Abstract
Calcium-mediated signaling regulates nuclear gene transcription by calcium/cAMP response element binding protein (CREB) via calcium-dependent kinases and phosphatases. This study tested the hypothesis that CREB is also present in mitochondria and subject to dynamic calcium-dependent modulation of its phosphorylation state. Antibodies to CREB and phosphorylated CREB (pCREB) were used to demonstrate the presence of both forms in isolated mitochondria and mitoplasts from rat brain. When energized mitochondria were exposed to increasing concentrations of Ca2+ in the physiological range, pCREB was lost while total CREB remained constant. In the presence of Ru360, an inhibitor of the mitochondrial Ca2+ uptake uniporter, calcium-dependent loss of pCREB levels was attenuated, suggesting that intramitochondrial calcium plays an important role in pCREB dephosphorylation. pCREB dephosphorylation was not, however, inhibited by the phosphatase inhibitors okadaic acid and Tacrolimus. In the absence of Ca2+, CREB phosphorylation was elevated by the addition of ATP to the mitochondrial suspension. Exposure of mitochondria to the pore-forming molecule alamethicin that causes osmotic swelling and release of intermembrane proteins enriched mitochondrial pCREB immunoreactivity. These results further suggest that mitochondrial CREB is located in the matrix or inner membrane and that a kinase and a calcium-dependent phosphatase regulate its phosphorylation state.
Keywords: adenosine triphosphate, kinase, phosphatase, transcription
Calcium-mediated signaling cascades play an important role in many cellular processes, including secretion of certain neurotransmitters and hormones, cell cycle control and muscle contraction. Regulation of these signaling cascades by Ca2+ is often as a result of activation of kinases, including protein kinase C (PKC) and calmodulin kinase (CAMK). Conversely, activation of phosphatases by Ca2+ also results in altering the balance of phosphorylated and non-phosphorylated target proteins. The Ca2+/cAMP response element binding protein (CREB) has been identified in several studies as a downstream target of Ca2+-mediated signaling events (Hahm et al. 2003; Mantelas et al. 2003).
Ca2+ regulates CREB-mediated signaling via activation of Ca2+-dependent kinases or by Ca2+-dependent phosphatases. The phosphorylation of CREB at a specific serine residue (Ser133) is the pivotal event regulating CREB’s ability to modulate nuclear gene transcription. CREB activation requires phosphorylation by kinases including cAMP stimulated protein kinase A (PKA), PKC, ribosomal S6 kinase (pp90RSK), CAMK, and mitogen-activated protein kinases (MAPK) (Ginty et al. 1994; de Groot et al. 1993; Brindle et al. 1995; Tan et al. 1996; Xing et al. 1998). Once phosphorylated, CREB forms a dimer via a conserved heptad repeat of leucine residues at the C-terminus then associates with CREB binding protein, which recruits and stabilizes RNA polymerase II, forming a transcription complex at the cAMP response element (CRE), an 8-bp motif 5′-TGACGTCA-3′ (Shaywitz and Greenberg 1999). Dephosphorylation of CREB by several phosphatases, including calcineurin, PP1, and PP2A, result in CREB returning to an inactive state (Choe et al. 2004; Zheng et al. 2004). Thus, the ratio of non-phosphorylated CREB to phosphorylated CREB (pCREB) becomes critical in maintaining a homeostatic cellular environment, not only physiologically, but also under pathological conditions including lack of neurotrophins or mitogens (Riccio et al. 1999), ischemia/reperfusion injury (Hu et al. 1999; Jin et al. 2001; Tanaka 2001) and other paradigms involving oxidative stress (Bedogni et al. 2003).
Most CREB studies, which included immunostaining of cells and immunoblotting of subcellular fractions, indicate that CREB is almost exclusively nuclear (Waeber and Habener 1991; Ginty et al. 1993). However, electron microscopy, gel shift and western blotting studies also identified the presence of CREB in rat brain mitochondria (Cammarota et al. 1999). While the functional significance of mitochondrial CREB is at this juncture unknown, preliminary evidence suggests that it is involved in regulation of mitochondrial gene expression (Ryu et al. Society for Neuroscience abstract, 2003). If transcription of one or more mitochondrial genes is regulated by CREB, mitochondrial CREB phosphorylation state would likely be regulated by Ca2+ and/or cAMP.
Intramitochondrial Ca2+ is known to regulate several mitochondrial metabolic enzymes, either directly or indirectly, via phosphorylation/dephosphorylation and responds to both physiological and pathological changes in cytosolic Ca2+ through Ca2+ influx and efflux pathways (for review see McCormack et al. 1990). This study tested the hypothesis that the phosphorylation state of mitochondrial CREB is regulated by extramitochondrial [Ca2+] in the physiological range and that this signal pathway is transduced through the mitochondrial Ca2+ uniport uptake mechanism.
