Abstract
Although it is known that interleukin-7 (IL-7) and IL-15 influence the survival and turnover of CD8+ T cells, less is known about how these cytokines affect different subsets during the course of the immune response. We find that IL-7 and IL-15 differentially regulate CD8+ T-cell subsets defined by KLRG1 and CD127 expression during the contraction phase of the immune response. The provision of IL-15, or the related cytokine IL-2, during contraction led to the preferential accumulation of KLRG1hiCD127lo CD8+ T cells, whereas provision of IL-7 instead favored the accumulation of KLRG1loCD127hi cells. While IL-7 and IL-15 both induced proliferation of KLRG1lo cells, KLRG1hi cells exhibited an extraordinarily high level of resistance to cytokine-driven proliferation in vivo despite their dramatic accumulation upon IL-15 administration. These results suggest that IL-15 and IL-2 greatly improve the survival of KLRG1hi CD8+ T cells, which are usually destined to perish during contraction, without inducing proliferation. As the availability of IL-15 and IL-2 is enhanced during periods of extended inflammation, our results suggest a mechanism in which a population of cytokine-dependent KLRG1hi CD8+ T cells is temporarily retained for improved immunity. Consideration of these findings may aid in the development of immunotherapeutic strategies against infectious disease and cancer.
Introduction
After acute infection or immunization, antigen-specific CD8+ T cells respond in 3 distinct phases: expansion, contraction, and memory.1–5 Antigen recognition initiates the expansion phase where individual CD8+ T-cell clones proliferate and acquire the ability to specifically lyse target cells and produce cytokines. After antigen clearance, the antigen-specific CD8+ T-cell population undergoes contraction, where the majority of effector cells die. A small number of effector CD8+ T cells survive, leading to stable levels of long-lived CD8+ memory T cells, which provide protection on reinfection
A number of studies suggest that cytokines, including interleukin-7 (IL-7) and IL-15, can play complimentary or overlapping roles in maintaining CD8+ T cells after antigen stimulation.4–20 Early after infection, CD8+ T-cell survival may be impaired in the absence of IL-7.21 Furthermore, it was reported that effector CD8+ T cells destined to become long-lived memory cells selectively express CD127, suggesting that these cells might be preferentially IL-7 dependent.22 In support of this, it was shown that IL-7 plays a dominant role over IL-15 in supporting memory cell formation from recently activated CD8+ T cells. Whether such memory precursor cells are solely dependent on IL-7, however, is not clear, as CD127hi effector cells develop after viral infection with the same kinetics in both IL-7-deficient and -sufficient mice, and IL-7-deficient mice develop memory CD8+ T cells.23 In the case of IL-15, several groups have reported reduced maintenance of antigen-experienced CD8+ T cells in IL-15-deficient mice, an effect that was much more severe in some cases11 than in others.12,24 In addition, it was found that acute homeostatic proliferation of memory CD8+ T cells displayed overlapping dependency on both endogenous IL-7 and IL-15.25–27 To gain insight into these findings, we investigated whether IL-7 and IL-15 may differentially regulate specific CD8+ T-cell subsets. We focused our study on the contraction phase of the immune response, which ultimately dictates the seeding of the memory CD8+ T-cell compartment.
As a model to evaluate how IL-7 and IL-15 regulate the survival and proliferation of distinct subsets of CD8+ effector T cells during contraction, we followed the CD8+ T-cell response to viral or intracellular bacterial infection in mice with or without administration of exogenous cytokines. Of most significance, we found that IL-15, and the related cytokine IL-2, induced the preferential accumulation of KLRG1hiCD127lo CD8+ T cells, whereas provision of IL-7 favored accumulation of KLRG1loCD127hi cells, and to a lesser extent, KLRG1hiCD127hi CD8+ T cells. Interestingly, these respective phenotypes are thought to be indicative of whether a CD8+ effector T cell will transition to a long-lived memory T cell or die during or in the months after contraction.22,28,29 Thus, KLRG1hiCD127lo CD8+ T cells are thought to be short-lived effector/memory cells, whereas KLRG1loCD127hi CD8+ T cells are thought to be long-lived memory precursor T cells. In the context of these CD8+ T-cell subsets, we find unexpected differences in the ability of IL-7, IL-15, and IL-2 to induce proliferation and survival.
Methods
Antibodies and recombinant proteins
Murine (m) IL-15 was purchased from eBioscience (San Diego, CA). Soluble mIL-15Rα-Fc and anti–human (h) IL-2 mAb (MAB602) were purchased from R&D Systems (Minneapolis, MN). Anti-hIL-7 mAb (500-M07) was purchased from PeproTech (Rocky Hill, NJ). Both hIL-2 and hIL-7 were provided by the NCI Biological Resources Branch Preclinical Repository (Frederick, MD), whereas hIL-15 was kindly provided by Amgen (Thousand Oaks, CA). Reagents and methodology used for routine flow cytometric analysis are described in “Flow cytometric analysis.”
