Abstract
Initiation of fibronectin (FN) matrix assembly is dependent on specific interactions between FN and cell surface integrin receptors. Here, we show that de novo FN matrix assembly exhibits a slow phase during initiation of fibrillogenesis followed by a more rapid growth phase. Mn2+, which acts by enhancing integrin function, increased the rate of FN fibril growth, but only after the initial lag phase. The RGD cell-binding sequence in type III repeat 10 is an absolute requirement for initiation by α5β1 integrin. To investigate the role of the cell-binding synergy site in the adjacent repeat III9, a full-length recombinant FN containing a synergy mutation, FN(syn−), was tested for its ability to form fibrils. Mutation of this site drastically reduced FN assembly by CHOα5 cells. Only sparse short fibrils were formed even after prolonged incubation, indicating that FN(syn−) is defective in progression of the assembly process. These results show that the synergy site is essential for α5β1-mediated accumulation of a FN matrix. However, the incorporation of FN(syn−) into fibrils and the deoxycholate-insoluble matrix could be stimulated by Mn2+. Therefore, exogenous activation of integrin receptors can overcome the requirement for FN’s synergy site as well as modulate the rate of FN matrix formation.
INTRODUCTION
The interaction of cell surface integrin receptors with the extracellular matrix plays a critical role in the regulation of a number of cellular functions including adhesion and migration, cytoskeletal organization, and cell cycle progression (reviewed in Hynes, 1990; 1992; Yamada and Miyamoto, 1995; Assoian, 1997). Fibronectin (FN) is an essential extracellular matrix component as demonstrated by the fact that null mutations in either FN or its integrin receptors result in embryonic lethality (George et al., 1993; reviewed in Fassler et al., 1996). Oncogenically transformed cells generally show reduced FN expression, further supporting a role for the FN matrix in regulating cell morphology and growth (Yamada et al., 1976; Ali et al., 1977; Giancotti and Ruoslahti, 1990).
The majority of integrin-mediated interactions with FN occur through the arg-gly-asp (RGD) cell-binding sequence in repeat III10 (Ruoslahti, 1991; Hynes, 1992). For some integrins, such as αvβ3, the RGD sequence alone is sufficient to support adhesion to FN (Bowditch et al., 1994; Danen et al., 1995). Other integrins, including α5β1 and αIIbβ3, require the presence of additional domains (Aota et al., 1991; Bowditch et al., 1991). For example, adhesion of cells to FN via α5β1 integrin requires not only the RGD site but a second synergy site corresponding to the sequence pro-his-ser-arg-asp (PHSRN) in repeat III9 (Aota et al., 1994). Both sites are also required for Xenopus gastrulation, providing additional functional evidence for the importance of the synergy site (Ramos and DeSimone, 1996). RGD-mediated adhesion can also be modulated by exogenous activators. For example, binding of certain antibodies to integrin extracellular domains can activate the receptors from a low- to high-affinity state for ligand binding (Frelinger et al., 1991; Arroyo et al., 1992; Kovach et al., 1992). Divalent cations such as Mn2+ can also stimulate integrin activity and interactions with ligand (Gailit and Ruoslahti, 1988; Bazzoni et al., 1995; Mould et al., 1995; reviewed in Humphries, 1996; Mould, 1996).
In addition to its role in cell adhesion, the RGD sequence is also essential for assembly of FN into a complex fibrillar matrix. Recombinant FNs (recFNs) containing a deletion of the RGD site are unable to initiate matrix assembly (Sechler et al., 1996). Furthermore, antibodies to α5β1 integrin or the cell-binding domain of FN can inhibit assembly (Akiyama et al., 1989; Roman et al., 1989; Fogerty et al., 1990; Nagai et al., 1991). Following integrin ligation, this multistep assembly process proceeds through specific interactions between individual FN molecules culminating in the formation of a detergent-insoluble matrix (McDonald, 1988; Schwarzbauer, 1991; Morla and Ruoslahti, 1992; Mosher, 1993; Aguirre et al., 1994; Hocking et al., 1994). Some of the interactions during these later stages are RGD independent, suggesting that integrins might not be involved in all stages of FN assembly.
