Abstract
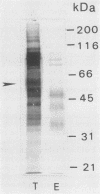
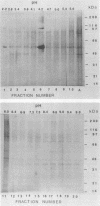
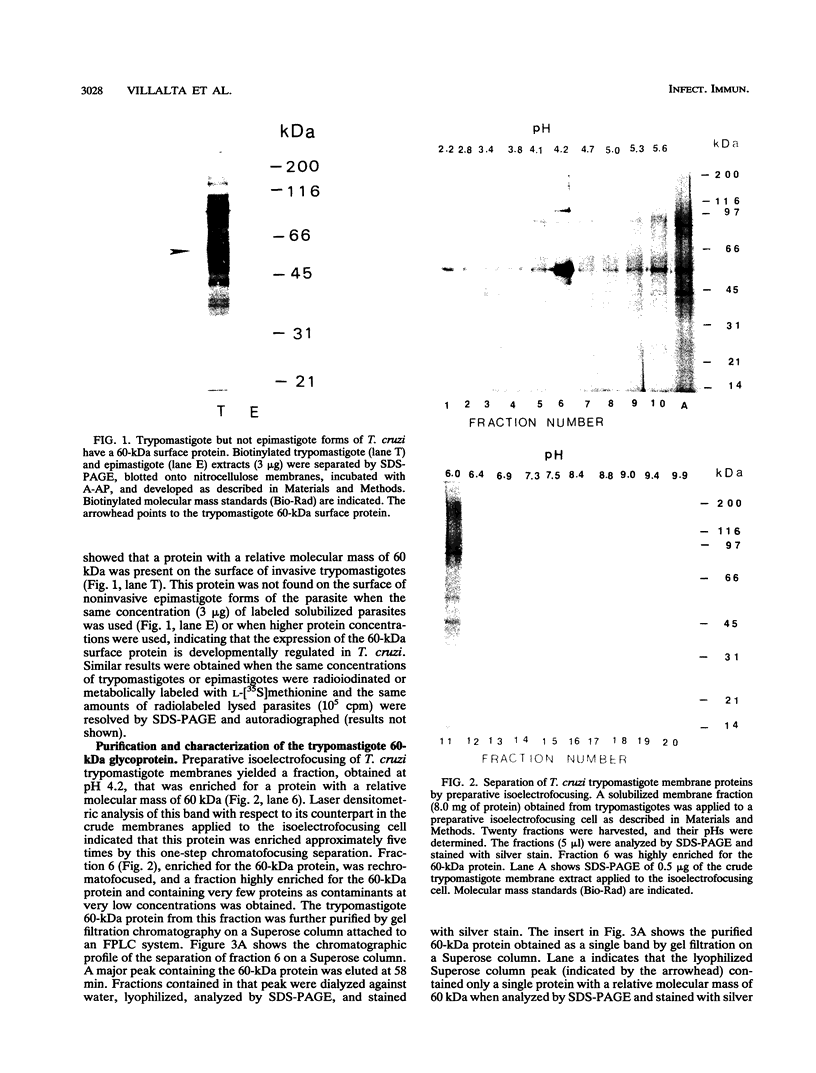
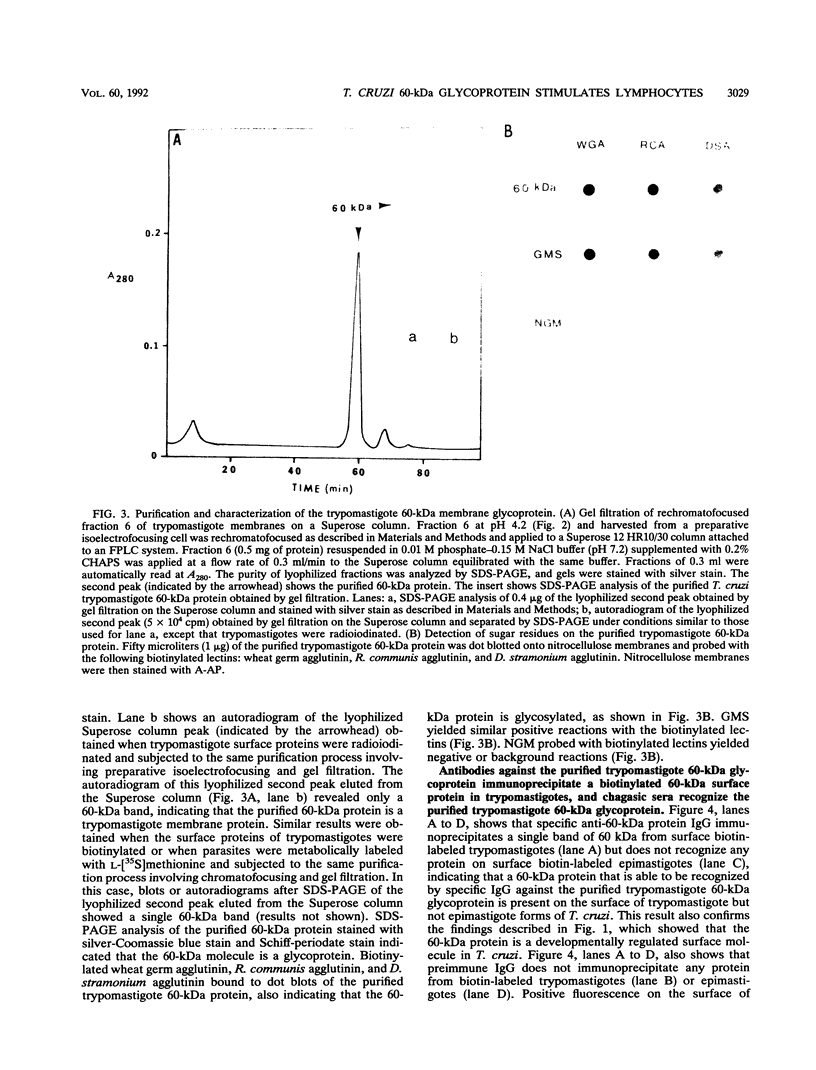
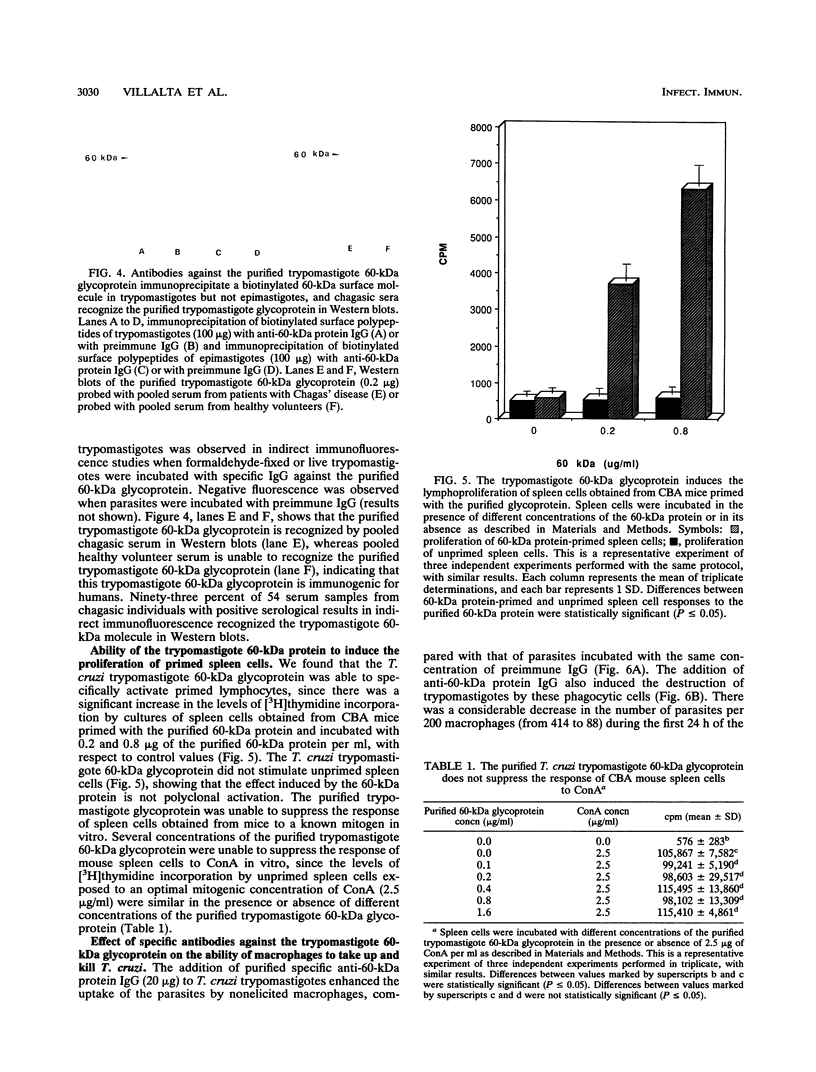
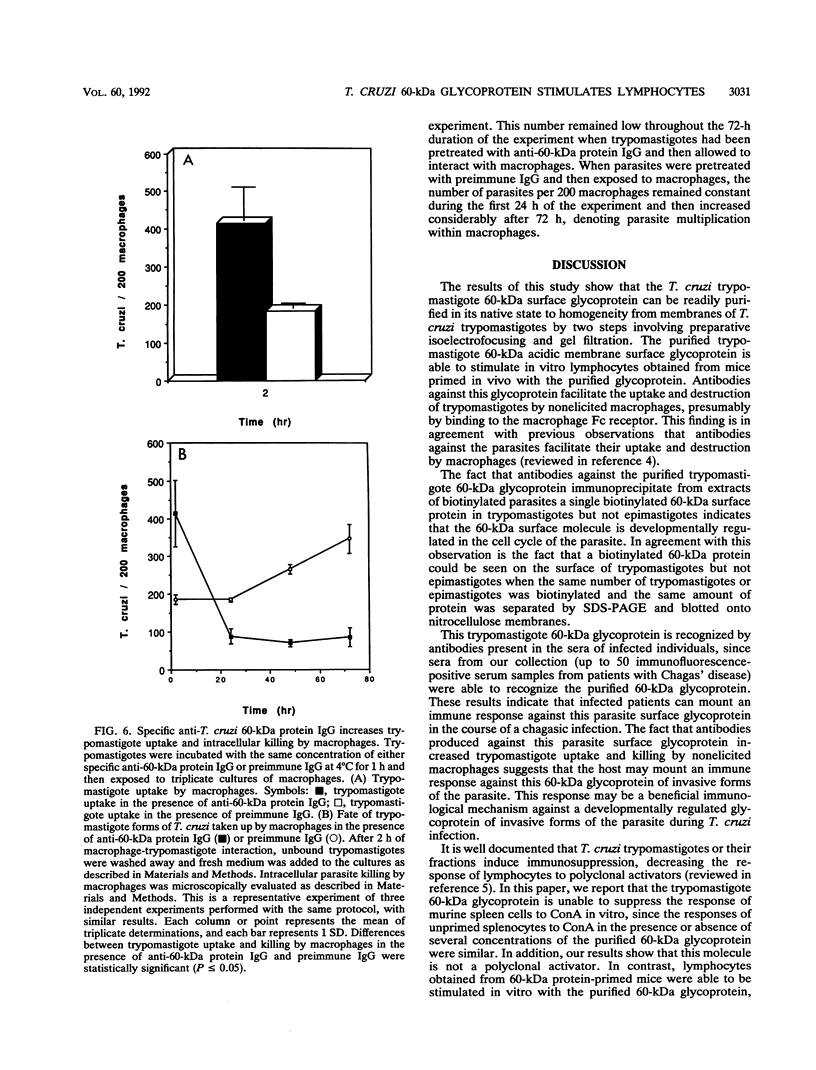
We have purified a glycoprotein with a relative molecular mass of 60 kDa and present on the surface of Trypanosoma cruzi trypomastigotes and studied its ability to prime and stimulate the proliferation of murine spleen cells. T. cruzi trypomastigote membrane proteins were separated by preparative isoelectrofocusing. A trypomastigote 60-kDa surface protein with an isoelectric point of 4.2 was enriched by chromatofocusing and was readily purified in native form to homogeneity by gel filtration on a Superose column by use of a fast protein liquid chromatography system. Biotinylated wheat germ agglutinin, Ricinus communis agglutinin, and Datura stramonium agglutinin bound to blots containing the purified trypomastigote 60-kDa surface protein, indicating that this protein was glycosylated. The purified trypomastigote 60-kDa glycoprotein was recognized by antibodies produced during human infection, and immunoglobulin G against the purified glycoprotein immunoprecipitated a biotinylated 60-kDa molecule from the surface of trypomastigotes but not epimastigotes. Specific immunoglobulin G against the 60-kDa glycoprotein also increased the uptake of trypomastigotes and promoted parasite killing by