Materials and methods
All reagents were purchased from Sigma (St Louis, MO, USA) unless otherwise stated.
Mitochondrial isolation
Forebrains removed from adult male Sprague–Dawley rats were rapidly dissected then further processed to isolate mitochondria using the percoll isolation method described by Sims (1990). Briefly, following decapitation the forebrain was rapidly removed and placed in ice-cold mannitol-sucrose (MS) buffer pH 7.4 (225 mM mannitol, 75 mM sucrose, 5 mM Hepes, 1 mM EGTA). The brain was homogenized then centrifuged twice at 1317 g for 3 min. Following a further 10-min centrifugation at 21 074 g, the pellet was re-suspended in 15% percoll (Amersham Biosciences, Piscataway, NJ, USA) then layered on a discontinuous percoll gradient and spun at 29 718 g for 8 min. The mitochondrial fraction was re-suspended in MS buffer containing 1 mg/mL BSA and centrifuged at 16 599 g for 10 min, then again at 6668 g for 10 min. The mitochondrial pellet was re-suspended in MS buffer without bovine serum albumin (BSA) or EGTA. Protein concentrations were determined by the Biuret method.
Mitoplast preparation
Isolated rat brain mitochondria (approximately 50 mg protein/mL) were diluted 1 : 1 with 12 mg/mL digitonin (Spectrum Chemical, Gardena, CA, USA) in MS isolation buffer and incubated for 20 min at 4°. The digitonin-treated mitochondria were diluted 1 : 4 in KCl buffer pH 7.0 (125 mM KCl ultrapure (Merck, Whitehouse Station, NJ, USA), 20 mM Hepes, 2 mM K2HPO4, 0.01 mM EGTA, 5 mM malate, 5 mM glutamate, 1 mM MgCl2, 3 mM ATP) and gently homogenized, then centrifuged at 18 522 g for 10 min at 4°. The supernatant was retained and the pellet re-suspended in KCl buffer then centrifuged at 18 522 g for 10 min. The pellet was re-suspended in KCl buffer and all fractions stored at −70° until utilized for western blot.
Western blot procedure
Isolated mitochondria were treated with 50 mM dithiothreitol (DTT) and NuPage 4 × LDS loading buffer (Invitrogen, Carlsbad, CA, USA) prior to heating at 70° for 10 min. The samples were rapidly centrifuged at 4° prior to separation by sodium dodecyl sulfate –polyacrylamide gel electrophresis (SDS–PAGE). Each lane was loaded with 25 μg of total protein, which was determined in separate studies to be in the linear range of the protein–immunoblot optical density relationship. Immunoblotting was performed as recommended by the manufacturers of the antibodies. Polyclonal rabbit anti-phospho-CREB (pCREB) and anti-CREB were purchased from Upstate Biotechnology (Lake Placid, NY, USA). Polyclonal rabbit anti-pCREB and anti-histone H3 were purchased from Cell Signaling Technology (Beverly, MA, USA). Cytochrome c oxidase subunit I (COX) monoclonal antibody was purchased from Molecular Probes (Eugene, OR, USA). Cytochrome c (Cyt c) monoclonal antibody was purchased from BD Biosciences (San Diego, CA, USA). Immunoreactivity was detected using the appropriate peroxidase-linked secondary antibody and enhanced chemiluminescence detection reagent purchased from Amersham Biosciences. Following detection of pCREB protein, membranes were stripped using Restore™ (Pierce, Rockford, IL, USA). The stripped membranes were re-probed with antibodies to COX, CREB, Cyt c, or histone H3. Optical densities of individual bands were quantified after subtraction of background levels of film exposure using the GelExpert software program (Nucleotech, San Carlos, CA, USA).
Enzyme-linked-immuno-sorbent assay (ELISA)
Mitochondrial samples previously assayed for immunoreactivity via western blot were also reassessed using an ELISA kit (BioSource International, Camarillo, CA, USA) that recognized pCREB phosphorylated at Ser133. pCREB levels were determined according to the manufacturer’s protocol.
Calcium-uptake assay
Freshly isolated rat brain mitochondria (0.25 mg/mL protein) in the presence of the fluorescent dye Fura 6F potassium salt (250 nM) purchased from Molecular Probes, were incubated with buffer (pH 7.0) 125 mM KCl, 20 mM Hepes, 2 mM KH2PO4, 1.0 μM EGTA, 5 mM malate, 5 mM glutamate, and 1 mM MgCl2. A further 3 mM MgCl2, 3 mM ATP and 20–400 μM Ca2+ were added to the cuvette, then the calcium concentrations in the buffer surrounding the mitochondria were measured for 5–10 min on an FL-2500 fluorescence spectrophotometer (Hitachi, Japan). The excitation wavelength for bound calcium was 340 nm and 380 nm for unbound calcium and the emission wavelength was 510 nm. Samples were then centrifuged at 18 522 g for 3 min and the pellet re-suspended in lysis buffer (pH 7.4) containing 0.5% non-idet p-40, 1% Triton X-100, 150 mM NaCl and 10 mM Tris. The aliquots were stored at −70° until western blotting or ELISA were performed.