Although α5β1 is the major receptor on cells that assemble FN fibrils, other integrins are also able to participate in this process (Wu et al., 1995, 1996; Wennerberg et al., 1996; Yang and Hynes, 1996). For αIIbβ3 and αvβ3 integrins, efficient assembly is dependent on antibody activation of the receptors to a high-affinity state for ligand binding. As αv integrins bind FN via the RGD sequence, this raises the question whether the synergy site plays a role in any of the stages of FN assembly.
In this report, we show that the dual interaction of the RGD and synergy sites with α5β1 integrin is required to fully support matrix assembly. De novo assembly of FN progressed through a slow phase of fibril initiation which was followed by a more rapid accumulation of a deoxycholate (DOC)-insoluble matrix. In contrast, assembly of FN(syn−), a recFN containing a mutation in the synergy site, was stalled during the initiation phase. Exogenous stimulation of α5β1 function with Mn2+ overcame the block in assembly of FN(syn−). Mn2+ activation of α5β1 also affected assembly of FN, resulting in a dramatic increase in the rate of conversion of fibrils into a DOC-insoluble matrix. With both native and mutant FNs, the stimulatory effect occurred only after the assembly of fibrils had been initiated. These results show that the required levels of integrin activity differ for α5β1-mediated initiation and for accumulation of dense matrix. In the absence of synergy site binding, the latter step can be induced by exogenous integrin activators. It is also possible that the assembly of a FN matrix may itself activate α5β1 in an RGD and synergy site-dependent process.
MATERIALS AND METHODS
Antibodies and Reagents
Ascites fluid was isolated from the previously described hybridoma cells producing rat-specific monoclonal antibody IC3 (Sechler et al., 1996; Sechler and Schwarzbauer, 1997). The function blocking antihamster α5 antibody PB1 (Brown and Juliano, 1985, 1988) and function blocking antihuman α5 antibody m16 (Akiyama et al., 1989) were kindly provided by R.L. Juliano (University of North Carolina-Chapel Hill, Chapel Hill, NC) and K. Yamada (National Institutes of Health, Bethesda, MD), respectively. The activating anti-β3 antibody LIBS6 (Frelinger et al., 1991) was provided by M.H. Ginsberg (Scripps Research Institute, La Jolla, CA). Fluorescein-conjugated goat anti-mouse IgG was purchased from Molecular Probes (Eugene, OR). Cycloheximide was purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Culture
CHOα5 cells, clone 17, transfected with a cDNA to the human α5 integrin subunit have been described previously (Sechler et al., 1996; Sechler and Schwarzbauer, 1997). For all experiments CHOα5 cells were cultured in DMEM, 2 mM glutamine, 1% nonessential amino acids, 100 μg/ml Geneticin (Life Technologies, Grand Island, NY), and 10% fetal calf serum (Hyclone Labs, Logan, UT) depleted of FN by gelatin-agarose affinity chromatography. The CHO K1 αvβ3 cell line (described in Wu et al., 1996) was kindly provided by M.H. Ginsberg (Scripps Research Institute) and maintained in DMEM supplemented with 10% fetal calf serum (Hyclone Labs), 2 mM glutamine, and 1% nonessential amino acids. For immunofluorescence experiments, FN-depleted fetal calf serum was used.
FN cDNA Construction and Recombinant Protein Purification
Mutation of the synergy site was generated using polymerase chain reaction (PCR) amplification with a mutant oligonucleotide. The mutant primer, FNPSR/GSE (5′-TAGAATTCTCTGAGCCCGGCACTCGG-3′), was prepared by the Synthesis and Sequencing Facility (Princeton University, Princeton, NJ) and spans nucleotides 4507 to 4482 of the rat FN cDNA. Base pair changes are underlined and change the codons from proline to glycine and from arginine to glutamate at amino acid positions 1498 and 1500, respectively. PCR amplification was performed using FNPSR/GSE and an upstream primer for 30 cycles under the following conditions: 95°C, 30 s; 37°C, 60 s; and 72°C, 60 s. The 690-bp product was digested with RsrII and EcoRI (within the primer) and the resulting 395-bp fragment was then used to insert the synergy site mutation into the FN cDNA in pVL1392. The mutation was confirmed following construction by restriction digests and sequencing.
