Abstract
Objectives
To determine if soluble CD40 ligand (sCD40L; formally CD154) levels vary with age and to identify age-dependent ranges in healthy pediatric and adult populations.
Study design
sCD40L was measured in 25 neonates, 74 children (3 months –15 years) and 20 adults using an enzyme-linked immunosorbent assay. For age group comparisons, Mann-Whitney tests were performed. Correlation coefficients assessed relationships between plasma and serum sCD40L.
Results
Plasma sCD40L levels were higher in neonates than in all other age groups, (p<0.001). All grouped pediatric plasma levels were significantly higher than in adults (p<0.0001). There were no significant differences in plasma sCD40L between pediatric age groups. Serum levels were significantly higher in neonates than in any other age group (p <0.0001). Pediatric and adult serum sCD40L levels were not significantly different.
Conclusions
Plasma sCD40L levels are highest at birth and remain higher than those in adults throughout childhood. Reasons for such developmental changes remain to be investigated. Age appropriate reference ranges should be used when sCD40L is being evaluated in pediatric disorders.
Keywords: platelets, platelet activation, pediatric, reference values
Originally identified on B lymphocytes, CD40 is a membrane receptor protein belonging to the tumor necrosis factor (TNF) receptor family (1,2,3). Its ligand, CD40L (formally CD154), is a TNF family trimeric transmembrane protein that was initially discovered on cells of the immune system (3,4). Both CD40 and CD40L have been identified on macrophages, endothelial cells, vascular smooth muscle, fibroblasts, and most notably, platelets (3,4,5,6,7).
When platelets are activated, CD40L moves from intracellular sites to the platelet surface (1,3,4). Membrane CD40L may then be cleaved, producing a soluble trimeric fragment known as sCD40L. Over 95% of circulating CD40L derives from platelets, suggesting that these cells contribute substantially to the biological functions initiated by CD40L (1,3).
CD40L has been shown to initiate inflammatory responses including the expression of adhesion receptors and tissue factor (TF), as well as the release of chemokines (3,8,9). CD40L also plays a role in thrombosis by inducing TF expression on endothelial cells and monocytes, operating as a platelet agonist, and stabilizing arterial thrombi (1,3,8,10). Notably, these functions are not mediated exclusively via CD40L-CD40 interactions, because the integrin receptors αIIb βIII and α5β1 have also been found to bind CD40L (10,11).
Given the involvement in inflammation and thrombosis, it is not surprising that CD40L has been implicated in the pathophysiology of many disorders. Increased sCD40L levels have been found in adults with acute coronary syndromes and peripheral arterial occlusive disease, and are strongly associated with an increased risk of cardiovascular events in healthy women and in adults with cardiovascular disease (8,12,13,14). Soluble CD40L has also been found to be elevated in adults with sickle cell anemia (15), systemic lupus erythematosus (16), rheumatoid arthritis (17) and some types of pulmonary arterial hypertension (18). Plasma levels of sCD40L are elevated following cardiopulmonary bypass (19) and are predictive of restenosis following percutaneous coronary angioplasty (20).
To date, the CD40L system is virtually unexplored in children and there are no published pediatric sCD40L reference ranges. It is unknown whether, like coagulation proteins and platelet function, there are developmental changes in sCD40L levels. As observed in adults, CD40L may also play a role in childhood inflammatory diseases and hypercoagulable states and levels may correlate with disease severity or risk. We therefore pursued a study of age dependent levels of sCD40L in healthy pediatric and adult populations in order to: (1) determine whether sCD40L levels vary with age and (2) identify reference ranges to be employed in future studies of childhood diseases.
METHODS
Subjects
Cord blood samples were obtained from 25 full-term newborns. The mean (± standard deviation) time between birth and specimen processing was 132 ± 43 minutes. Blood was also obtained from 74 healthy pediatric patients (3 months –14.99 years) presenting for elective day-surgery. Twenty healthy adult volunteers (age 22–58 years) also provided specimens.
Procedures
Study approval was obtained from the Research Subjects Review Board at the University of Rochester Medical Center (URMC). Parents provided witnessed written informed consent for their child’s participation at the outpatient visit that preceded the pediatric surgical procedure. For children over 13 years, witnessed written assent was acquired. An approved informed consent procedure was also pursued for the procurement of cord blood samples. The adult volunteers were recruited by the URMC blood bank for the purpose of sCD40L assay quality control.