Statistical analysis
Ca2+ uptake data are expressed as the mean ± SE and the statistical significance was determined by one-way ANOVA with the Tukey post hoc test. Statistical significance was assumed as p < 0.05. Results from tests on the effects of ATP and alamethicin are expressed as the mean ± SE and the statistical significance determined by Student t-test.
Results
Isolated rat brain mitochondria and mitoplasts possess phosphorylated CREB (pCREB) immunoreactivity
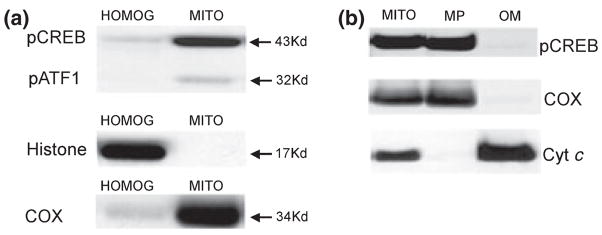
Isolated mitochondria from rat brain were analyzed by western blot using an antibody specific for CREB phosphorylated at Ser133 (pCREB). A strong band was revealed at 43 kDa with a less prominent band at 32 kDa in the mitochondrial extract. The band at 32 kDa likely represents cross reactivity to phospho-ATF1 (pATF1), as reported earlier (Huang et al. 2001). The brain homogenate at a protein level equivalent to that of isolated mitochondria exhibited a relatively weaker band at 43 kDa (Fig. 1a). The blots were then stripped and re-probed for the nuclear specific marker histone H3 to assess nuclear contamination of the mitochondrial separation. The brain homogenate extract had a very strong band at 17 kDa (Fig. 1a), whereas the mitochondrial extract had no detectable signal indicating a lack of nuclear contamination. Similar samples that were probed with histone H3 antibody then stripped and re-probed for pCREB yielded the same results (data not shown). Membranes were also stripped and re-probed with the mitochondrial marker COX revealing strong immunoreactivity for the mitochondrial extract and a relatively light band in the brain homogenate (Fig. 1a). These results suggest that pCREB immunoreactivity is present in rat brain mitochondria, in accordance with an earlier study (Cammarota et al. 1999), and that these mitochondrial extracts are not contaminated with nuclear components.
Fig. 1.
pCREB immunoreactivity in rat forebrain homogenates, isolated mitochondria. (a) Brain homogenates and isolated non-synaptosomal brain mitochondria were treated with lysis buffer. Samples (25 μg protein) were applied to each lane of the electrophoresis gel. Immunoblots for pCREB (top), the nuclear marker histone H3 (middle), and the mitochondrial inner membrane marker cytochrome c oxidase subunit I (COX, bottom) were performed. (b) Isolated non-synaptosomal brain mitochondria, mitoplasts and outer membrane fractions (25 μg protein) were electrophoretically separated then blotted for pCREB (top), COX (middle) and the intermembrane marker cytochrome c (Cyt c, bottom).
To further assess the localization of mitochondrial pCREB, isolated rat brain mitochondria were treated with the steroid glycoside digitonin to remove the outer membrane (OM), intermembrane proteins, and peripheral inner membrane proteins, e.g. cytochrome c, creating mitoplasts consisting of inner membrane and matrix proteins. Analysis by western blotting for pCREB revealed a strong band at 43 kDa in the mitoplasts (MP), with a barely detectable signal in the supernatant (OM) (Fig. 1b). The blots were then stripped and re-probed for COX, revealing a similar distribution (Fig. 1b). Conversely, the blots were stripped and re-probed for the intermembrane-localized cytochrome c (Cyt c) revealing a very strong band in the OM fraction with a barely detectable band in the MP (Fig. 1b). Taken together, these results suggest that pCREB is localized in the inner membrane or in the matrix.