Creation of recombinant baculovirus and purification of recombinant protein were performed as described (Aguirre et al., 1994; Sechler et al., 1996). For purification of recombinant FN protein, baculovirus-infected BTI-TN-5B-4 (High Five) insect cells (Invitrogen Corp., San Diego, CA) were grown in Express Five serum-free medium (Life Technologies). Rat plasma FN (pFN) and recombinant FN (recFN) were purified by gelatin agarose chromatography. Approximately 1 mg of recFN was purified from 5 × 107 infected cells. Since insect cells do not synthesize endogenous FN, recFN preparations were free of contaminating FN. RecFNs were stored in 10 mM 3-[cyclohexylamino]-1-propanesulfonic acid (pH 11) and 150 mM NaCl at −80°C.
Immunofluorescence
CHOα5 cells were seeded in medium containing FN-depleted serum onto glass coverslips in 24-well dishes at a concentration of 2.0 × 105 cells/cm2. A nearly confluent monolayer resulted after an overnight incubation. pFN or FN(syn−) was added to cells along with fresh medium and incubated for the specified period of time. Cells were then washed with phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde for 15 min at room temperature. Coverslips were washed with PBS and incubated for 30 min at 37°C with IC3 ascites diluted 1:1000 in PBS with 2% ovalbumin. Following incubation with IC3, coverslips were washed with PBS and incubated with fluorescein-conjugated goat anti-mouse secondary antibody at a concentration of 1:400. After a final wash with PBS, coverslips were mounted onto microscope slides with FITC-guard (Testog, Inc., Chicago, IL).
CHO K1 αvβ3 cells were seeded at a concentration of 5 × 105 cells/cm2 onto glass coverslips in a 24-well dish with medium containing FN-depleted fetal calf serum. Cells were allowed to attach for 1 h, washed with medium containing 20 μg/ml cycloheximide, and incubated with fresh cycloheximide-containing medium for an additional hour. After another wash, medium containing 20 μg/ml cycloheximide, 40 μg/ml PB1 antihamster α5 antibody, 300 μg/ml LIBS6 anti-β3-activating antibody, and either 75 μg/ml pFN or FN(syn−) was added to the cells and incubated for an additional 6 or 22 h. Cycloheximide treatment decreased the production of endogenous FN to undetectable levels as determined by labeling with [35S]methionine and gelatin binding. Immunofluorescence staining was then performed as described above. Staining was visualized with a Nikon Optiphot-2 microscope using a 40× plan-apochromatic objective, and photography was performed as described in the study by Schwarzbauer (1991).
Isolation, Detection, and Quantitation of DOC-soluble and -insoluble Material
CHOα5 cells were cultured in a 24-well dish as described above except in the absence of glass coverslips. pFN and FN(syn−) were incubated with the cells for defined time periods. After the incubation period, cells were washed with serum-free DMEM and lysed in 200 μl of DOC lysis buffer (2% DOC, 0.02 M Tris-HCl, pH 8.8, 2 mM phenylmethylsulfonyl fluoride, 2 mM EDTA, 2 mM iodoacetic acid, and 2 mM N-ethylmaleimide) per well. DOC-insoluble material was isolated and aliquots of DOC-soluble and -insoluble material were separated by SDS-PAGE. Immunodetection and quantitation were performed as described (Sechler et al., 1996) with the exception that IC3 ascites was used at a dilution of 1:10,000. In initial experiments, immunoblots were developed with chemiluminescent reagents (Pierce, Rockford, IL) and repeated at least twice. To quantify results, experiments were repeated in at least two separate trials using 125I-labeled protein A as described (Sechler et al., 1996).
