Abstract
Platelet-derived growth factor (PDGF) exerts potent effects on wound healing including the regeneration of tooth-supporting structures. Limitations of topical protein delivery to periodontal osseous defects include transient biological activity and the bioavailability of PDGF at the wound site. The objective of this investigation was to determine the feasibility of in vivo PDGF-B gene transfer to stimulate periodontal tissue regeneration in large tooth-associated alveolar bone defects in rats. Periodontal lesions (0.3 × 0.2 cm in size) were treated with a 2.6% collagen matrix alone or a matrix containing adenoviruses encoding luciferase (control), a dominant negative mutant of PDGF-A (PDGF-1308), or PDGF-B. Block biopsies were harvested at 3, 7, and 14 days post-gene delivery and descriptive histology and histomorphometric analyses were performed. The defects treated with Ad-PDGF-B demonstrated greater proliferating cell nuclear antigen positively stained cells and strong evidence of bone and cementum regeneration beyond that of Ad-luciferase and Ad-PDGF-1308 groups. Quantitative image analysis showed a nearly fourfold increase in bridging bone and sixfold increase in tooth-lining cemental repair in the Ad-PDGF-B-treated sites compared to lesions treated with Ad-luciferase or collagen matrix alone, which showed limited hard tissue neogenesis. In addition, the Xenogen In Vivo Imaging System revealed sustained and localized gene expression of the luciferase reporter at the periodontal lesions for up to 21 days after gene transfer. These results indicate that in vivo direct gene transfer of PDGF-B stimulates alveolar bone and cementum regeneration in large periodontal defects. Gene therapy utilizing PDGF-B may offer the potential for periodontal tissue engineering applications.
Keywords: gene therapy, platelet-derived growth factor, tissue engineering, periodontal disease, biomimetics, wound repair
Introduction
Periodontitis, one of the most common oral inflammatory infectious diseases and the leading cause of tooth loss, is characterized by the destruction of tooth-supporting tissues, including alveolar bone, periodontal ligament, and cementum [1]. Conventional mechanical or anti-infective periodontal therapies are focused on eliminating inflammatory sources and halting disease progression. To restore lost tooth-supporting structures, various regenerative procedures have been developed, such as osteoconductive biomaterials or cell occlusive barrier membranes [2]. However, outcomes of these therapies are limited and unpredictable in terms of regeneration. The utilization of gene therapy to sustain the release and bioavailability of osteogenic growth factors offers potential for dental tissue engineering applications [3-5].
Platelet-derived growth factor (PDGF) is a member of a multifunctional polypeptide family, which is composed of A, B, C, and D polypeptide chains that form homo- or heterodimeric molecules [6]. PDGF binds to two structurally related intrinsic tyrosine kinase receptors (PDGF-Rα and PDGF-Rβ) and subsequently exerts its biological effects on cell migration, proliferation, extracellular matrix synthesis, and anti-apoptosis [7-11]. PDGF not only plays a crucial role in the development of the heart, kidney, and vasculature [12], but also contributes to tissue repair [3]. PDGF-α and -β receptors are induced in regenerating periodontal soft and hard tissues [13,14]. In addition, PDGF initiates tooth-supporting periodontal ligament (PDL) cell chemotaxis [15], mitogenesis [16], matrix synthesis [17,18], and attachment to tooth dentinal surfaces [19]. More importantly, in vivo application of PDGF alone or in combination with insulin-like growth factor-I results in partial repair of periodontal tissues as shown in preclinical and clinical investigations [20-23].
Although clinical trial results have offered promising findings, the degree of tooth-supporting tissue regeneration is suboptimal. A possible reason for these results may be related to the short half-life of PDGF in vivo. This could be a result of proteolytic degradation, rapid diffusion, or the solubility of the delivery matrix within periodontal lesions [24]. Therefore, gene transfer may be an alternative for growth factor application to tooth-supporting defects. Delivery of PDGF by gene transfer has been shown to stimulate gingival fibroblast, PDL, and tooth-lining cell (cementoblast) mitogenesis and proliferation above that of continuous PDGF administration in vitro [25,26]. Adenovirus-mediated PDGF-B gene transfer accelerates gingival soft tissue wound healing in an ex vivo wound repair model [27]. Therefore, to explore the role of PDGF transgenes in affecting periodontal tissue repair in vivo, we applied Ad-PDGF-B or a dominant-negative mutant of the PDGF-A gene (PDGF-1308) to tooth-supporting periodontal osseous defects. The results indicate that in vivo PDGF-B gene transfer promotes regeneration of tooth-supporting structures.