Generation of cytokine complexes
Cytokine complexes were generated by incubation of cytokine and either soluble receptor or antibody for 20 minutes at 37°C and diluted in phosphate-buffered saline before intraperitoneal injection.30,31 For generation of IL-15 complexes, recombinant mIL-15 or hIL-15 was used at doses that yielded similar biologic activity. The following concentrations were used per injection of cytokine complex: mIL-15 (1.5 μg) with msIL-15Rα-Fc (7 μg), hIL-15 (0.5 μg) with msIL-15Rα-Fc (2.3 μg), hIL-7 (1.5 μg) with anti-hIL-7 mAb (7.5 μg), or hIL-2 (1.5 μg) with anti-hIL-2 mAb (7.5 μg).
Mice, adoptive transfers, infections
C57BL/6 (B6), B6.PL (Thy1.1), CD45.1, RAG1−/−, and OT-I mice were obtained from The Jackson Laboratory (Bar Harbor, ME). IL-15−/− mice were kind gifts of Amgen. All animal work was performed in accordance with the guidelines outlined by the Institutional Animal Care and Use Guidelines at the University of California–San Diego (San Diego, CA). For adoptive T-cell transfer, CD8+ T cells were injected intravenously one day before infection or cytokine treatment. For analysis of CD8+ T-cell contraction, mice were infected intravenously with either 5 × 103 colony forming units of Listeria monocytogenes expressing ovalbumin (LM-OVA)32 or 105 plaque forming units of vesicular stomatitis virus expressing ovalbumin (VSV-OVA).33 In a small percentage of mice, we observed rejection of donor OT-I+ CD8+ T cells after infection despite a pure B6 background, and were thus excluded from analysis. To ensure complete clearance of Listeria, where indicated, mice were injected intraperitoneally with 1 mg of ampicillin on day 5 of infection and maintained for 48 hours on drinking water with 2 mg/mL of ampicillin, as described.34 To block IL-7-receptor, mice were treated with 500 μg anti–IL-7Rα mAb (A7R34) (intraperitoneally) on days 2, 4, 7, 10, and 13 after infection as previously described.26
Cell isolation, sorting, and CFSE labeling
Before cell sorting, T cells were enriched by negative selection using a mouse T-cell enrichment column (MTCC-25, R&D Systems). Enriched T cells were labeled with antibodies, and CD45.1+KLRG1hi or CD45.1+KLRG1lo cell populations sorted to more than 98% purity using a BD FACSAria. In experiments involving the transfer of OT-I effector cells into IL-7−/− mice, effector OT-I CD8+ T cells were enriched by positive selection, using anti-Thy1.1-biotin (H1S51), streptavidin particles, and an IMag magnet, following instructions provided by the manufacturer, BD Biosciences (San Jose, CA). For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, T cells were labeled with 1.5 μM of CFSE (Invitrogen, Carlsbad, CA) following the manufacturer's directions.
Flow cytometric analysis
Cells were analyzed by flow cytometry using standard techniques. Briefly, cells were washed in staining buffer containing 2% bovine growth serum and 0.01% sodium azide, and stained with fluorescently labeled antibodies as indicated. The antibodies used in these studies were as follows: B220-fluorescein isothiocyanate (FITC; RA3-6B2; eBioscience), CD8-FITC and -PerCP (53-6.7; BD Biosciences PharMingen, San Diego, CA), CD25-FITC and -phycoerythrin (PE PC61.5; eBioscience), CD27-PE (LG.7F9; eBioscience), CD43-FITC (1B11; BioLegend, San Diego, CA), CD44-FITC and -allophycocyanin (APC; IM7; eBioscience), CD62L-FITC and -PE (MEL-14; eBioscience), CD122-PE (TM-β1; eBioscience), CD127-FITC and -PE (A7R34; eBioscience), CXCR3-PE (220803; R&D Systems), interferon-γ (IFN-γ)–PE (XMG1.2; eBioscience), KLRG1-biotin and -APC (2F1; eBioscience), CD45.1-FITC, -PE, -PE-Cy7, and -APC (A20; eBioscience), CD45.2-FITC, -PE, and -APC (104; eBioscience), CD11b-PE (M1/70; eBioscience), CD49β-APC (DX5; eBioscience), Gr1-FITC (RB6-8C5; eBioscience), IFN-γ–PE mAb (XMG1.2; eBioscience), IgM-PE (II/41; eBioscience), Ly6C-FITC (AL-21; BD Biosciences PharMingen), and NK1.1-PE (PK136; eBioscience). We also used the polyclonal antibody, anti–IL-15Rα-biotin (BAF551; R&D Systems). Streptavidin-PE and -APC were obtained from eBioscience. H-2Kb tetramers bound with the OVA-peptide, SIINFEKL, and conjugated with PE, were purchased from Beckman Coulter (Fullerton, CA). Staining for intracellular bromodeoxyuridine (BrdU) was performed with reagents and according to the instructions outlined in the FITC BrdU flow kit (559619; BD Biosciences). Flow cytometry was performed with a BD FACSCalibur, BD FACSAria, or BD LSR II cytofluorometer (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Results