Mn2+ Stimulation and Integrin Function Blocking
CHOα5 cells were seeded in a 24-well dish with glass coverslips (for immunofluorescence experiments) or without coverslips (for biochemical analysis) and incubated overnight. Fresh medium containing MnCl2 and either pFN or FN(syn−) was then added to the CHOα5 cells and incubated for defined time periods. Incubations in the presence of 0.1 and 0.2 mM MnCl2 were performed for up to 16 h, while exposure to 1 mM MnCl2 was limited to a maximum of 4 h. Anti-integrin function blocking experiments were performed by adding FN(syn−) to CHOα5 cells and incubating the cells for 16 h. One millimolar MnCl2 was then added to culture medium either alone or with 50 μg/ml m16 and 20 μg/ml PB1 anti-α5 antibodies. Immunofluorescence and isolation of DOC-insoluble and -soluble material was then performed as described above.
RESULTS
The Synergy Site Is Required for FN Fibril Assembly
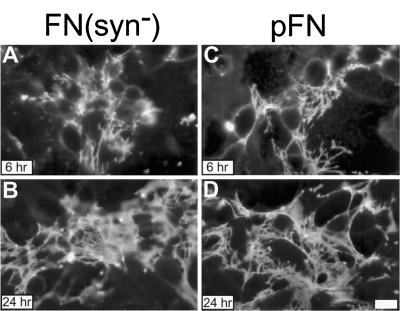
A full-length recFN was constructed to contain a PPSRN to PGSEN mutation in repeat III9 of rat FN in a region corresponding to the human FN PHSRN synergy site (Aota et al., 1994). Recombinant protein [FN(syn−)] was generated with the baculovirus expression system and purified by gelatin-agarose chromatography. FN(syn−) and native pFN were added to the culture medium of CHOα5 cells at a concentration of 25 μg/ml and incubated for the times specified (Figure 1). These cells lack endogenous FN but will assemble a matrix when provided with exogenous FN (Sechler et al., 1996). FN fibrils were then visualized by indirect immunofluorescence. pFN was assembled into short fibrils by 1 h of incubation (Figure 1A), and an increase in the density and length of these fibrils was observed after longer periods of incubation (Figure 1, B and C). In contrast, at least 6 h of incubation were required to detect any FN(syn−) at the cell surface (Figure 1D). By 16 h, FN(syn−) was assembled into very sparse short fibrils (Figure 1E), suggesting that mutation of the synergy site delays the assembly of this recFN. Increasing the concentration of FN(syn−) to 50 μg/ml also gave a similar sparse matrix. There was no significant increase in length or number of fibrils after 48 h of incubation (Figure 1F), demonstrating that the absence of a synergy site prevents rather than delays assembly.
Figure 1.
Time course of fibril formation by CHOα5 cells. CHOα5 cells were cultured in medium containing FN-depleted serum and incubated with 25 μg/ml pFN (A–C) and FN(syn−) (D–F) for the times indicated. FN fibrils were visualized by indirect immunofluorescence with monoclonal antibody IC3 specific for rat FN. Bar, 10 μm.
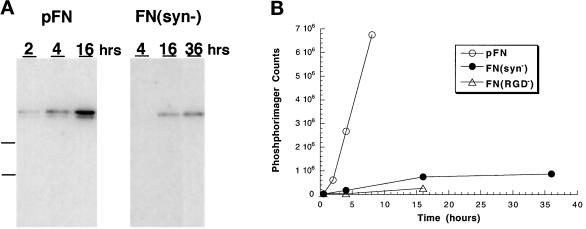
During FN matrix assembly, fibrils are gradually converted from a DOC-soluble into a DOC-insoluble form. The amount of DOC-insoluble matrix after 16 h of incubation with FN(syn−) was similar to DOC-insoluble pFN after only 2 h (Figure 2,A and B). Little change was observed in FN(syn−) accumulation after 16 h. Furthermore, the amount of FN(syn−) DOC-insoluble matrix was only slightly greater than that isolated from cells with FN(RGD−) (Figure 2B). These results show that FN(syn−) is defective in the initiation and progression of assembly.