Results
Adenovirus transduction efficiency
To confirm the transduction efficiency of Ad-PDGF-B into cells of rat origin, we measured PDGF-B RNA transcripts in primary cultures of rat dermal fibroblasts (DFs) transduced with Ad-PDGF-B in vitro. We used Ad-luciferase, Ad-PDGF-1308, and the cells without any adenovirus transduction (NT) as controls. As shown in Fig. 1A, the expression of PDGF-B RNA was demonstrated only in DFs transduced with Ad-PDGF-B and not in extracts from cells transduced by Ad-luciferase or Ad-PDGF-1308 or NT.
FIG. 1.
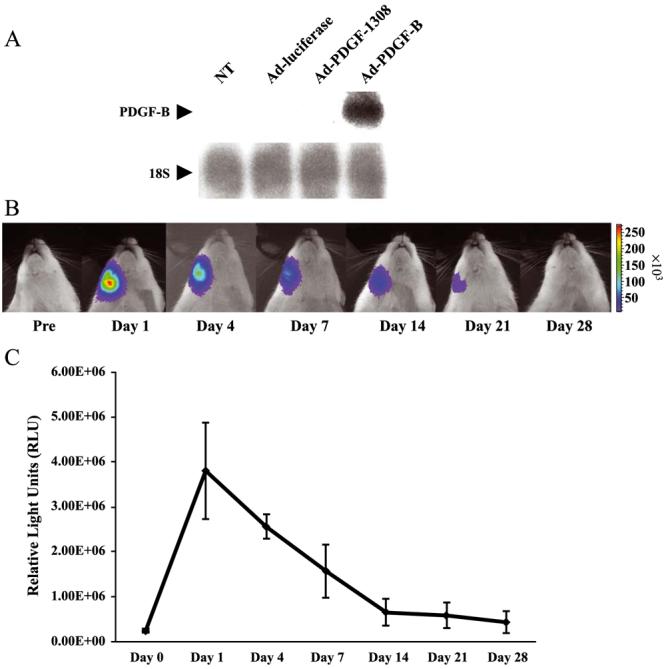
Adenovirus transduction efficiency in vitro and in vivo. (A) The transduction efficiency of Ad-PDGF-B in rat dermal fibroblasts was evaluated by Northern blotting and compared to Ad-luciferase, Ad-PDGF-1308, and no treatment (NT). Forty-eight hours after transduction, a high intensity of PDGF-B gene expression was noted following Ad-PDGF-B gene transfer, while other groups failed to exhibit PDGF-B gene expression. (B) Images of a representative rat receiving Ad-luciferase to the mandible depict the kinetics of luciferase expression for a period of 28 days. The alveolar bone of the mandibulae received 20 μl of 2.5 × 1011 PN/ml Ad-luciferase contained in a 2.6% collagen matrix. Evaluation of luciferase expression was then assessed by the use of a Xenogen In Vivo Imaging System. (C) Quantitative intensity of luciferase expression was measured as relative light units at days 1 –28 post-gene transfer. The highest level of luciferase expression occurred at day 1 post-gene transfer and rapidly decreased to 20% by day 14 compared with the expression at day 1 post-gene transfer. Luciferase expression continued to decline over time and was undetectable 28 days following gene delivery. Bars represent standard deviation (n = 3).
Kinetics of adenovirus transduction in vivo
To assess the kinetics of adenovirus transduction in vivo when delivered in the collagen matrix, we used Adluciferase to visualize transgene expression by optical imaging utilizing a charge-coupled device camera (Fig. 1B). As shown in Fig. 1C, luciferase expression was the highest at day 1 post-gene delivery and decreased at days 4 and 7 by 40 and 65%, respectively, compared to the levels of expression seen at day 1. At 14 and 28 days post-gene transfer, the luciferase expression subsequently decreased by 80 and 87% compared to the levels found at day 1, respectively.