Both IL-7 and IL-15 rescue antigen-specific CD8+ T cells during contraction
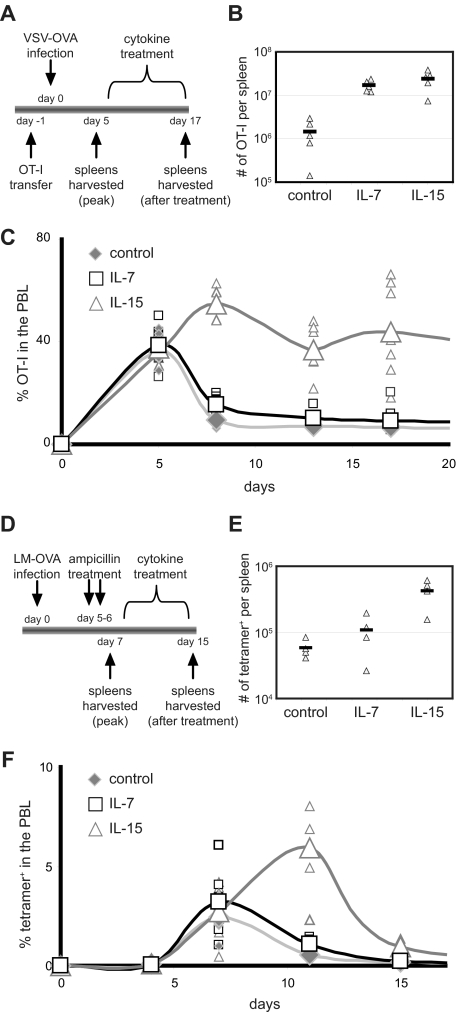
To evaluate the effects of exogenous IL-7 and IL-15 during CD8+ T-cell contraction, ovalbumin-specific CD8+ T cells (OT-I RAG1−/−CD45.1+) were transferred into B6 recipient mice. At the peak of the CD8+ T-cell response to VSV-OVA infection, mice were treated with IL-7 or IL-15 for 12 days (Figure 1A). Here we used cytokine complexes as these reagents provide dramatically enhanced in vivo delivery of cytokines30,31,35–39 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both IL-7 and IL-15 increased the number of antigen-specific CD8+ T cells by approximately 10-fold in the spleen by the end of treatment (Figure 1B) in agreement with previous studies.11–18 Interestingly, in the spleen (data not shown) and peripheral blood lymphocytes (PBLs; Figure 1C), the percentage of donor T cells was much higher with IL-15 complexes than with IL-7 complexes. This difference highlights the ability of IL-7 complexes to increase total splenocyte cell numbers without as great a proportional impact on the percentage of antigen-specific CD8+ T cells, possibly the result of the distinct ability of IL-7 complexes to expand immature B-cell precursors (Figure S1C,D). Of note, the increased numbers and percentages of donor OT-I CD8+ T cells observed after cytokine treatment were sustained in the PBLs and spleen (Figure S2) at approximately 2 months after infection in agreement other studies.11,14,16,17,40 However, the enhanced percentage of donor OT-I CD8+ T cells induced after cytokine administration did appear to wane over extended periods of time (Figure S2E), as has also been observed with other studies.16,17,40
Figure 1.
IL-7 and IL-15 complexes augment CD8+ T-cell accumulation during contraction. (A) OT-I CD8+ T cells (105) were adoptively transferred into B6 mice followed by infection with VSV-OVA. On days 5 through 17 of infection, mice were treated every 48 hours with IL-7 complexes, IL-15 complexes, or vehicle alone as a control. Antigen-specific donor CD8+ T cells in the spleens and PBLs were identified by CD45.1 expression and assessed at indicated time points. (B) Triangles represent OT-I CD8+ T cells per spleen at day 17. Bar represents the mean. (C) The percentage of donor OT-I cells present among PBLs is shown. Small symbols represent individual mice; larger symbols connected with a line represent the mean. (D) The endogenous response to LM-OVA was assessed with Kb OVAp-bound tetramers. Mice were treated with cytokine complexes from day 7 through day 15 after infection. (E) Triangles represent the number of OVAp-specific CD8+ T cells per spleen on day 15 as indicated by tetramer staining. Bar represents the mean. (F) The percentage of tetramer+ CD8+ T cells present among PBLs is shown. Small symbols represent individual mice; larger symbols connected with a line represent the mean. All data are representative of at least 2 independent experiments.
We also evaluated the response of endogenous CD8+ T cells in mice infected with LM-OVA using H-2Kb tetramers bound with the OVA-peptide (OVAp; Figure 1D). Here, we were able to ensure that cytokine treatment did not affect pathogen clearance by pretreating mice with ampicillin. At the onset of contraction, infected mice were treated with IL-7 complexes, IL-15 complexes, or vehicle alone. Similar to experiments with donor OT-I cells, IL-7 induced approximately 2-fold and IL-15 induced approximately 7-fold increases in absolute numbers of endogenous, antigen-specific CD8+ T cells in the spleen on day 15 (Figure 1E) and increased the percentage of endogenous OVAp-specific CD8+ T cells in the PBLs (Figure 1F).