macrophages. The purified 60-kDa glycoprotein was able to specifically activate primed lymphocytes, since there was a significant increase in [3H]thymidine incorporation by spleen cells obtained from CBA mice primed with this glycoprotein, with respect to control values. Furthermore, the 60-kDa glycoprotein did not stimulate unprimed spleen cells, indicating that the lymphoproliferation induced by this glycoprotein was specific and was not due to polyclonal activation. Our findings indicate that this T. cruzi trypomastigote 60-kDa surface glycoprotein primes and activates lymphocytes, which could lead to a beneficial immune response in the host.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Grant I. H., Gold J. W., Wittner M., Tanowitz H. B., Nathan C., Mayer K., Reich L., Wollner N., Steinherz L., Ghavimi F. Transfusion-associated acute Chagas disease acquired in the United States. Ann Intern Med. 1989 Nov 15;111(10):849–851. doi: 10.7326/0003-4819-111-10-849. [DOI] [PubMed] [Google Scholar]
- Hagar J. M., Rahimtoola S. H. Chagas' heart disease in the United States. N Engl J Med. 1991 Sep 12;325(11):763–768. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- Hudson L., Britten V. Immune response to South American trypanosomiasis and its relationship to Chagas' disease. Br Med Bull. 1985 Apr;41(2):175–180. doi: 10.1093/oxfordjournals.bmb.a072046. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Villalta F., Tai P. C. Role of inflammatory cells in Chagas' disease. III. Kinetics of human eosinophil activation upon interaction with parasites (Trypanosoma cruzi). J Immunol. 1986 Jan;136(2):662–666. [PubMed] [Google Scholar]