Figure 2.
Native and recFN incorporation into DOC-insoluble matrix. (A) Immunoblot analysis of incorporation of pFN and FN(syn−) into DOC-insoluble matrix. DOC-insoluble material was extracted from CHOα5 cells incubated with 25 μg/ml pFN or FN(syn−) at the times indicated (h) and separated by 5% SDS-PAGE under reduced conditions. FN was detected with monoclonal antibody IC3. Molecular weight markers 180 and 116 kDa are indicated by dashed lines. (B) Quantification of DOC-insoluble FN isolated from CHOα5 cells incubated with 25 μg/ml pFN (open circles), FN(syn−) (closed circles), or FN(RGD−) (open triangles). The amount of DOC-insoluble FN was quantitated using a Molecular Dynamics PhosphorImager, and values are expressed as total phosphorimager counts. Data are from a single experiment and are representative of results from at least four independent experiments as described in MATERIALS AND METHODS.
Activated αvβ3 Integrin Supports FN(syn−) Matrix Assembly
Function blocking anti-α5 integrin antibodies inhibit FN fibril formation by CHOα5 cells, indicating that the α5β1 integrin is the principle receptor supporting matrix assembly in these cells (our unpublished observations). However, activated αvβ3 and αIIbβ3 integrins can also participate in FN matrix assembly (Wu et al., 1995, 1996). Unlike α5β1 which requires both the RGD and synergy sites for maximal cell adhesion to FN, αvβ3 recognizes only the RGD sequence (Bowditch et al., 1994; Danen et al., 1995). Although CHOα5 cells showed a 50% reduction in adhesion to FN(syn−) compared with pFN, αvβ3-mediated adhesion of CHO-αvβ3 cells was not significantly different. CHO-αvβ3 cells were used to determine whether FN(syn−) is capable of forming fibrils. An anti-β3-activating antibody (LIBS6) was included to enhance αvβ3 activity. As shown in Figure 3, when αvβ3 integrin was used as the FN receptor, FN(syn−) and pFN were assembled into comparable matrices as assessed by both the amount and morphology of fibrils formed. Therefore, the synergy site is not essential for fibril assembly by activated αvβ3 receptor.
Figure 3.
FN(syn−) and pFN matrix assembly supported by αvβ3. αvβ3-transfected CHO K1 cells were incubated with 20 μg/ml cycloheximide to inhibit endogenous FN synthesis, monoclonal antihamster α5 integrin function blocking antibody, activating LIBS6 anti-β3 antibody, and 25 μg/ml of either FN(syn−) (A and B) or pFN (C and D) for 6 and 24 h. FN fibrils were visualized by indirect immunofluorescence using monoclonal antibody IC3. Bar, 10 μm.
Rate of FN Assembly Can Be Increased with the Addition of Mn2+
Activating antibodies can induce high-affinity binding of integrins to their ligands (O’Toole et al., 1990; Faull et al., 1993). Similarly, divalent cations such as Mn2+ have been shown to change integrin binding affinity (Gailit and Ruoslahti, 1988; Bazzoni et al., 1995) and Mn2+ can increase the binding of α5β1 integrin receptor to fibronectin (Gailit and Ruoslahti, 1988; Mould et al., 1995). To determine whether Mn2+ stimulation of α5β1 integrin can enhance FN assembly, MnCl2 was added to CHOα5 culture medium along with 25 μg/ml pFN. No noticeable difference in assembly between Mn2+-treated and untreated cells was observed after 0.5 h of incubation (Figure 4, A and C). However, by 4 h, the pFN matrix assembled by Mn2+-treated cells appeared to be more dense than that of untreated cells (Figure 4, B and D). Wild-type recFN (FNA−B−; Sechler et al., 1996) showed the same Mn2+-stimulated increase in matrix assembly as pFN. The presence of Mn2+ was not required for the entire 4-h incubation. A matrix comparable to that shown in Figure 4D was assembled when Mn2+ was added 2 h after pFN and incubated for 2 additional h.