Exogenous IL-7 and IL-15 differentially regulate contraction of CD8+ T-cell subsets
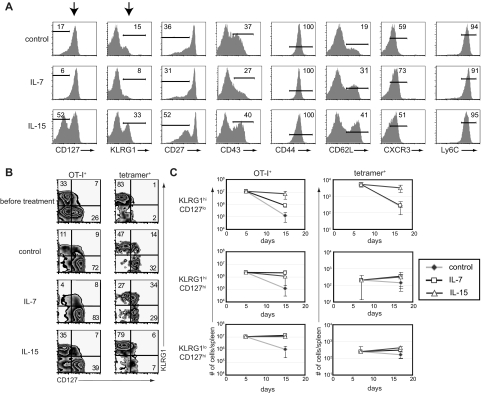
Interestingly, we found that IL-7 and IL-15 rescued different CD8+ T-cell subsets based on expression of KLRG1 and CD127 during the contraction phase after infection (Figures 2A, S3). However, statistically significant differential induction by IL-7 and IL-15 was not observed for other markers tested, including CD27, CD43, CD44, CD62L, CXCR3, and Ly6C. Administration of IL-15 complexes resulted in approximately a 2- to 3-fold increase in the percentages of KLRG1hiCD127lo CD8+ T cells and administration of IL-7 complexes induced increases in the percentages of the reciprocal KLRG1loCD127hi and KLRG1hiCD127hi CD8+ T-cell subsets, compared with control treatment (Figure 2A,B). These cytokine-induced trends were evident when we assessed the phenotype of either donor OT-I CD8+ T cells responding to VSV-OVA infection or endogenous CD8+ T cells responding to LM-OVA infection (Figure 2B, left and right), although for IL-7 complexes, the preferential induction of KLRG1loCD127hi CD8+ T cells was much more prevalent in experiments using donor OT-I CD8+ T cells. It is noteworthy that at the memory time point, we continued to see the preferential retention of the KLRG1hiCD127lo CD8+ T-cell subset after IL-15 administration (Figure S2D). However, these KLRG1hiCD127lo CD8+ T cells diminish over time,22,28,29 perhaps explaining why administration of IL-15, and the related cytokine IL-2, fail to result in sustained increases in memory CD8+ T cell numbers.11,16,17,40
Figure 2.
Administration of IL-7 or IL-15 during contraction rescues different CD8+ T-cell subsets. (A) Splenocytes were harvested on day 17 of the experiment outlined in Figure 1A, and expression of the indicated cell surface markers on donor cells was analyzed by flow cytometry. (B) CD127 and KLRG1 coexpression on antigen-specific donor and endogenous CD8+ T cells at days 17 and 15, respectively, as described in Figure 1A and D. (C) Absolute numbers of donor OT-I (left) or tetramer+ (right) CD8+ T-cell subsets as gated in panel B. All data are representative of 2 independent experiments.
Accumulation of absolute numbers of antigen-specific cells during cytokine treatment similarly revealed that KLRG1hiCD127lo CD8+ T cells contracted approximately 10 times more than KLRG1loCD127high CD8+ T cells in the spleen (Figure 2C). Importantly, we found that administration of IL-15, but not IL-7 complexes, reduced the contraction of KLRG1hiCD127lo CD8+ T cells. Thus, in IL-15-treated mice, there was a less than 2-fold decrease in numbers of either donor or endogenous KLRG1hiCD127lo CD8+ T cells, whereas these cells decreased 90- and 20-fold, respectively, in mice not receiving cytokine treatment. In contrast, both IL-7 and IL-15 complexes helped reduce the more limited contraction of KLRG1loCD127hi CD8+ T cells. Interestingly, both IL-7 and IL-15 complexes also reduced the contraction, and even promoted, the accumulation of KLRG1hiCD127hi CD8+ T cells. Cumulatively, these results show that, whereas IL-15 complexes broadly promote the retention of all the relevant cell populations, IL-7 complexes selectively promote accumulation of KLRG1loCD127hi and KLRG1hiCD127hi CD8+ T cells.