Proliferating cell nuclear antigen (PCNA) immunolocalization at periodontal defects
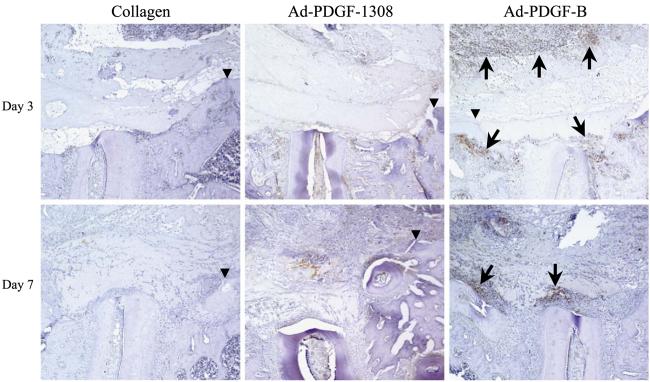
To determine the effect of the Ad-PDGF-B treatment on local DNA synthesis in the early phases of periodontal wound healing, we performed PCNA immunostaining on the specimens harvested at days 3, 7, and 14 (Fig. 2). In the Ad-PDGF-B-treated group, we found greater numbers of positively stained cells on the surfaces of the alveolar bone and denuded tooth roots, as well as the tissues surrounding the collagen matrix containing Ad-PDGF-B, compared to the other treatments at both days 3 and 7. In addition, the number and distribution of positively staining cells in the other groups (collagen alone, Ad-luciferase, and Ad-PDGF-1308) revealed no apparent differences at both 3 and 7 days postsurgery (Ad-luciferase data not shown). At day 14, we noted minimal PCNA staining for all groups, including Ad-PDGF-B (data not shown).
FIG. 2.
Photomicrographs of PCNA immunohistochemistry at days 3 and 7 postsurgery and gene delivery. Positively stained cells can be visualized by the brown nuclei. At 3 days postsurgery, most of the positively stained cells were seen along the defect periphery. However, more positive cells were detected along the defect and the carrier peripheries following Ad-PDGF-B treatment compared with the other treatment groups. At day 7, a larger number of positively stained cells were associated with the defects treated with Ad-PDGF-B compared to other groups (100× original magnification). Arrows indicate positively stained cells. Arrowheads indicate the edges of defects.
Effects of PDGF-B and PDGF-1308 gene therapy vectors on periodontal engineering
At 3 days after treatment, we observed no significant evidence of bone or cementum formation in any of the treatment groups, and very few cells invaded into the adenovirus–collagen implant (Fig. 2). In addition, we noted an inflammatory cell infiltrate in close proximity to the collagen matrix in nearly all the specimens. However, in lesions treated with Ad-PDGF-B, we found more cells on the surfaces of alveolar bone and denuded roots, as well as around the collagen matrix. At 7 days post-gene delivery, we noted some bone formation at the surface of the native alveolar bone in each group. Although we found no evidence of cementogenesis, we saw increased numbers of root-associated cells, as well as greater evidence of cellular invasion into the collagen matrix, especially in the Ad-PDGF-B group. For all groups, the inflammatory cell infiltrate persisted but at a reduced level compared to day 3. At 14 days after surgery, bone bridging and cementogenesis with fiber insertion could be seen in most of the defects treated by Ad-PDGF-B compared to the other groups (Fig. 3). In the Ad-PDGF-B group, the majority of the collagen matrix was resorbed, whereas more remnants of the collagen matrix could be found in the lesions treated by collagen alone, collagen with Ad-luc, or collagen with Ad-PDGF-1308. At day 14, we could see no significant evidence of an inflammatory cell infiltrate for all of the groups. Moreover, we saw greater evidence of vascularization in the newly formed periodontium in the Ad-PDGF-B-treated group compared to the other groups. One specimen in the collagen matrix group displayed a limited zone of ankylosis. However, in none of the specimens was any evidence of root resorption noted.
FIG. 3.
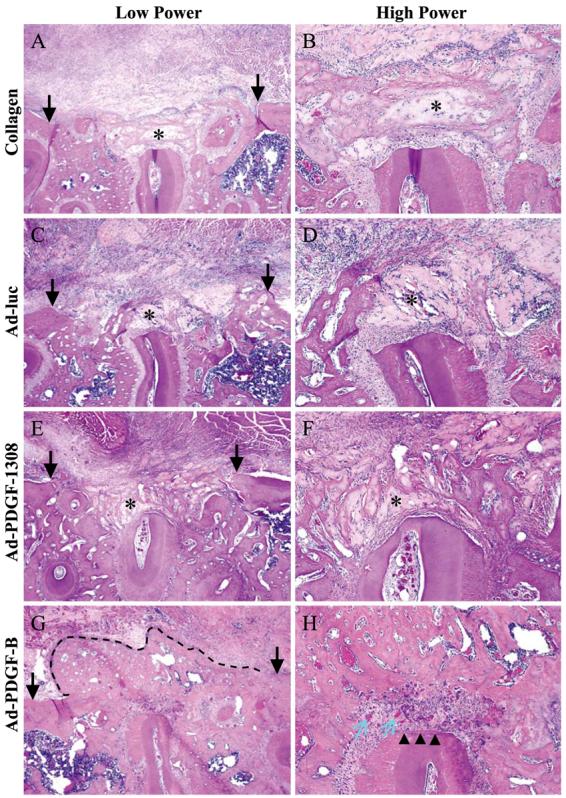
Histological microphotographs of periodontal alveolar bone defects treated for 14 days after gene delivery of Ad-PDGF-B, Ad-PDGF-1308, or Ad-luciferase or collagen matrix only (40× original magnification, left; 200× original magnification, right). Arrows on left side indicate alveolar bone wound edges. Limited alveolar bone formation occurred in the collagen, Ad-luc, and Ad-PDGF-1308 defects, while significant bone bridging was noted most extensively in sites treated with Ad-PDGF-B (dashed line). A thin layer of newly formed cementum (arrowheads) was observed only in the Ad-PDGF-B-treated defects. In addition, more vascularization (blue arrows) was seen in the periodontal ligament region of the Ad-PDGF-B-treated lesions. Asterisks indicate the collagen carrier.