Accumulation of Ca2+ in isolated mitochondria decreases pCREB levels
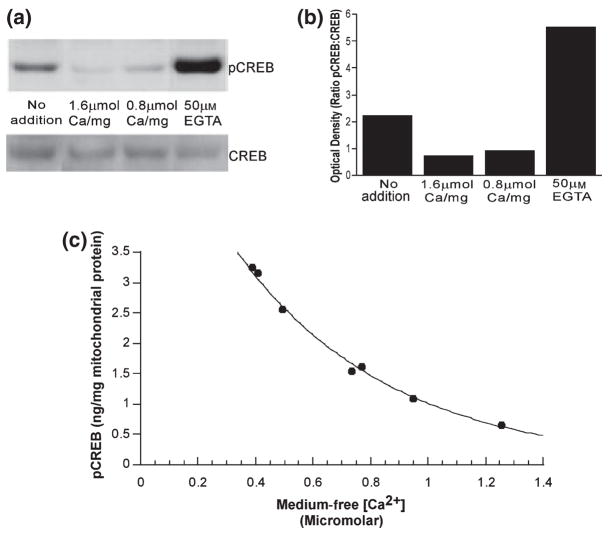
Ca2+ has been identified as a potent inducer of signal transduction pathways that have CREB as a downstream target. We therefore next determined if addition of exogenous Ca2+ to freshly isolated rat brain mitochondria would affect CREB/pCREB immunoreactivity. Isolated brain mitochondria were incubated for 5 min at 37° in a KCl-based medium containing respiratory substrates, ATP, phosphate and Mg2+ in the presence or absence of 50 μM EGTA and either 0.8 or 1.6 μmol Ca2+/mg protein. These conditions support the mitochondrial uptake and retention of over 2 μmol Ca2+/mg mitochondrial protein (Chinopoulos et al. 2003). The suspensions were then centrifuged and the mitochondrial pellets frozen and stored at −70° in lysis buffer. The mitochondrial lysates were later electrophoretically separated and immunoblotted with antibodies specific to pCREB (Fig. 2a, upper panel) then stripped and re-probed with an antibody that recognizes CREB regardless of phosphorylation state (total CREB, Fig. 2a, lower panel). Densitometric analysis of the pCREB to CREB ratio data indicates a 68% decrease in pCREB levels in isolated mitochondria exposed to 1.6 μmol Ca2+/mg mitochondrial protein as compared with basal pCREB levels (no addition). Conversely, there was a 250% increase in pCREB levels following exposure to 50 μM EGTA (Fig. 2b). As there was no consistent difference in total CREB immunoreactivity between the treatment groups (Fig. 2a, lower panel), the effect of exposure to Ca2+ or EGTA on pCREB is not because of an effect on total CREB protein levels. Subsequent measurements of pCREB utilized an enzyme-linked-immunosorbent assay (ELISA) specific for CREB phosphorylated at Ser133 as the results obtained with this convenient assay correlate well with those obtained using immunoblots (data not shown).
Fig. 2.
pCREB and total CREB Immunoreactivity in brain mitochondria following incubation in the absence and presence of Ca2+. (a) Representative immunoblots of mitochondrial lysates probed for pCREB (upper panel), then stripped and re-probed for total CREB (lower panel). (b) Optical density of bands from immunoblots (Fig. 2a) of mitochondrial lysates exposed to 50 μM EGTA or Ca2+ (0.8–1.6 μmol/mg mitochondrial protein). Data are expressed as the ratio of the pCREB : CREB optical densities and are representative of four separate experiments. (c) pCREB (ng/mg mitochondrial protein) as assessed by ELISA following exposure of isolated mitochondria to EGTA (1–101 μM). The medium free [Ca2+] was determined from Fura 6F fluorescent measurements according to Grynkiewicz et al. (1985).
To further assess the effects of extramitochondrial [Ca2+] at physiological, submicromolar concentrations, we exposed isolated rat brain mitochondria to medium containing concentrations of EGTA ranging from 1 to 101 μM. The initial medium free [Ca2+] was measured using Fura 6F and calculated according to the method of Grynkiewicz et al. (1985). Analysis of the mitochondrial samples by ELISA indicates that mitochondrial pCREB is substantially reduced by increasing extramitochondrial Ca2+ in a physiological range of approximately 0.4–1.2 μM (Fig. 2c).
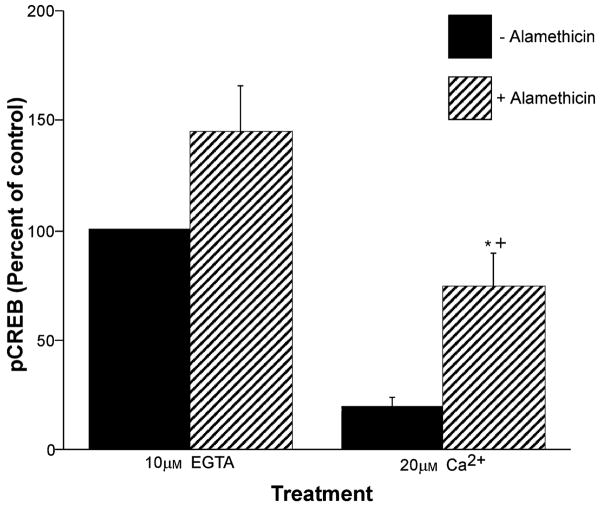
The effect of Ca2+ on pCREB phosphorylation state was also tested in the presence of the non-selective pore-forming molecule alamethicin (80 μg/mL) which causes osmotic swelling and equilibration of ions and small molecules across the mitochondrial inner membrane. Following incubation, the suspension was centrifuged and the mitochondrial pellet was used for ELISA measurements of pCREB. As in the absence of alamethicin, pCREB was significantly lower in the presence of Ca2+ compared with EGTA (Fig. 3). The additional finding that the ratio of pCREB to pellet protein was higher following alamethicin treatment is further evidence that pCREB is located on or within the mitochondrial inner membrane.