Differential dependence of KLRG1hiCD127lo and KLRG1loCD127hi CD8+ T-cell subsets on endogenous IL-7 and IL-15
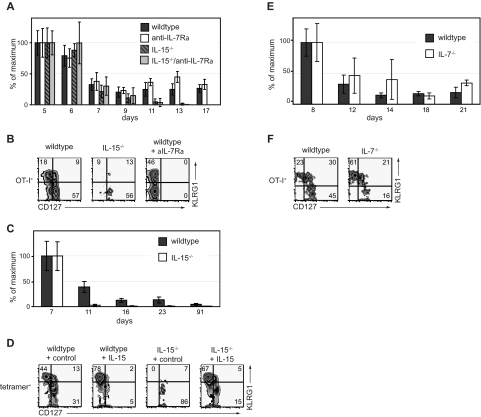
Although our results show that administration of exogenous cytokines can modulate the distribution of CD8+ T-cell subsets during contraction, an important question was whether endogenous IL-7 and IL-15 play similar roles in regulating these subsets. Thus, we examined the contraction phase of the OT-I CD8+ T-cell response to VSV-OVA in wild-type or IL-15−/− mice. In addition, in a subset of mice, IL-7Rα signaling was blocked with an anti–IL-7Rα antibody as previously described.25,26 With these conditions, we found that contraction of total CD8+ effector T cells was greater in the absence of IL-15 than IL-7 (Figure 3A). Without endogenous IL-15, contraction of the KLRG1hiCD127lo CD8+ T-cell subset was particularly severe (Figure 3B), in agreement with recently published results.29 In contrast, when we blocked IL-7 in wild-type hosts, KLRG1hi CD8+ T cells were present at higher proportions (Figure 3B). (Note that CD127 levels during antibody administration cannot be determined as the antibody used for blocking cytokine signaling also blocks flow cytometric staining of the receptor.) In the IL-15–deficient hosts that received IL-7 blockade, we did not obtain sufficient donor cells to assess their phenotype, indicating that IL-7 and IL-15 collaborate in rescuing CD8+ T cells from contraction. Similar to experiments with donor OT-I T cells, we found accelerated contraction of OVA-specific tetramer+ KLRG1hiCD127lo CD8+ T cells in the absence of IL-15 during LM-OVA infection (Figure 3C,D). Treatment of IL-15−/− mice with IL-15 complexes rescued the KLRG1hiCD127lo antigen-specific CD8+ T cells (Figure 3D).
Figure 3.
Endogenous IL-7 and IL-15 regulate different CD8+ T-cell subsets during contraction. (A) OT-I CD8+ T cells (105) were adoptively transferred into B6 or IL-15−/− mice followed by infection with VSV-OVA. Beginning on day 2, mice were treated with anti–IL-7Rα mAb. Percentage maximal response of donor OT-I among PBLs is shown at the indicated time points. (B) Phenotype of donor OT-I CD8+ T cells at day 16 in mice treated as in panel A. (C) B6 or IL-15−/− mice were infected with LM-OVA. Percentage maximal response of tetramer+ CD8+ T cells is shown. (D) Phenotype of tetramer+ T cells on day 16 as shown in panel C. Mice treated with IL-15 complexes from day 7 through day 16 are indicated. (E) OT-I T cells (105) were adoptively transferred into B6 mice followed by infection with LM-OVA. On day 7 of infection, donor T cells were isolated and transferred into secondary B6 or IL-7−/− hosts. Percentage maximal response of donor OT-I among PBLs are shown at indicated time points. (F) Phenotype of donor OT-I T cells at day 21 as in panel E. All data are representative of 2 independent experiments.
Because there may be limitations in blocking IL-7 signaling by antibody administration, we also followed the contraction of antigen-specific CD8+ T cells in IL-7−/− mice. Here, we adoptively transferred day 7 OT-I effector cells generated in B6 hosts, into wild-type or IL-7−/− hosts. Of note, contraction of the OT-I T cells was not enhanced in IL-7−/− mice (Figure 3E). However, KLRG1hiCD127lo CD8+ T cells dominated the percentage of surviving CD8+ effector T cells (Figure 3F), suggesting that, whereas the antigen-specific CD8+ T-cell population could survive in the absence of IL-7, the remaining cells were phenotypically distinguishable.
IL-15 promotes survival, but not proliferation, of KLRG1hiCD127loCD8+ T cells
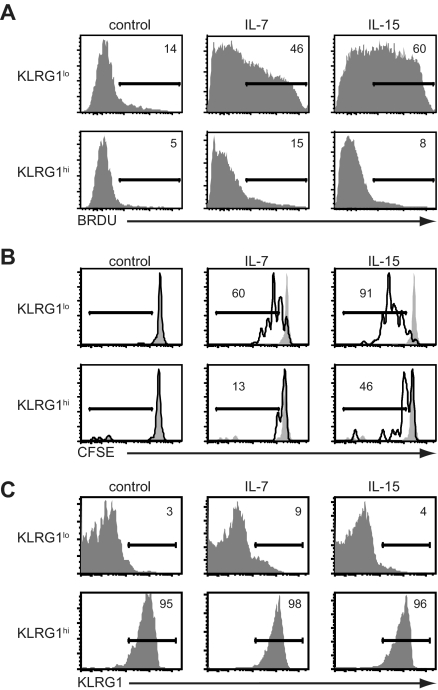
The substantial increase in percentage and absolute numbers of KLRG1hiCD127lo CD8+ T cells after treatment with IL-15 complexes suggested proliferation and/or survival were enhanced. To directly assess the proliferation of KLRG1 subsets, we adoptively transferred OT-I CD45.1+ CD8+ T cells into B6 mice and infected them with LM-OVA. Nine days after infection, BrdU was given for 3 days. We observed no BrdU incorporation by splenocytes in either KLRG1hi or KLRG1lo CD8+ T-cell subsets in the absence of cytokine treatment (Figure 4A). Treatment with IL-7 or IL-15 complexes induced 3- to 7-fold increases in BrdU incorporation by the donor KLRG1lo CD8+ T cells. However, despite being present at a higher proportion after IL-15 complex administration, donor KLRG1hi CD8+ T cells were essentially refractory to either IL-15- or IL-7–induced proliferation (Figure 4A). Although KLRG1hi CD8+ T cells were resistant to cytokine-mediated proliferation in vivo, they produced IFN-γ after stimulation with OVAp in agreement with others (Figure S4).28,29,41
Figure 4.