The histomorphometric analysis of the specimens at day 14 postsurgery is shown in Fig. 4. There were no statistically significant differences for any measured parameter between the collagen- and the Ad-luciferase-treated groups. Ad-PDGF-B treatment increased the length of the newly formed bone in the defects by 2.5- to 4.25-fold compared to the collagen alone or the Ad-luciferase treatment. The Ad-PDGF-B treatment also enhanced the alveolar bone fill by 2- to 3-fold more than that in the Adluciferase or Ad-PDGF-1308 groups, while no difference was seen in alveolar bone fill between the Ad-PDGF-B group and the collagen alone. In addition, Ad-PDGF-B treatment increased cementum formation by 3- to 10-fold, compared to the other groups.
FIG. 4.
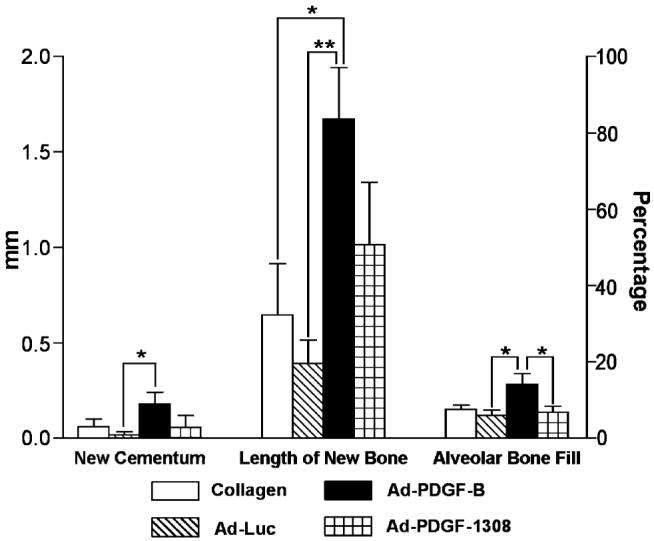
Histomorphometric analysis of the specimens 14 days postsurgery and gene delivery. Ad-PDGF-B treatment increased cementum formation, compared to Ad-luciferase treatment (*P < 0.05). Furthermore, Ad-PDGF-B treatment not only improved the bridging length of newly formed alveolar bone compared to Ad-luciferase (**P < 0.01) and collagen matrix alone (*P < 0.05) groups, but also enhanced the percentage of alveolar bone fill compared to Ad-luciferase and Ad-PDGF-1308 treatments (*P < 0.05) (n = 6–8 specimens/group).
Discussion
Restoration of tooth-supporting structures previously destroyed by chronic periodontitis is a major goal of therapy. A considerable obstacle in attempts to achieve predictable periodontal tissue regeneration is to target growth factors to periodontal lesions. Various growth factors such as PDGF-BB [21], bone morphogenetic proteins (BMPs) [28], or fibroblast growth factor-2 [29] have been shown to stimulate partial periodontal tissue regeneration. To overcome the limitations of topical growth factor protein administration, gene therapy has been considered for the restoration of lost bone and periodontal supporting structures. In this study, in vivo gene therapy was used as a mode of PDGF-BB delivery to achieve periodontal tissue regeneration. The results demonstrated that in vivo PDGF-B gene transfer enhances alveolar bone formation and cementogenesis in large periodontal bone defects.
Adenoviral vectors are a promising means of delivering genes to a wide range of cell types and tissues [30]. Adenovirus has a high transduction efficiency and does not integrate into the host genome of transduced cells [31]. Although adenovirus induces a cytotoxic T cell response, the effort to refine vector design (e.g., development of “gutted” vectors) will aid in adenovirus utility for clinical application [32].