Figure 4.
Effect of Mn2+ on FN matrix assembly. CHOα5 cells were incubated with 25 μg/ml pFN in the presence or absence of 1 mM MnCl2 for 0.5 h (A and C) and 4 h (B and D) followed by staining for FN fibrils by immunofluorescence. Bar, 10 μm.
De novo FN assembly into DOC-insoluble matrix follows a pattern similar to formation of a linear polymer with a slow initiation phase followed by a more rapid growth phase. In untreated CHOα5 cells, the accumulation of total cell-associated FN increased steadily over time in direct proportion to the input FN concentration (Figure 5A). Incorporation into DOC-insoluble matrix, however, occurred slowly at early times but showed an increase after a lag period of several hours (Figure 5B). The initiation phase is more clearly evident with the addition of Mn2+ which accelerated total FN binding and incorporation into DOC-insoluble material after 2 h but had no apparent effect before that time (Figure 5B). Mn2+ stimulation had a more dramatic effect on the proportion of DOC-insoluble FN. More than 80% of the total FN was DOC insoluble with Mn2+ as compared with 35% without Mn2+ (Figure 5C). Therefore, Mn2+ speeds up the conversion from DOC-soluble to DOC-insoluble matrix. This is not simply due to an increase in the amount of FN bound to the cell surface because doubling the FN concentration did not significantly increase the proportion of DOC-insoluble matrix.
Figure 5.
Rate of incorporation of FN into DOC-insoluble matrix in the presence or absence of Mn2+. Quantitative immunoblot analysis was performed on DOC-insoluble and -soluble extracts isolated from CHOα5 cells incubated with 25 μg/ml pFN alone (open circles), 25 μg/ml with 1 mM MnCl2 (closed circles), or 50 μg/ml pFN alone (open triangles) over the indicated periods of time. Accumulation of total cell-associated FN (A), DOC-insoluble FN matrix (B), and DOC-insoluble FN as percentage of total (C) was determined. Data are from a single experiment and are representative of resuts from at least four independent experiments as described in MATERIALS AND METHODS.
Mn2+ Activation of α5β1 Allows Assembly of FN(syn−)
An initiation phase of several hours is observed before significant conversion of FN fibrils into DOC-insoluble matrix. FN(syn−) appears to be arrested in this phase, since little DOC-insoluble material is recovered even after prolonged incubation (see Figure 2). Because Mn2+ can increase matrix accumulation of pFN by CHOα5 cells, we tested its effects on FN(syn−) assembly. Incubation for up to 4 h in the presence of 1 mM MnCl2 did not increase incorporation of FN(syn−) into fibrils or DOC-insoluble material. However, a longer 16-h incubation with 0.2 mM MnCl2 did stimulate assembly of FN(syn−) into a fibrillar matrix (Figure 6). Apparently, Mn2+ cannot exert an effect on FN(syn−) assembly until significantly later due to the extended initiation phase observed with the mutant protein.
Figure 6.
FN(syn−) assembly in response to Mn2+ addition. Twenty-five micrograms per milliliter FN(syn−) were added to CHOα5 cells along with no MnCl2 (A) or 0.2 mM MnCl2 (B) and incubated for 16 h. Cells were then fixed and FN fibrils were detected by immunofluorescence. Bar, 10 μm.