Antigen-specific KLRG1hiCD8+ T cells are resistant to cytokine-mediated proliferation in vivo. (A) Mice were infected with VSV-OVA and treated with cytokine complexes as outlined in Figure 1A. Mice were pulsed with BrdU from days 9 to 12 of infection. On day 12, BrdU incorporation in either KLRG1lo or KLRG1hi donor CD8+ splenocytes was determined by flow cytometry. (B) OT-I T cells (105) were adoptively transferred into B6 followed by infection with LM-OVA. On day 10 of infection, spleens were harvested and KLRG1lo and KLRG1hi donor OT-I CD8+ T cells were isolated by cell sorting, labeled with CFSE, and transferred into secondary B6 recipients. Mice were then treated for 6 days with cytokine complexes or vehicle control, and CFSE dilution among donor splenocytes assessed by flow cytometry. (C) Same as in panel B, except that cells were not labeled with CFSE. All data are representative of 2 independent experiments.
To rule out the possibility that KLRG1hi CD8+ T cells convert to KLRG1lo CD8+ T cells on cell division, we sorted KLRG1lo and KLRG1hi effector subsets on day 10 of LM-OVA infection. The sorted KLRG1lo and KLRG1hi CD8+ T cells were labeled with CFSE and adoptively transferred into secondary B6 recipients that were then treated with cytokine complexes for 6 days. As we observed with the BrdU analysis, both IL-7 and IL-15 complexes induced rapid proliferation of the KLRG1lo CD8+ T cells, but neither induced significant proliferation of the KLRG1hi subset as assessed by CFSE dilution (Figure 4B). Furthermore, there was no conversion in phenotype between KLRG1lo and KLRG1hi populations (Figure 4C). These results show that the accumulation of KLRG1hi antigen-specific T cells during contraction with IL-15 complex treatment is the result of increased survival of KLRG1hi CD8+ T cells, not the result of proliferation or conversion between the KLRG1lo and KLRG1hi populations.
Survival of KLRG1hi CD8+ T cells depends on stimulation through the IL-2/IL-15 receptor
The simplest explanation for why IL-7 and IL-15 differentially affect KLRG1hi and KLRG1lo CD8+ T cells is by virtue of their expression of the receptor subunits necessary for cytokine responsiveness. KLRG1hi and KLRG1lo CD8+ T cells both express the IL-2/IL-15 receptor components necessary for survival and cytokine signaling (CD122 and CD132; Figure S5). However, as shown earlier, KLRG1hi CD8+ T cells, but not KRLG1lo CD8+ T cells, are largely IL-7Rα (CD127) low. This differential expression of IL-7Rα provides a simple explanation for why KLRG1hi CD8+ T cells do not respond functionally to IL-7, but it fails to explain why KLRG1hi and KRLG1lo CD8+ T cells respond differently to IL-15. Addressing this question will require elucidation of the intracellular signals involved in these 2 cell types, although it has been suggested that expression of p27kip and Bmi-1 may be relevant.15,42
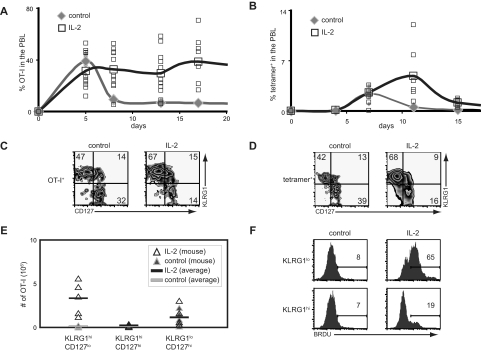
Given that the receptors for IL-15 also function in IL-2 signaling, our results predicted that administration of IL-2 would rescue cells during contraction in a manner similar to IL-15. To test this, we administered IL-2 complexes after infection and assessed the response of donor OT-I CD8+ T cells and endogenous antigen-specific CD8+ T cells after infection. In both cases, IL-2 not only increased the absolute numbers and percentages of responding antigen-specific CD8+ T cells, but also preferentially expanded the KLRG1hiCD127lo CD8+ T-cell subset (Figure 5A-E). IL-2 complexes also induced enhanced BrdU-incorporation in KLRG1lo CD8+ T cells but not KLRG1hi CD8+ T cells (Figure 5F). Thus, similar to IL-15, IL-2 also preferentially induces the accumulation of KLRG1hiCD127lo CD8+ T cells through enhanced survival as opposed to increased proliferation.
Figure 5.