Recently, our group reported that dermal fibroblasts (DFs) transduced with adenovirus encoding BMP-7 delivered to alveolar bone wounds stimulated periodontal tissue regeneration [33]. However, the ex vivo gene therapy approach possesses the limitations of cell procurement issues and the need for an additional surgical procedure for biopsy harvest. Therefore, an in vivo viral gene delivery approach offers a simpler method for targeting genes to periodontal defects for tissue engineering.
Development of optimized carriers for adenovirus delivery is a critical factor in gene therapy applications. Our results demonstrated that collagen will immobilize adenovirus and allow for localized in vivo transgene expression for up to 21 days (Fig. 1). Doukas et al. reported that 2.6% collagen containing Ad-luciferase had the lowest release rate (most immobilization capacity) in vitro during the first 24 h, compared to samples formulated with 0.15% collagen or 2.5% carboxymethylcellulose [34]. Recently, Beer et al. have reported that poly(lactic-glycolic) acid copolymer can be used in adenovirus-mediated direct gene delivery in vivo [35]. However, this method had a low yield in adenovirus-polymer formulation (∼10%), an organic-agent treatment process that reduces adenovirus in vivo transduction efficiency to some extent. Our study showed that the peak of luciferase expression occurred 1 day after gene transfer, which may be due to the direct diffusion of adenovirus from the 2.6% collagen matrix. Luciferase expression decreased significantly over time, but could still be detected as late as 21 days after delivery, which may be a result of collagen immobilization effect or simply reduction in transgene expression over time.
Periodontal tissue regeneration is a complex process that entails the precise regulation of various wound healing cascades [13]. Previous investigations have shown that the first step in periodontal repair is reoccupation of the defect region and the surface of the tooth root dentin by PDL cells or undifferentiated mesenchymal cells [36]. Many in vitro studies have demonstrated that PDGF not only stimulates chemotactic migration of PDL cells and osteoblastic cells, but also increases PDL cell attachment to the dentinal surface [15,19,37]. In addition, PDGF has powerful stimulatory effects on cell proliferation [38,39]. PCNA immunostaining results indicated that Ad/PDGF-B promoted greater cell PCNA via an autocrine and/or a paracrine fashion. Therefore, the effect of PDGF on PDL cell recruitment into the lesion and enhancement of cell proliferation may be a mechanism of PDGF's ability to promote periodontal tissue regeneration. Our group has previously shown that long-term delivery of PDGF by gene transfer in vitro was more effective in stimulating mitogenesis and proliferation of gingival fibroblasts, PDL cells, and cementoblasts than continuous PDGF protein application [25]. The in vivo results here showed that Ad-PDGF-B gene transfer not only enhanced the alveolar bone fill by two- to threefold, but also increased cementum formation by greater than threefold, compared to controls (Fig. 4). Thus, gene delivery may provide a more effective method for PDGF delivery to promote periodontal tissue regeneration.
Moreover, PDGF has an important effect on angiogenesis. PDGF-BB is produced by blood vessel endothelium and PDGF-Rβ has been found in pericytes [40,41]. The blood vessel maturation process, including the longitudinal spreading of pericytes or vascular smooth muscle cells (PC/vSMC) along growing blood vessels depends on PDGF-BB and PDGF-Rβ. Targeted disruption of both PDGF-BB and PDGF-Rβ genes leads to the formation of blood vessels lacking PC/vSMCs and the alteration of the integrity of vessel architecture [42]. Indeed, lesions treated with Ad-PDGF-B tended to display increased evidence of new blood vessel formation, especially in the PDL region (Figs. 3G and 3H).
PDGF-1308 is a dominant negative mutant that possesses antagonistic effects through formation of inactive and unstable heterodimers with wild-type PDGF-B or -A chains [43]. The percentage of alveolar bone fill was significantly less in the Ad-PDGF-1308-treated defects compared to the Ad-PDGF-B group. The biological activities of PDGF are exerted through the autophosphorylation of its tyrosine kinase receptors [11]. It has been shown that Ad-PDGF-1308 completely abolishes the activated state of PDGF-Rs by blocking phosphorylation of the receptor, thereby disrupting PDGF bioactivity, including its effect on cell proliferation [43-45]. In the present study, Ad-PDGF-1308 was incorporated in the collagen matrix, therefore the Ad-PDGF-1308 may infect the cells located in the defect border and exert its biological action in an autocrine fashion at the initial healing phase [45]. The mechanism involved in the retardation of alveolar bone fill in the Ad-PDGF-1308 group may be mediated by suppression of cell proliferation as shown by a low level of PCNA staining in the Ad-PDGF-1308 defects. In addition, Ad-PDGF-1308 may reduce bone formation by the suppression of extracellular bone matrix molecules such as bone sialoprotein (BSP) and osteocalcin (OCN). We have previously shown that cloned root-lining cells (cementoblasts) transduced by Ad-PDGF-1308 displayed decreased expression of the bone-associated genes BSP and OCN, which resulted in the reduction of cementoblast-induced mineral formation in vivo [46]. Although the total bone formation was reduced in the Ad-PDGF-1308 group compared to the Ad-PDGF-B-treated group (data not shown), no significant difference was seen in terms of length of new bone. One possible explanation was that more bone formation was seen in the Ad-PDGF-1308-treated group at the defect periphery, where the area may not have been influenced by Ad-PDGF-1308, while less dense bone was formed in the alveolar bone defect.