- Kirchhoff L. V., Gam A. A., Gilliam F. C. American trypanosomiasis (Chagas' disease) in Central American immigrants. Am J Med. 1987 May;82(5):915–920. doi: 10.1016/0002-9343(87)90152-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Villalta F. Host-cell attachment by Trypanosoma cruzi: identification of an adhesion molecule. Biochem Biophys Res Commun. 1988 Aug 30;155(1):256–262. doi: 10.1016/s0006-291x(88)81077-5. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Villalta F. Trypanosoma cruzi receptors for human transferrin and their role. Mol Biochem Parasitol. 1990 Jan 15;38(2):245–252. doi: 10.1016/0166-6851(90)90027-j. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Villalta F. Trypanosoma cruzi trypomastigote clones differentially express a parasite cell adhesion molecule. Mol Biochem Parasitol. 1989 Mar 1;33(2):159–170. doi: 10.1016/0166-6851(89)90030-3. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Wallace G. R., Clarke J. L. Multiple peptide synthesis (Pepscan method) for the systematic analysis of B- and T-cell epitopes: Application to parasite proteins. Parasitol Today. 1989 Dec;5(12):397–400. doi: 10.1016/0169-4758(89)90307-4. [DOI] [PubMed] [Google Scholar]
- Nickerson P., Orr P., Schroeder M. L., Sekla L., Johnston J. B. Transfusion-associated Trypanosoma cruzi infection in a non-endemic area. Ann Intern Med. 1989 Nov 15;111(10):851–853. doi: 10.7326/0003-4819-111-10-851. [DOI] [PubMed] [Google Scholar]
- Noisin E. L., Villalta F. Fibronectin increases Trypanosoma cruzi amastigote binding to and uptake by murine macrophages and human monocytes. Infect Immun. 1989 Apr;57(4):1030–1034. doi: 10.1128/iai.57.4.1030-1034.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Cross-reactivity of vector-borne metacyclic forms of Trypanosoma cruzi with mammalian and culture stages. J Protozool. 1983 May;30(2):329–331. doi: 10.1111/j.1550-7408.1983.tb02924.x. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of cell surface mannose residues in host cell invasion by Trypanosoma cruzi. Biochim Biophys Acta. 1983 Dec 7;736(1):39–44. doi: 10.1016/0005-2736(83)90167-0. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of inflammatory cells in Chagas' disease. II. Interactions of mouse macrophages and human monocytes with intracellular forms of Trypanosoma cruzi: uptake and mechanism of destruction. J Immunol. 1984 Dec;133(6):3338–3343. [PubMed] [Google Scholar]
- Villalta F., Lima M. F., Zhou L. Purification of Trypanosoma cruzi surface proteins involved in adhesion to host cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):925–931. doi: 10.1016/0006-291x(90)90764-e. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J Immunol. 1988 Jul 1;141(1):265–272. [PubMed] [Google Scholar]