IL-2 complexes preferentially induce the accumulation of KLRG1hiCD127loCD8+ T cells. (A) B6 mice were treated with or without IL-2 complexes as outlined in Figure 1A. The percentage of donor OT-I cells present among PBLs is shown. (B) B6 mice were treated with or without IL-2 complexes as outlined in Figure 1D. The percentage of tetramer+ CD8+ T cells present among PBLs is shown. (C) CD127 and KLRG1 coexpression on donor OT-I T cells in the spleen on day 17 after infection as described in panel A. (D) CD127 and KLRG1 coexpression on tetramer+ CD8+ T cells in the spleen on day 15 after infection as described in panel B. (E) Absolute numbers of donor OT-I CD8+ T-cell subsets on day 17 after infection as described in panel A. (F) BrdU incorporation on donor OT-I T cells from mice with or without treatment with IL-2 complexes as outlined in Figure 4A.
Discussion
This study provides novel insights into cytokine-regulated survival of different CD8+ T-cell subsets during the contraction phase and seeding of the memory T-cell compartment after infection. Using expression of KLRG1 and CD127, we identify distinct populations of CD8+ T cells with differential responsiveness to cytokines. Administration of IL-15 or IL-2 complexes during contraction drives the preferential accumulation of KLRG1hiCD127lo CD8+ T cells while also promoting increased survival of other CD8+ T-cell subsets. In contrast, IL-7 complexes preferentially supported the accumulation of KLRG1loCD127hi and KLRG1hiCD127hi CD8+ T cells, with very limited impact on CD127lo CD8+ T-cell subsets. The preferential ability of IL-15 and IL-2 complexes to expand KLRG1hiCD127lo CD8+ T cells was not expected as these populations express similar levels of receptor components, whereas the ability of IL-7 to selectively drive CD127hi CD8+ T cells was more predictable based on IL-7Rα expression. Strikingly, we observed that KLRG1hi but not KLRG1lo CD8+ T cells exhibited a broad resistance to IL-15- and IL-2-mediated proliferation in vivo despite the use of cytokine complexes, which exhibit enhanced biologic activity. Combined with evidence that cytokine administration does not induce rapid conversion of KLRG1lo to KLRG1hi CD8+ T cells, our results show that IL-15 and IL-2 complexes dramatically improve the survival of KLRG1hi CD8+ T cells. Consideration of these findings is useful in understanding the basic role of cytokines in promoting CD8+ T cell responses after infection and relevant to efforts that augment the efficacy of CD8+ T cell–directed therapies.
The functional properties and significance of CD8+ T-cell subsets based on KLRG1 and CD127 expression are not fully understood. For example, expression of KLRG1 on CD8+ T cells has been reported to be a marker of cellular senescence and an indicator of the inability to respond to antigenic challenge.41 A recent study found that forced expression of the IL-7Rα subunit on KLRG1hiCD127lo CD8+ T cells failed to restore IL-7–driven proliferation, suggesting these cells may have inherent deficiencies in cytokine-induced proliferation that extend beyond the simple absence of IL-7Rα expression.15 However, whereas some studies find that these cells exhibit a proliferative defect, others have reported that KLRG1hi CD8+ T cells can undergo vigorous proliferation after antigen challenge.43 Indeed, we find that day 10 KLRG1hi effector CD8+ T cells can undergo significant proliferation after VSV-OVA infection (data not shown). The differences in these findings have yet to be explained; however, there is agreement that KLRG1hi CD8+ T cells are not impaired in effector functions, such as IFN-γ production and cytotoxic killing compared with KLRG1lo CD8+ T cells.28,29,41 Relevant to our findings, it has been shown that the KLRG1hiCD127lo phenotype is associated with CD8+ T cells that are selectively lost over a period of months after the onset of contraction, as opposed to KLRG1loCD127hi phenotype that contains longer-lived memory CD8+ T cells.22,28,29
Our observation that IL-15 and IL-2 levels can preferentially impact the survival of these KLRG1hi CD8+ T cells provides a new perspective on the significance of this T-cell subset. IL-15 and IL-2 can be elevated during periods of extended inflammation; our data suggest that such an environment would favor the maintenance of these KLRG1hi CD8+ T cells. As these cells are functional in terms of both cytotoxicity and cytokine production, the increased numbers of KLRG1hi CD8+ T cells could play a vital role in enhancing the clearance of prolonged infection. Importantly, as the survival of these KLRG1hi CD8+ T cells appears tightly linked to the presence of elevated levels of IL-15 and IL-2, these cells would probably die soon after cytokine availability is reduced and the activity of these T cells might be harmful. In agreement, antibiotic treatment of Listeria-infected mice, which reduces inflammation, results in enhanced frequencies of KLRG1loCD127hi CD8+ T cells.44 However, if antibiotic-treated mice are given the IL-15-inducing agent, CPG,45 CD127lo (and presumably KLRG1hi) CD8+ T cells emerge. Similar results extended these findings and linked T-bet as a critical factor transcription factor in maintaining the functional characteristics of KLRG1hi CD8+ T cells including expression of the IL-15 receptor subunit, CD122.29
IL-2, IL-7, and IL-15 have all garnered interest as reagents for augmenting CD8+ T-cell immunity either in treating disease or enhancing the recovery of lymphocytes after cytoreductive therapy.6,7,46–48 In these regards, one critical issue is the selection of cytokine. Our results would suggest prioritizing the use of IL-7 where long-term immunity is the primary objective, as the KLRG1lo CD8+ T-cell subset, which is thought to be long-lived and seed the memory compartment, is selectively expanded with this cytokine. In contrast, IL-2 or IL-15 should more efficiently increase effector cell numbers, adding to the functional arsenal, by supporting survival of KLRG1hi CD8+ T cells. Indeed, the latter 2 cytokines have proven particularly useful at augmenting tumor immunotherapy, a situation where the expansion of effector function to eliminate tumor is important. It is worth noting, though, that vaccination or antigen-stimulation during IL-2 administration has in some cases been shown to be counterproductive.49,50 Thus, it will be important to determine the optimal timing of vaccination in conjunction with cytokine administration to best enhance the desired CD8+ subset.