In conclusion, the results from this study demonstrated that a 2.6% collagen matrix was an effective matrix for the delivery of adenovirus particles in vivo and allowed for localized transgene expression for up to 3 weeks. Direct gene therapy of PDGF-B not only stimulated alveolar bone neogenesis and periodontal ligament formation, but also enhanced cementogenesis. PDGF gene therapy may offer promise for optimizing growth factor or cytokine delivery to the periodontium by regulating the extent and duration of factor expression to putative tissue repair cells. More studies will be needed to determine the clinical application of gene therapy for periodontal tissue engineering.
Materials and Methods
Adenovirus transduction efficiency in vitro and in vivo
To confirm adenovirus transduction efficiency, primary rat DFs were used as previously reported [44]. Briefly, rat DFs were plated at a density of 50,000 cells/cm2 in Dulbecco's modified Eagle medium (DMEM) (Gibco BRL Life Technologies, Inc., Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA, USA), antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin), and 2 mM glutamine. After overnight incubation, the cells were transduced at a multiplicity of infection of 200 in serum-free DMEM for 48 h with one of three different recombinant adenoviruses driven by the cytomegalovirus promoter, encoding human PDGF-B (Ad-PDGF-B), firefly luciferase (Ad-lucif-erase) (kind gifts from Selective Genetics, Inc., San Diego, CA, USA), or PDGF-1308 (dominant negative mutant of PDGF-A; Ad-PDGF-1308) [26]. Then, the total cellular RNA of the dermal fibroblasts transduced by the adenoviruses was extracted using a modified guanidine thiocyanate procedure with Trizol reagent [47].
Seven micrograms of total RNA was denatured, fractionated on 6% formaldehyde/1.2% agarose gels, transferred onto nylon membranes (Duralon-UV; Stratagene, Inc., La Jolla, CA, USA), and immobilized using Stratalink (Stratagene). Blots were then hybridized with 32P-labeled PDGF-B and 18S probes, generated from randomly primed cDNA (Rediprime; Amersham, Arlington Heights, IL, USA), and exposed to Kodak X-OMAT film with intensifying screens at −70°C for 6–24 h. The human PDGF-B cDNA probe, an ∼570-bp product, was obtained from a human PDGF-B-inserted pBSIISK(+) plasmid (Selective Genetics) following linearization with HindIII and BamHI. 18S was used to evaluate the relative loading and transfer efficiency of RNA samples.
Kinetics of luciferase expression by Ad-luciferase–collagen matrix in vivo
Ad-luciferase was formulated with 6.5% collagen matrix (Matrix Pharmaceutical, Inc., Fremont, CA, USA) as previously reported [34] to a final adenovirus–collagen mixture containing 5 × 109 particles of Adluciferase in 20 μl 2.6% collagen. Then, 20 μl of the adenovirus–collagen mixture was delivered to the alveolar bone buccal sides of the mandible of Sprague–Dawley rats. At 1, 4, 7, 14, 21, and 28 days postadministration, the luciferase expression was measured with the Xenogen In Vivo Imaging System (Xenogen Corp., Alameda, CA, USA) 12–15 min after luciferase substrate luciferin injection (4 mg luciferin/25 g body weight).
PDGF gene transfer to periodontal alveolar bone wounds
Immediately prior to implantation into the wounds, three different adenovirus–collagen formulations were prepared, containing 2.5 × 1011 viral particles (PN)/ml 2.6% collagen of Ad-luciferase, Ad-PDGF-B, or Ad-PDGF-1308. A periodontal alveolar bone defect model was used as previously described [33]. In brief, a total of 30 Sprague–Dawley rats (260–350 g; Harlan, Indianapolis, IN, USA) were anesthetized with ketamine and xylazine, and bilateral 0.3 × 0.2-cm mandibular osseous defects were created in the buccal plate overlying the first molar and second molar tooth roots. The exposed tooth roots were carefully denuded of periodontal ligament, cementum, and superficial dentin. Then, ∼20 μl of the adenovirus–collagen construct containing one of the three adenoviruses (Ad-luciferase, Ad-PDGF-B, or Ad-PDGF-1308) or 2.6% collagen matrix alone was delivered to the defects. After surgery, the rats were administered supplemental antibiotics (ampicillin 268 μg/ml of drinking water) daily for 14 days. Animals were sacrificed and block biopsies harvested at day 3 (n = 6), day 7 (n = 10), and day 14 (n = 14) post-gene delivery. Each treatment was introduced randomly into one defect on one side of the rat mandibulae (providing n = 6–8 defects/group).