Fig. 3.
Enrichment of pCREB by mitochondrial osmotic lysis. Mitochondria were incubated for 5 min in the presence of either EGTA (10 μM) or Ca2+ (20 μM) in the absence or presence of the pore-forming molecule alamethicin (80 μg/mL). Following centrifugation, mitochondrial pellets were analyzed for pCREB using ELISA. Data are expressed as the mean percentage of control values on each ELISA plate ± SE for n = 3–5 separate experiments. +Significantly different (p < 0.01) from Ca2+ treatment alone; *significantly different (p < 0.05) from EGTA with alamethicin treatment.
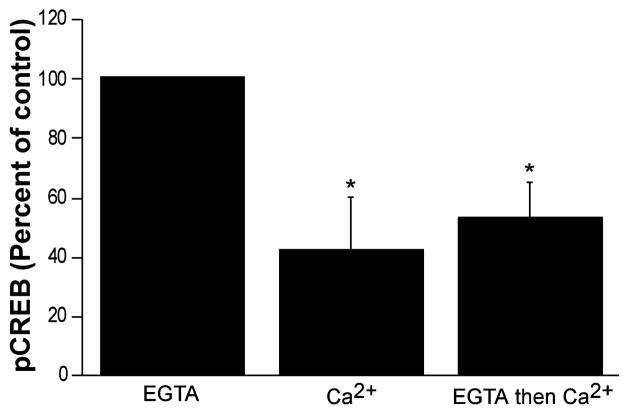
To investigate the potential dynamic regulation of Ca2+-sensitive changes in the phosphorylation state of CREB, isolated respiring rat brain mitochondria were first exposed to EGTA (10 μM) and then incubated in the presence of excess Ca2+ (20 μM) to determine if CREB phosphorylation is reversible. As shown in Fig. 4, 5 min exposure to EGTA followed by 5 min exposure to Ca2+ resulted in a loss of CREB phosphorylation that was essentially as great as a 10 min exposure to Ca2+.
Fig. 4.
Calcium-induced pCREB dephosphorylation following EGTA-induced phosphorylation. Mean pCREB levels as assessed by ELISA following exposure of isolated mitochondria to EGTA (10 μM) or Ca2+ (20 μM) alone, or addition of Ca2+ after 5 min of EGTA treatment. Data are expressed as a percentage of the control values (EGTA) on each ELISA plate. Each bar is the mean of four to five separate experiments; error bars are SEM. *Significantly different (p < 0.05) from control.
Fig. 5.
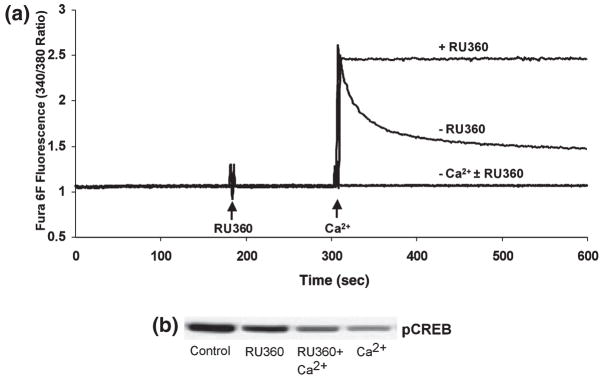
Effect of mitochondrial calcium uptake inhibition on pCREB. (a) Fura 6F fluorescent recordings of the ambient free [Ca2+] in the standard reaction medium containing isolated rat brain mitochondria (0.25 mg/mL), in the presence of the oxidizable substrates malate (5 mM) plus glutamate (5 mM) plus ATP (3 mM). The medium contained either EGTA (10 μM) or Ca2+ (20 μM) ± the mitochondrial Ca2+ uptake inhibitor RU360 (500 nM). (b) Typical immunoblot of pCREB in mitochondrial pellets obtained following centrifugation of suspensions as shown in (a), representative of three separate experiments.