It is relevant to mention that, in our study, we administered cytokine complexes instead of free cytokines. Although the mechanism of how cytokine complexes exhibit improved biologic activity is not fully understood, it is thought that this may relate to improved half-life. Although it has been shown that administration of IL-15 cytokine complexes can result in improved donor CD8+ T-cell immune responses,30 our study shows, for the first time, that the response of endogenous antigen-specific CD8+ T cells present at physiologic levels can also be dramatically augmented after administration of IL-2, IL-7, or IL-15 cytokine complexes. These findings represent an important step in translating the use of such reagents to the clinic where low numbers of antigen-specific T cells will probably be present.
It is becoming increasingly well recognized that the administration of cytokines to modulate immune responses has great potential in medicine. Although many cytokines are often viewed as having overlapping properties suggesting the potential for interchangeable use, our study finds distinct differences between IL-2, IL-7, and IL-15, not previously appreciated. Consideration of our findings, combined with a further understanding of the functional roles of these distinct CD8+ T-cell subsets, will aid in the development of therapies for augmenting T cell–based responses against infectious disease and cancer.
Supplementary Material
Acknowledgments
The authors thank Karen Ong for excellent help in flow cytometric analysis and cell sorting and K. Cheung, A. Doedens, W. D'Souza, L. D'Cruz, M. Salem, and M. Werneck for critical review of this manuscript.
This work was supported by the National Institutes of Health (NIH; grant RO1AI67545), Cancer Research Institute, V Foundation for Cancer Research, California Breast Cancer Research Program, and Pew Scholars Program (A.W.G.). M.P.R. is supported by a fellowship from the Prevent Cancer Foundation. J.A.B. is supported by the University of California, San Diego Immunology training grant from the NIH (National Research Service Award T32AI060536-02). J.F.P. is supported by a CJ Martin Fellowship from the Australian National Health and Medical Research Council.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.P.R. designed and performed research, interpreted data, and wrote the paper; N.A.L., J.F.P., P.F., J.A.B., and P.A.M. designed and performed research and interpreted data; and C.D.S. and A.W.G. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: C.D.S. is a shareholder in Nascent Biologics Inc. The remaining authors declare no competing financial interests.
Correspondence: Ananda W. Goldrath, 9500 Gilman Drive, University of California, San Diego, La Jolla, CA 92093-0377; e-mail: agoldrath@ucsd.edu.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 4.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 6.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 8.Maeurer MJ, Trinder P, Hommel G, et al. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect Immun. 2000;68:2962–2970. doi: 10.1128/iai.68.5.2962-2970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan IA, Casciotti L. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J Immunol. 1999;163:4503–4509. [PubMed] [Google Scholar]
- 10.Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 11.Yajima T, Yoshihara K, Nakazato K, et al. IL-15 regulates CD8+ T-cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 12.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen CL, Salem ML, Rubinstein MP, et al. Mechanisms of enhanced antigen-specific T cell response following vaccination with a novel peptide-based cancer vaccine and systemic interleukin-2 (IL-2). Vaccine. 2003;21:2318–2328. doi: 10.1016/s0264-410x(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 14.Nanjappa SG, Walent JH, Morre M, Suresh M. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest. 2008;118:1027–1039. doi: 10.1172/JCI32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- 18.Yajima T, Nishimura H, Ishimitsu R, et al. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]
- 19.Kasper LH, Matsuura T, Khan IA. IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- 20.Moniuszko M, Fry T, Tsai WP, et al. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 22.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 23.Klonowski KD, Williams KJ, Marzo AL, Lefrançois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 25.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldrath AW, Sivakumar PV, Glaccum M, et al. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T-cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T-cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 32.Dudani R, Chapdelaine Y, Faassen Hv H, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 33.Kim SK, Reed DS, Olson S, et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci U S A. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 35.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 37.Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 38.Kamimura D, Sawa Y, Sato M, Agung E, Hirano T, Murakami M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J Immunol. 2006;177:306–314. doi: 10.4049/jimmunol.177.1.306. [DOI] [PubMed] [Google Scholar]
- 39.Finkelman FD, Madden KB, Morris SC, et al. Anti-cytokine antibodies as carrier proteins: prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 40.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 41.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 42.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci U S A. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 44.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 45.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 46.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 48.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrikant P, Mescher MF. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- 50.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.