Histology
At the time of sacrifice, the mandibulae were placed into Bouin's fixative (Polysciences, Warrington, PA, USA), decalcified with 10% vol/vol acetic acid, 4% vol/vol formaldehyde, 0.85% NaCl for 2 to 3 weeks, and then embedded in paraffin. The specimens were cut into 4-to 5-μm coronal sections and were either stained with hematoxylin and eosin or prepared for immunohistochemical staining.
Immunohistochemical staining for PCNA
The effect of the PDGF transgenes on a marker for cell proliferation was assessed using immunostaining for PCNA from tissue specimens at days 3, 7, and 14. The tissue specimens were deparaffinized in xylene and rehydrated with gradient alcohol. Antigen retrieval was performed by incubating the specimens in 10 mM sodium citrate (pH 6) at 90–95°C for 10 min. The specimens were allowed to cool to room temperature (RT) and washed twice with 50 mM Tris–HCl buffer. Endogenous peroxidase was blocked with 3% H2O2 in 50 mM Tris–HCl buffer for 5 min, followed by washing. Nonspecific binding was blocked by incubating the specimens with 2% normal goat serum in 50 mM Tris–HCl buffer for 30 min. Mouse monoclonal anti-rat PCNA (LSAB2 kit; Dako Corp., Carpinteria, CA, USA) was applied onto the specimens at 1:200 dilution in 50 mM Tris–HCl buffer containing 1% bovine serum albumin for 10 min at RT. Mouse monoclonal IgG2a diluted at the same concentration was used as the negative control. The specimens were washed three times. The secondary antibody staining method followed the manufacturer's protocol. Color was developed using a DAB staining solution (Dako Corp.) for 3–5 min at RT. The specimens were washed two times, counterstained with Gill's hematoxylin formulation No. 1 (Fisher Scientific, Fair Lawn, NJ, USA) for 1 min, rinsed in running water, dehydrated through a series of gradient alcohol and xylene, and then mounted with mounting medium (Fisher Scientific).
Histomorphometry
Computer-assisted image analysis was utilized to determine new cementum formation, length of new bone, and bone defect fill, indicators of periodontal tissue repair. Images of specimens were captured using a Nikon Eclipse E800 microscope (Nikon, Inc., Melville, NY, USA) fitted with a SPOT-2 camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) for analysis using Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). Images of coded specimens were captured at 20, 40, 100, and 200× magnification for histomorphometric analysis. A single masked, calibrated examiner evaluated all of the slides and demonstrated a pre- and poststudy calibration inter- and intraexaminer error of <5% compared to a standard examiner. Parameters of periodontal regeneration were measured as previously described [33]: (1) for new cementum, length of new cementum on the distal root of the first molar was measured as the total length of mineral-like tissue on the denuded root surface in millimeters; (2) for length of new bone, length of new bridging bone was measured from the borders of the original osseous defect (mesially–distally) in millimeters; and (3) for alveolar bone defect fill, percentage of area of new bone filling the original bone defect area not comprising the zone of newly formed PDL was assessed. Mean values were generated for each of the groups evaluated. The decoded specimens were then analyzed using one-way analysis of variance and Bonferroni's multiple comparison test to measure statistical differences among groups.
Acknowledgments
This study was supported by NIH/NIDCR Grants DE 11960 and DE 13397 to W.V.G., as well as by NIH/NCI Grant R24CA83099 to Dr. Brian D. Ross from the Center for Molecular Imaging. The authors thank Mr. Christopher Strayhorn for expert histological assistance and Mr. Dan Hall for luciferase imaging assistance. The authors thank Dr. Lois Chandler for critically reading the manuscript.