For all subjects, exclusion criteria included: (1) chronic medical conditions; (2) medication use; (3) recent (preceding week) or current fevers; (4) acute illness; (5) recent (preceding week) or current use of non-steroidal anti-inflammatory drugs; and (6) aspirin use in the past month. Participating children were re-evaluated at the time of surgery to ensure that they were still eligible. Cord bloods were excluded from analysis if they were greater than 3 hours old or of indeterminate age.
Within approximately 1–2 minutes following delivery, cord blood (from both the umbilical veins and arteries) was dripped into an untreated (red top) and ethylenediaminetetraacetic acid (EDTA) treated (lavender top) tube using a plastic funnel. Samples were then sent to the blood bank where they were immediately centrifuged at a relative centrifugal force of 1300g for 5 minutes. Platelet poor supernatants were separated and stored at −80°C for later analysis.
For pediatric collections, a tourniquet was applied to the upper arm and a 20- or 22-gauge angiocatheter was inserted into a peripheral vein. Blood was allowed to flow directly into an untreated (red-top) and citrated (blue-top) tube (1–2 ml in each). Care was taken to ensure that only blood from the initial angiocatheter insertion was utilized so as to avoid any confounding effects that might be produced by prolonged tourniquet use. In some instances, for children under 8 years, inhalation anesthetics (but no other medications) were given prior to the blood draw. Samples were transported within minutes to the blood bank for processing in the manner described above. In all pediatric cases, care was taken not to collect or transfer blood through a needle as such a maneuver could potentially activate platelets, causing enhanced sCD40L or cytokine release. Samples were processed quickly to minimize platelet activation.
In adults, a 21G vacutainer blood collection needle (Becton Dickinson and Company, Franklin Lakes, NJ) was inserted into an antecubital vein and blood was drawn into untreated (red-top), citrated (blue-top), and EDTA treated (lavender top) tubes. A tourniquet was employed and samples were drawn exclusively from the first attempt. Specimens were then handled as previously outlined.
Soluble CD40L was assayed using an enzyme-linked immunosorbent assay (ELISA) that captures monomeric and multimeric forms of the protein. (21).
Statistical Methods
Sample data were grouped according to subject age (Newborn, 0–5 years, 6–10 years, 11–14 years, 20 years and above) and the median, quartiles, and range (5th to 95th percentiles) of sCD40L values were determined. Individual group comparisons were made using the Mann-Whitney rank order tests. In order to assess the linear relationship between serum and plasma sCD40L, as well as the relationship between platelet count and serum or plasma sCD40L, correlation coefficients were determined. A correlation coefficient was also calculated to assess the linear relationship between citrate and EDTA prepared sCD40L plasma levels in adults. For pair-wise comparisons among the five age groups, a Bonferroni-adjusted significance level of 0.05/10 = 0.005 was used. For all other analyses, a significance level of ≤ 0.05 was employed. Analyses were performed using the statistical programs MedCalc version 9.2 for Windows (Mariakerke, Belgium), and SPSS 14.0 for Windows, Chicago, Illinois.
RESULTS
De-identified cord blood was used for the newborn sCD40L assays. Although samples likely reflect the demographics of our local population, actual male/female and racial/ethnic percentages are unknown. Of the 74 pediatric subjects, 29 were female and 45 were male. Of the 20 adults, 11 were female and 9 were male.
The median, quartiles, and range (5th to 95th percentiles) of sCD40L for each group are presented in the Table. Comparisons of median values between groups are illustrated in Figure 1. Median plasma sCD40L was significantly higher in newborns than in all other age groups (p<0.001). Median plasma sCD40L levels were significantly higher in each of the pediatric groups than in the adult cohort (p<0.0001). Median levels did not differ significantly between the pediatric age groups. Median serum levels were significantly higher in cord blood than in any other age group (p <0.0001). Pediatric and adult serum sCD40L levels were not significantly different.
Figure 1.