Inhibition of the mitochondrial Ca2+ uniporter attenuates Ca2+-dependent pCREB loss
Ca2+ and other divalent cations enter the mitochondrial matrix via a uniporter that can be blocked by RU360, a derivative of ruthenium red (Matlib et al. 1998). To assess the role of intramitochondrial Ca2+ in altering mitochondrial pCREB levels, isolated rat brain mitochondria were exposed to 500 nM RU360 in the absence or presence of 20 μM Ca2+. RU360 inhibited the mitochondrial uptake of exogenous Ca2+ from the medium, as measured using the fluorimetric Ca2+ indicator Fura 6F (Fig. 5a). At the end of these measurements, the mitochondrial suspensions were centrifuged and the pellets used for immunoblot analysis of pCREB. The presence of RU360 partially inhibited the loss of pCREB immunoreactivity caused by exposure to Ca2+ (Fig. 5b). The inhibitory effect of RU360 on Ca2+-induced dephosphorylation may be partially masked by the small decrease in pCREB levels caused by exposure to RU360 in the absence of Ca2+.
Effect of exogenous ATP on mitochondrial pCREB
Mitochondrial CREB phosphorylation and its response to Ca2+ was measured in the absence and presence of ATP to further characterize the factors responsible for its regulation. Isolated rat brain mitochondria were exposed to either 10 or 1 μM EGTA or 20 μM Ca2+ in the presence or absence of 3 mM ATP. pCREB levels were approximately 70% lower (p < 0.05) in mitochondria exposed to EGTA in the absence of exogenous ATP compared with mitochondria exposed to EGTA in the presence of exogenous ATP (Table 1). Additionally, 20 μM Ca2+ without exogenous ATP decreased pCREB levels, although not significantly (p = 0.07) in mitochondria as compared with mitochondria exposed to Ca2+ in the presence of exogenous ATP (Table 1). Taken together, these results suggest that Ca2+-sensitive decreases in mitochondrial pCREB levels may be as a result of activation of a Ca2+-regulated phosphatase as such activity is not ATP dependent.
Table 1.
Mean pCREB values as assessed by ELISA of mitochondrial lysates in the presence or absence of ATP (3 mM) and EGTA (10 μM) or Ca2+ (20 μM)
| pCREB (ng/mg mitochondrial protein)
|
||
|---|---|---|
| − ATP | + ATP | |
| 10 μM EGTA | 1.01 ± 0.32* | 3.39 ± 0.90 |
| No addition | 0.77 ± 0.16 | 1.72 ± 0.42 |
| 20 μM Ca2+ | 0.48 ± 0.08 | 0.83 ± 0.14 |
Data are expressed as mean ng pCREB/mg mitochondrial protein from four separate experiments ± SEM.
Significantly different (p < 0.05) from control (EGTA with ATP).
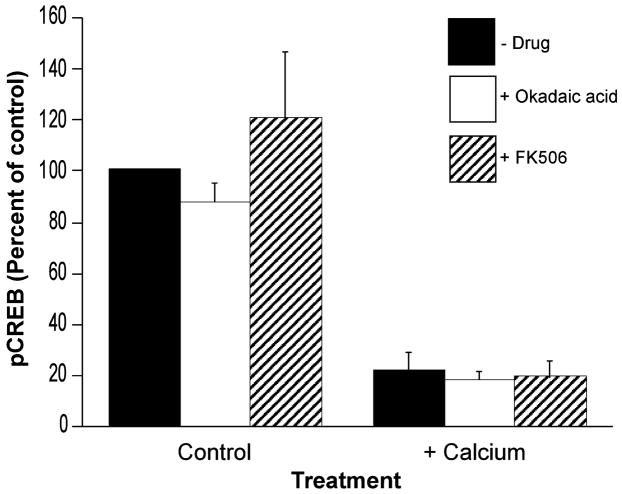
Effect of phosphatase inhibitors on Ca2+-dependent loss of mitochondrial pCREB
To determine if the Ca2+-sensitive reduction in mitochondrial pCREB immunoreactivity is because of Ca2+-dependent phosphatase activity, experiments were performed in the presence of okadaic acid (OA, 10 nM), a broad spectrum phosphatase inhibitor, and Tacrolimus (FK506, 100 nM), an inhibitor of the Ca2+-dependent phosphatase calcineurin. As expected, neither agent affected mitochondrial pCREB in the presence of EGTA (Fig. 6). These agents were, however, also ineffective at attenuating the Ca2+-dependent loss of pCREB, as determined by ELISA (Fig. 6). Cyclosporin A (CsA, 1 μM), another inhibitor of calcineurin, was also not effective at reversing the Ca2+-dependent loss of pCREB (data not shown).
Fig. 6.
