM, Cunningham M, et al. Fibroblast growth factor 2-retargeted adenoviral vectors exhibit a modified biolocalization pattern and display reduced toxicity relative to native adenoviral vectors. Hum Gene Ther. 2000;11(1):191–204. doi: 10.1089/10430340050016265. [DOI] [PubMed] [Google Scholar]
- 118.Zhu Z, Lee CS, Tejeda KM, et al. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J Dent Res. 2001;80(3):892–7. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giannobile WV, Lee CS, Tomala MP, et al. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72(6):815–23. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFalphaR and prolongs ERK and Akt/PKB activation. Am J Physiol Cell Physiol. 2002;282(3):C538–44. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anusaksathien O, Webb SA, Jin QM, et al. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9(4):745–56. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anusaksathien O, Jin Q, Zhao M, et al. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J Periodontol. 2004;75(3):429–40. doi: 10.1902/jop.2004.75.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81(7):905–17. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 124.Baltzer AW, Lattermann C, Whalen JD, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7(9):734–9. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 125.Cheng SL, Lou J, Wright NM, et al. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68(2):87–94. [PubMed] [Google Scholar]
- 126.Lee JY, Musgrave D, Pelinkovic D, et al. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83A(7):1032–9. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 127.Lee JY, Peng H, Usas A, et al. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13(10):1201–11. doi: 10.1089/104303402320138989. [DOI] [PubMed] [Google Scholar]
- 128.Musgrave DS, Bosch P, Ghivizzani S, et al. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24(6):541–7. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 129.Huang YC, Simmons C, Kaigler D, et al. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12(5):418–26. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 130.Jin QM, Zhao M, Economides AN, et al. Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res. 2004;45(1):50–9. doi: 10.1080/03008200490278142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dunn CA, Jin Q, Taba M, Jr, et al. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11(2):294–9. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