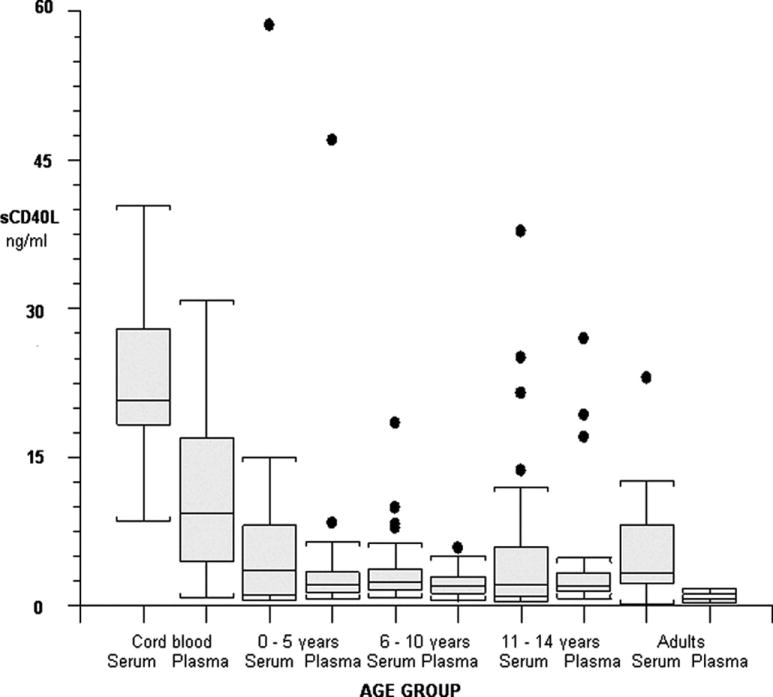
sCD40L in serum and plasma by age group. The line in each box represents the median value. The 25th and 75th percentile are at each perimeter and range/outliers are displayed.
There was a statistically significant correlation between plasma and serum sCD40L levels in cord blood specimens and in those from each pediatric age group: cord blood group, n = 25, r = 0.98 (95% CI 0.95 to 0.99), p < 0.0001; 0–5 year group, n = 23, r = 0.99 (95% CI 0.97 to 0.99), p < 0.0001; 6–10 year group, n = 27, r = 0.95 (95% CI 0.89 to 0.98), p < 0.001; 11–14 year group, n = 23, r = 0.97 (95% CI 0.93 to 0.99), p <0.0001. Median serum values were always higher than median plasma values. No correlation was apparent between plasma and serum sCD40L in adults [n = 20, r = 0.045 (95% CI −0.44 to 0.45), p = 0.98]. Notably, adult plasma obtained from EDTA and citrate anti-coagulated samples were highly correlated [n = 20, r = 0.90 (95% CI 0.75–0.95)] (Figure 2; available at www.jpeds.com).
Figure 2.
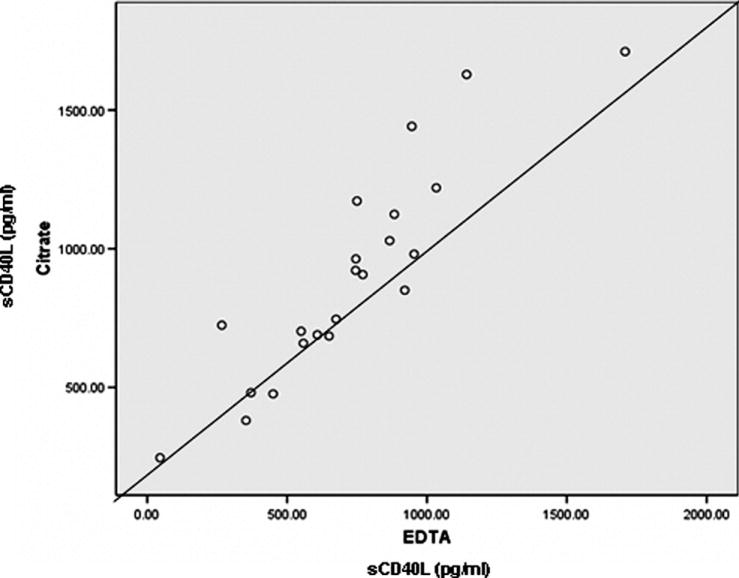
sCD40L in EDTA vs. Citrate treated adult samples.