References
- 1.Williams RC. Periodontal disease. N. Engl. J. Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Anusaksathien O, Jin Q, Ma P, Giannobile WV. Scaffolding in periodontal engineering. In: Ma PX, Eliseeff J, editors. Scaffolding in Tissue Engineering. Dekker; New York: 2004. [Google Scholar]
- 3.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr. Pharm. Biotechnol. 2002;3:129–139. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 5.Baum BJ, Mooney DJ. The impact of tissue engineering on dentistry. J. Am. Dent. Assoc. 2000;131:309–318. doi: 10.14219/jada.archive.2000.0174. [DOI] [PubMed] [Google Scholar]
- 6.Ostman A, Heldin CH. Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv. Cancer Res. 2001;80:1–38. doi: 10.1016/s0065-230x(01)80010-5. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043–1052. [PubMed] [Google Scholar]
- 8.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J. Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem. J. 1989;258:919–922. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 11.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski WE, et al. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood. 2001;97:1990–1998. doi: 10.1182/blood.v97.7.1990. [DOI] [PubMed] [Google Scholar]
- 13.Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch. Oral Biol. 2001;46:275–284. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 14.Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J. Periodontal Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J. Dent. Res. 1996;75:986–992. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- 16.Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J. Periodontol. 1993;64:142–148. doi: 10.1902/jop.1993.64.2.142. [DOI] [PubMed] [Google Scholar]
- 17.Haase HR, Clarkson RW, Waters MJ, Bartold PM. Growth factor modulation of mitogenic responses and proteoglycan synthesis by human periodontal fibroblasts. J. Cell. Physiol. 1998;174:353–361. doi: 10.1002/(SICI)1097-4652(199803)174:3<353::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J. Periodontol. 1992;63:515–525. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 19.Zaman KU, Sugaya T, Kato H. Effect of recombinant human platelet-derived growth factor-BB and bone morphogenetic protein-2 application to demineralized dentin on early periodontal ligament cell response. J. Periodontal Res. 1999;34:244–250. doi: 10.1111/j.1600-0765.1999.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 20.Lynch SE, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J. Periodontol. 1991;62:458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 21.Giannobile WV, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal. Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 22.Howell TH, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 23.Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int. J. Periodont. Restor. Dent. 2003;23:213–225. [PubMed] [Google Scholar]
- 24.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 25.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J. Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J. Dent. Res. 2001;80:892–897. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–756. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannobile WV, et al. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J. Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 29.Murakami S, et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J. Periodontal Res. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 30.Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. Adv. Pharmacol. 1997;40:137–206. doi: 10.1016/s1054-3589(08)60140-4. [DOI] [PubMed] [Google Scholar]
- 31.Bromberg JS, Debruyne LA, Qin L. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv. Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- 32.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 33.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J. Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doukas J, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum. Gene Ther. 2001;12:783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 35.Beer SJ, et al. Poly(lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 36.Isidor F, Karring T, Nyman S, Lindhe J. The significance of coronal growth of periodontal ligament tissue for new attachment formation. J. Clin. Periodontol. 1986;13:145–150. doi: 10.1111/j.1600-051x.1986.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 37.Godwin SL, Soltoff SP. Extracellular calcium and platelet-derived growth factor promote receptor-mediated chemotaxis in osteoblasts through different signaling pathways. J. Biol. Chem. 1997;272:11307–11312. doi: 10.1074/jbc.272.17.11307. [DOI] [PubMed] [Google Scholar]
- 38.Canalis E. Effect of platelet-derived growth factor on DNA and protein synthesis in cultured rat calvaria. Metabolism. 1981;30:970–975. doi: 10.1016/0026-0495(81)90094-9. [DOI] [PubMed] [Google Scholar]
- 39.Saygin NE, Tokiyasu Y, Giannobile WV, Somerman MJ. Growth factors regulate expression of mineral associated genes in cementoblasts. J. Periodontol. 2000;71:1591–1600. doi: 10.1902/jop.2000.71.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994;125:917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmgren L, Glaser A, Pfeifer-Ohlsson S, Ohlsson R. Angio-genesis during human extraembryonic development involves the spatiotemporal control of PDGF ligand and receptor gene expression. Development. 1991;113:749–754. doi: 10.1242/dev.113.3.749. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 43.Mercola M, et al. Dominant-negative mutants of a platelet-derived growth factor gene. Genes Dev. 1990;4:2333–2341. doi: 10.1101/gad.4.12b.2333. [DOI] [PubMed] [Google Scholar]
- 44.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF down-regulates gas gene product PDGFalphaR and prolongs ERK and Akt/PKB activation. Am. J. Physiol. Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shamah SM, Stiles CD, Guha A. Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Mol. Cell. Biol. 1993;13:7203–7212. doi: 10.1128/mcb.13.12.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anusaksathien O, Jin QM, Zhao M, Somerman MJ, Giannobile WV. Effect of sustained gene delivery of platelet-derived growth factor (PDGF) or its antagonist (PDGF-1308) on tissue-engineered cementum. J. Periodontol. 2004;75:429–440. doi: 10.1902/jop.2004.75.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie WQ, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991;11:324–327. [PubMed] [Google Scholar]