Effect of phosphatase inhibitors on mitochondrial pCREB. Mitochondria were incubated for 5 min in the presence of either EGTA (10 μM) or Ca2+ (20 μM) in the absence or presence of okadaic acid (10 nM) or FK506 (100 nM). Following centrifugation, mitochondrial pellets were analyzed for pCREB using ELISA. Data are expressed as the mean percentage of control values on each ELISA plate ± SE for n = 3–12 separate experiments.
Discussion
Our results confirm an earlier finding (Cammarota et al. 1999) that CREB and pCREB immunoreactivity is present in isolated mitochondria. Additionally, following digitonin permeabilization of mitochondria, we demonstrate that pCREB immunoreactivity is present in mitoplasts, but not outer membrane fractions that contain intermembrane-associated proteins (Fig. 1b), suggesting inner membrane or matrix localization of pCREB. Recent studies describe the mitochondrial localization of other proteins involved in transcriptional regulation, including NF-κB (Cogswell et al. 2003), estrogen receptor β (Yang et al. 2004) and Smad5 (Jullig and Stott 2003). These findings suggest that mitochondrial gene regulation may be controlled by a number of heretofore uncharacterized factors.
This study is the first to demonstrate that the phosphorylation state of mitochondrial CREB is sensitive to changes in extramitochondrial free [Ca2+] in the physiological range. Blockade of the inner mitochondrial membrane uniporter by RU360 was shown in this study to attenuate, but not completely reverse, Ca2+-sensitive loss of pCREB (Fig. 5b). Relatively small changes in intramitochondrial free Ca2+ levels would be sufficient to decrease pCREB levels based on our findings in this study (Fig. 2c). The incomplete inhibition of Ca2+-dependent pCREB dephosphorylation could therefore be due to leakage of Ca2+ into the matrix.
The matrix localization of pCREB is further supported by the finding that pCREB is enriched in the mitochondrial pellet following the addition of alamethicin to the mitochondrial suspension (Fig. 3). Alamethicin releases mitochondrial intermembrane proteins e.g. cytochrome c, but many matrix proteins are retained following this treatment. The additional observation that sensitivity of pCREB dephosphorylation to Ca2+ is maintained in the presence of alamethicin indicates that the effect of Ca2+ is not because of its effects on mitochondrial bioenergetics or membrane permeability.
A partial CRE site has been demonstrated previously to be present in the regulatory D-loop of mitochondrial DNA (Ogita et al. 2002), indicating the potential for pCREB-induced mitochondrial gene transcription. Although at present a function for mitochondrial pCREB has not been elucidated, the possibility arises for mitochondrial pCREB to function as a transcription factor directly altering mitochondrial DNA or by cross-talk between the mitochondrion and the nucleus. Cross-talk between mitochondria and the nucleus as a result of cellular stress has been previously described (Biswas et al. 1999; Sawa 2001; Delsite et al. 2002). We estimate the mitochondrial pCREB contribution to total cellular pCREB is in the range of 15–20% based on relative levels of pCREB and COX immunoreactivity in the brain homogenates and isolated mitochondria (Fig. 1a). Mitochondrial pCREB therefore constitutes a significant fraction of total tissue pCREB in the rat brain.
It is well established that CREB plays a role in many diverse cellular activities, from cell growth and differentiation to apoptosis. In addition, neurodegenerative diseases, e.g. Alzheimer’s and Huntington’s diseases, and acute brain injury, have been associated with alterations in CREB-regulated gene expression. The finding that CREB is present in mitochondria, whose altered activities and gene expression are strongly implicated in these disorders, warrants further investigation into the role of mitochondrial CREB and its regulation by Ca2+ in both normal cell homeostasis and response to pathological stress.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences Grant ES07263 to RAS and NIH NS34152 and the US Army Medical Research and Material Command Neurotoxin Research Program Grant DAMD 17-99-1-9483 to GF.