Platelet counts were only available in the adult group. There were no statistically significant correlations between platelet count and serum or plasma sCD40L, although the existence of these relationships cannot be ruled out given the small sample size and correspondingly wide confidence intervals (data not shown).
DISCUSSION
The present study demonstrates that plasma sCD40L levels are high at birth and remain significantly higher than those of healthy adults throughout childhood. The median cord plasma sCD40L level is also significantly higher than the median level of any pediatric group. Neonatal serum sCD40L is significantly higher than in all of the older age groups.
When compared with those in older subjects, decreased neonatal platelet responses have repeatedly been identified. Larger, more active von Willebrand multimers in newborn plasma, as well as a higher hematocrit at birth, are believed to offset this deficit, resulting in shorter cord blood Platelet Function Analyser –100 closure times (22,23). It is conceivable that, given its ability to bind platelet associated CD40L, αIIb βIII, and α5β1, the increased neonatal sCD40L found in this study may further contribute to the newborn’s ability to form a primary hemostatic plug (3,10,11).
Although lower than in cord blood, pediatric plasma sCD40L levels are still significantly higher than in normal adults. During childhood and adolescence many platelet and coagulation functions become more like those of adults so that higher sCD40L during this period is not as easily ascribed to compensating hemostatic abilities. When considering developmental changes in coagulant and inhibitor levels, Monagle and colleagues hypothesized that these proteins might have additional functions that dictate age-related differences in concentration (24). It could be that there is a greater necessity at birth and during the early years of life for certain non-hemostatic CD40L activities, mandating higher levels during these periods.
Children and neonates may have fewer CD40, αIIb βIII, and α5β1expressing cells (although platelet counts are higher than in adults) or fewer receptors per cell, resulting in higher levels of circulating ligand. Alternatively, differences in ligand levels might be simply attributed to differences in platelet number because historic studies of platelet ranges reveal that platelet counts in all pediatric age groups are statistically higher than in adults (25). Although there appeared to be no correlation between an individual adult’s platelet count and the plasma sCD40L level in this study, the small sample size and wide confidence intervals undermine the confidence with which such a statement can be made. Correlations have been noted in the setting of thrombocytosis (26), but other investigations have failed to show a direct relationship between normal platelet counts and sCD40L levels (27).
Mean serum sCD40L was higher than mean plasma sCD40L in all specimens, although the degree of difference was not consistent. In addition, several of the statistically significant differences in pediatric versus adult plasma sCD40L levels were not recapitulated in the serum specimen results. Soluble CD40L is released into the supernatant during in vitro clotting, which likely accounts for more elevated serum levels. The process of clotting, and associated platelet activation, is no doubt prone to some variability, making differences between serum and plasma, as well as between age groups, less easily identified. In all, serum measurements may not provide an accurate representation of in vivo circumstances. Furthermore, although serum sCD40L is frequently reported, its validity has previously been called into question. Conde et al found that most of the CD40L in serum was associated with microparticles instead of actually representing the soluble form (28). Conversely, practically all of the CD40L in plasma was soluble, and plasma sCD40L is likely the more reliable measure.
The study has some limitations. Newborn samples were obtained from cord blood and may not represent levels present in the first few days of life. Further studies will be needed to clarify whether such high sCD40L levels are sustained during this time period. Our oldest pediatric subjects ranged from 11–14.99 years; therefore many were likely pre-pubertal. As it is possible that pubertal changes may result in lower sCD40L levels more similar to those of adults, the inclusion of an older adolescent age group would have been informative.
Pediatric and adult plasma specimens were collected in citrate, whereas cord blood samples were anticoagulated with EDTA (cord blood was collected for a number of other studies that required EDTA). Although some investigators have questioned the suitability of EDTA prepared plasma (29) for the sCD40L assay used in this study, Blake et al compared anti-coagulants and found that though citrate provided the highest reproducibility, the variation coefficient for EDTA treated samples was < 10% (30). We compared plasma sCD40L from the same adult subject’s blood separately treated with citrate or EDTA, and the results were well correlated (r = 0.90). In addition, both adult plasma EDTA and citrate samples produced significantly lower levels than cord plasma EDTA samples (p = 0.0001).
When compared to pediatric and adult levels, plasma sCD40L is high at birth. Although lower than in the neonate, plasma sCD40L levels in children remain higher than those of adults. Future research may help to answer questions regarding the underlying reasons for developmental changes in the concentration of this plasma protein. Age appropriate reference ranges should be used when sCD40L is being evaluated in pediatric disorders.
Table 1.
Plasma and serum sCD40L (ng/ml) values.
| Age group | Sample | 1st Quartile 25th percentile | Median 50th percentile | 3rd Quartile 75th percentile | 5th–95th percentiles |
|---|---|---|---|---|---|
| Cord blood N = 25 | Plasma
Serum |
5.0
18.3 |
9.4
20.9 |
15.9
27.0 |
1.5–25.8
12.3–33.8 |
| 0–5 years N = 23 | Plasma
Serum |
1.5
1.3 |
2.5
3.7 |
3.4
8.0 |
0.6–8.4
0.5–15.0 |
| 6–10 years N = 28 | Plasma
Serum |
1.3
1.7 |
2.2
2.6 |
2.9
3.6 |
0.6–5.0
0.8–9.9 |
| 11–14 years N = 23 | Plasma
Serum |
1.4
1.3 |
2.1
2.3 |
3.3
5.7 |
0.7–19.3
0.8–25.1 |
| Adultsα N = 20 | Plasma
Serum |
0.7
2.4 |
0.9
3.4 |
1.1
7.8 |
0.5–1.6
0.8–12.6 |
Represents citrate-treated samples. For EDTA treated samples: median = 0.7, range =0.3–1.1 ng/ml.
Acknowledgments
This study was funded in part by a NINDS/NIH Mid-Career Investigator Patient-Oriented award (K24 NS048323) (NBL), and by NIH grants: HL086367 (RPP, NB), HL 078603 (RPP, NB), and ES 01247 (RPP).
We would like to thank Julie Pietraszewski, PNP, for her assistance with consent/assent procedures. We also thank Walter Pegoli MD, George Drugas MD, and Mary Santos MD, for permitting us to request consent from their patients.
List of Abbreviations
- sCD40L
soluble CD40 ligand
- ELISA
enzyme-linked immunosorbent assay
- EDTA
ethylenediaminetetraacetic acid
- URMC
University of Rochester Medical Center
- TNF
tumor necrosis factor
- TF
tissue factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 2.Phipps RP. Atherosclerosis: The emerging role of inflammation and the CD40–CD40 ligand system. PNAS. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-Derived CD40L The Switch-Hitting Player of Cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 4.Wagner DD. New Links Between Inflammation and Thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 5.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(5):591–593. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 6.Henn V, Steinbach S, Bucher K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporarily limited by coexpression CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman J, Sime PJ, Phipps RP. Expression of CD154 (CD40 ligand) by human lung fibroblasts: differential regulation by IFN-gamma and IL-13, and implications for fibrosis. J Immunology. 2004;172:1862–71. doi: 10.4049/jimmunol.172.3.1862. [DOI] [PubMed] [Google Scholar]
- 8.Schonbeck U, Mach F, Sukhova GK, Herman M, Graber P, Kehry MR, et al. CD40 Ligation Induces Tissue Factor Expression in Human Vascular Smooth Muscle Cells. Am J Pathol. 2000;156:7–14. doi: 10.1016/S0002-9440(10)64699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, et al. Soluble CD40 Ligand in Acute Coronary Sundromes. N Engl J Med. 2003;348:1104–11. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 10.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a beta3 integrin-dependent mechanism. Nat Med. 2002;8:247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 11.Leveille C, Bouillon M, Guo W, Bolduc J, Sharif-Askari E, El-Fakhry Y, et al. CD40 Ligand Binds to α5β1 Integrin and Triggers Cell Signaling. J Biol Chem. 2007;282:5143–5151. doi: 10.1074/jbc.M608342200. [DOI] [PubMed] [Google Scholar]