Abbreviations used
- CAMK
calmodulin kinase
- COX
cytochrome c oxidase
- CRE
cAMP response element
- CREB
calcium/cAMP response element binding protein
- CsA
cyclosporin A
- Cyt c
cytochrome c
- FK506
Tacrolimus
- MAPK
mitogen-activated protein kinase
- OA
okadaic acid
- pCREB
phosphorylated calcium/cAMP response element binding protein
- PKA
cAMP stimulated protein kinase A
- PKC
protein kinase C
- pp90RSK
ribosomal S6 kinase
References
- Bedogni B, Pani G, Colavitti R, Riccio A, Borello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganese superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278:16 510–16 519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle P, Nakajima T, Montminy M. Multiple protein kinase A-regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci USA. 1995;92:10 521–10 525. doi: 10.1073/pnas.92.23.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Paratcha G, Bevilaqua L, Levi de Stein M, Lopez M, Pellegrino de Iraldi A, Izquierdo I, Medina JH. Cyclic AMP-responsive element binding protein in brain mitochondria. J Neurochem. 1999;72:2272–2277. doi: 10.1046/j.1471-4159.1999.0722272.x. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Starkov AA, Fiskum G. Cyclosporin A-insensitive permeability transition in brain mitochondria. J Biol Chem. 2003;278:27 382–27 389. doi: 10.1074/jbc.M303808200. [DOI] [PubMed] [Google Scholar]
- Choe ES, Parelkar NK, Kim JY, Cho HW, Kang HS, Mao L, Wang JQ. The protein phosphatase 1/2A inhibitor okadaic acid increases CREB and Elk-1 phosphorylation and c-fos expression in the rat striatum in vivo. J Neurochem. 2004;89:383–390. doi: 10.1111/j.1471-4159.2003.02334.x. [DOI] [PubMed] [Google Scholar]
- Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS. NF-κB and IκBα are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-κB. J Biol Chem. 2003;278:2963–2968. doi: 10.1074/jbc.M209995200. [DOI] [PubMed] [Google Scholar]
- Delsite R, Kahhap S, Anbazhagan R, Gabrielson E, Singh KK. Nuclear genes involved in mitochondria-to-nucleus communication in breast cancer cells. Mol Cancer. 2002;1:1–10. doi: 10.1186/1476-4598-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- de Groot RP, den Hertog J, Vandenheede JR, Goris J, Sassone-Corsi P. Multiple and cooperative phosphorylation events regulate the CREM activator function. EMBO J. 1993;12:3903–3911. doi: 10.1002/j.1460-2075.1993.tb06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hahm SH, Chen Y, Vinson C, Eiden LE. A calcium-initiated signaling pathway propagated through calcineurin and cAMP response element binding protein activates proenkephalin gene transcription after depolarization. Mol Pharmacol. 2003;64:1503–1511. doi: 10.1124/mol.64.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Fux CM, Martone ME, Zivin JA, Ellisman MH. Persistent phosphorylation of cyclic AMP responsive element-binding protein and activating transcription factor-2 transcription factors following transient cerebral ischemia in rat brain. Neuroscience. 1999;89:437–452. doi: 10.1016/s0306-4522(98)00352-2. [DOI] [PubMed] [Google Scholar]
- Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ. PTEN induces chemosensitivity in PTEN-mutated prostrate cancer cells by suppression of Bcl-2 expression. J Biol Chem. 2001;276:38 830–38 836. doi: 10.1074/jbc.M103632200. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao X, Simon R, Greenberg D. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global ischemia. J Mol Neurosci. 2001;16:49–56. doi: 10.1385/JMN:16:1:49. [DOI] [PubMed] [Google Scholar]
- Jullig M, Stott NS. Mitochondrial localization of Smad5 in a human chondrogenic cell line. Biochem Biophys Res Comm. 2003;307:108–113. doi: 10.1016/s0006-291x(03)01139-2. [DOI] [PubMed] [Google Scholar]
- Mantelas A, Stamatakis A, Kazanis I, Philippidis H, Stylianopoulou F. Control of neuronal nitric oxide synthase and brain-derived neurotrophic factor levels by GABA-A receptors in the developing rat cortex. Dev Brain Res. 2003;145:185–195. doi: 10.1016/j.devbrainres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10 223–10 231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Ogita K, Okuda H, Kitano M, Fujinami Y, Ozaki K, Yoneda Y. Localization of activator protein-1 complex with DNA binding activity in mitochondria of murine brain after in vivo treatment with kainite. J Neurosci. 2002;22:2561–2570. doi: 10.1523/JNEUROSCI.22-07-02561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Sawa A. Mechanisms for neuronal cell death and dysfunction in Huntington’s disease: pathological cross-talk between the nucleus and the mitochondria? J Mol Med. 2001;79:375–381. doi: 10.1007/s001090100223. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Combs MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Alteration of second messengers during acute cerebral ischemia – adenylate cyclase, cyclic AMP-dependent protein kinase, and cyclic AMP response element binding protein. Prog Neurobiol. 2001;65:173–207. doi: 10.1016/s0301-0082(01)00002-8. [DOI] [PubMed] [Google Scholar]
- Waeber G, Habener JF. Nuclear translocation and DNA recognition signals colocalized within the bZIP domain of cyclic adenosine 3′,5′-monophosphate response element-binding protein CREB. Mol Endocrinol. 1991;5:1431–1438. doi: 10.1210/mend-5-10-1431. [DOI] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Wang Z, Delbono O. Ca2+ calmodulin kinase and calcineurin mediate IGF-1-induced skeletal muscle dihydro-pyridine receptor α1S transcription. J Membrane Biol. 2004;197:101–112. doi: 10.1007/s00232-003-0645-8. [DOI] [PubMed] [Google Scholar]