- 12.Aukrust P, Damas JK, Solum NO. Soluble CD40 ligand and platelets: self-perpetuating pathogenic loop in thrombosis and inflammation? J Am Coll Cardiol. 2004;43:2326–2328. doi: 10.1016/j.jacc.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Garlichs CD, Eskafi S, Raaz D, Schmidt A, Ludwig J, Herrmann M, et al. Patients with acute coronary syndromes express enhanced CD40 ligand/CD154 on platelets. Heart. 2001;86:649–655. doi: 10.1136/heart.86.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and Cardiovascular Risk in Women. Circulation. 2001;104:2266–2268. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- 15.Lee SP, Ataga KI, Orringer EP, Philliips DR, Parise LV. Biologically Active CD40 Ligand is Elevated in Sickle Cell Anemia. Potential role for Platelet-Mediated Inflammation. Thromb Vasc Biol. 2006;26:1626–1631. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Santana-Sahagun E, Rassenti LZ, Weisman MH, Tamura N, Kobayashi S, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest. 1999;104:947–955. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura T, Kobayashi S, Kato K, Bando H, Haruta K, Oyanagi M, et al. Soluble CD154 in Rheumatoid Arthritis: Elevated Plasma Levels in Cases with Vasculitis. J Rheumatol. 2001;28:2583–2590. [PubMed] [Google Scholar]
- 18.Damas JK, Otterdal K, Yndestad A, Aass H, Solum NO, Froland SS, et al. Soluble CD40 Ligand in Pulmonary Arterial Hypertension: Possible Pathogenic Role of the Interaction Between Platelets and Endothelial Cells. Circulation. 2004;110:999–1005. doi: 10.1161/01.CIR.0000139859.68513.FC. [DOI] [PubMed] [Google Scholar]
- 19.Nannizzi-Alaimo L, Rubenstein MH, Alves VL, Leong GY, Phillips DR, Gold HK. CardioPulmonary Bypass Induces Release of Soluble CD40 Ligand. Circulation. 2002;105:2849–2854. doi: 10.1161/01.cir.0000019068.32280.b3. [DOI] [PubMed] [Google Scholar]
- 20.Cipollone F, Ferri C, Desideri G, Paloscia L, Materazzo G, Mascellanti M, et al. Restenosis After Coronary Angioplasty. Circulation. 2003;108:2776–2782. doi: 10.1161/01.CIR.0000103700.05109.0D. [DOI] [PubMed] [Google Scholar]
- 21.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps RP, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40 and is a cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israels SJ, Michelson AD. Antiplatelet therapy in children. Thromb Res. 2006;118:75–83. doi: 10.1016/j.thromres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Israels SJ, Cheang T, McMillan-Ward EM, Cheang M. Evaluation of primary hemostasis in neonates with a new in vitro platelet function analyzer. J Pediatr. 2001;138:116–9. doi: 10.1067/mpd.2001.109794. [DOI] [PubMed] [Google Scholar]
- 24.Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis: Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro A, Nakahata T, Matsubara K, Hayashi Y, Kato T, Suzuki Y, et al. Age-related changes in thrombopoietin in children: reference interval for serum thrombopoietin levels. Br J Haematol. 1999;106:884–888. doi: 10.1046/j.1365-2141.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 26.Viallard JF, Solanilla A, Gauthier B, Contin C, Dechanet J, Grosset C, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99:2612–14. doi: 10.1182/blood.v99.7.2612. [DOI] [PubMed] [Google Scholar]
- 27.Mason PJ, Chakrabarti S, Albers AA, Rex S, Vitseva O, Varghese S, et al. Plasma, serum, and platelet expression of CD40 ligand in adults with cardiovascular disease. Am J Cardiol. 2005;96:1365–1369. doi: 10.1016/j.amjcard.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Conde ID, Kleiman NS. Soluble CD40 Ligand in Acute Coronary Syndromes. N Engl J Med. 2003;348:2575–2577. [PubMed] [Google Scholar]
- 29.Bereczki D, Nagy E, Pal A, Magyar MT, Balla J. Should Soluble CD40 Ligand Be Measured From Serum or Plasma Samples? Arterioscler Thromb Vasc Biol. 2003;23:1129–1130. doi: 10.1161/01.ATV.0000072368.37740.8E. [DOI] [PubMed] [Google Scholar]
- 30.Blake GJ, Otsfeld RJ, Yucel EK, Varo N, Schonbeck U, Blake MA, et al. In Response to: Should Soluble CD40 Ligand Be Measured From Serum or Plasma Samples? Arterioscler Thromb Vasc Biol. 2003;23:1130–1131. doi: 10.1161/01.ATV.0000072368.37740.8E. [DOI] [PubMed] [Google Scholar]