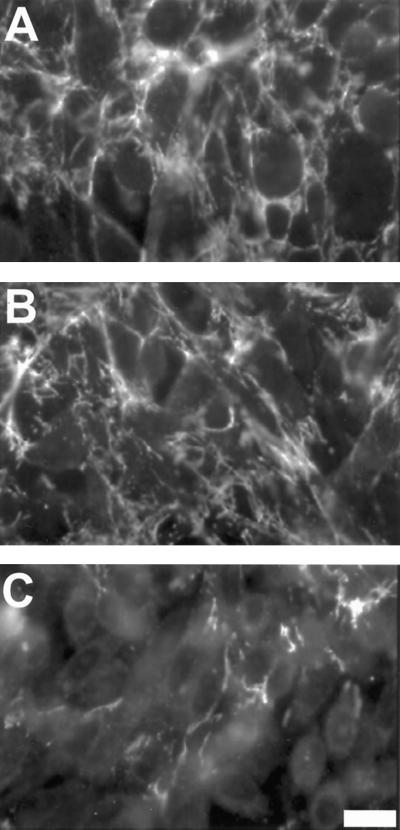
To test the effects of Mn2+ after initiation of FN(syn−) fibril assembly, 1 mM MnCl2 was added to CHOα5 cells that had already formed sparse fibrils of FN(syn−). A substantial increase in the amount of fibrillar FN(syn−) was evident after only 4 h of incubation with Mn2+ (Figure 7A). The increase in fibrillar FN(syn−) also corresponded to a significant accumulation of DOC-insoluble material (Figure 7B). Mn2+ stimulation failed to enhance the assembly of FN(RGD−) into a matrix.
Figure 7.
Mn2+ stimulation of assembly after initiation of a FN(syn−) matrix. (A) Twenty-five micrograms per milliliter FN(syn−) were added to CHOα5 cells and incubated for 16 h to allow for the formation of FN(syn−) fibrils. Cells were then incubated for an additional 4 h in either the absence (top) or presence (bottom) of 1 mM MnCl2. FN fibrils were detected by immunofluorescence with IC3 antibody. Bar, 10 μm. (B) Twenty-five micrograms per milliliter FN(syn−) and FN(RGD−) were added to CHOα5 cells and cultured under conditions described in A. DOC-insoluble matrix was isolated and the amount of FN detected by quantitative immunoblot analysis. Results are the average of three experiments for FN(syn−) and two experiments for FN(RGD−).
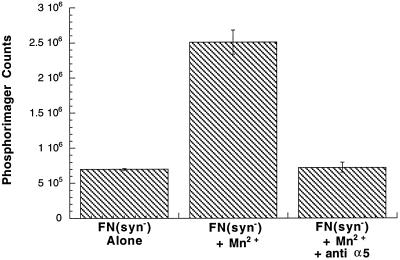
To confirm that α5β1 is responsible for Mn2+ stimulation of FN(syn−) matrix assembly, function blocking anti-integrin antibodies and 1 mM MnCl2 were added simultaneously to CHOα5 cells with FN(syn−) matrix. As shown in Figure 8, antihuman and antihamster α5 integrin antibodies inhibited FN(syn−) matrix formation. Together, these results demonstrate that the block in progression of FN(syn−) matrix assembly can be reversed by altering α5β1 integrin affinity by Mn2+ stimulation.
Figure 8.
Mn2+ stimulates α5β1 integrin and enhances FN(syn−) matrix assembly. CHOα5 cells were incubated with FN(syn−) for 16 h followed by an additional 4 h of incubation under the following conditions: no MnCl2 or antibodies [FN(syn−) alone], 1 mM MnCl2 [FN(syn−) + Mn2+], and 1 mM MnCl2 and function blocking anti-α5 integrin antibodies [FN(syn−) + Mn2+ + anti-α5]. DOC-insoluble matrix was isolated and the amount of FN present was determined by quantitative immunoblot analysis. Results are the average of two experiments.
PMA Does Not Stimulate FN Matrix Assembly by CHOα5 Cells
Other agents such as phorbol esters have been shown to increase cell adhesion (Danilov and Juliano, 1989; Vuori and Ruoslahti, 1993; Faull et al., 1994) and matrix assembly (Somers and Mosher, 1993) by integrin receptors. Phorbol 12-myristate 13-acetate (PMA) increases cell adhesion to FN without inducing an increase in the affinity of integrin receptors for ligand (Faull et al., 1994). Although treatment of CHOα5 cells with PMA resulted in an increase in cell spreading (our unpublished observations), it had no effect on the assembly of pFN into fibrils (Figure 9,A and B). PMA was also unable to enhance the incorporation of FN(syn−) into a matrix (Figure 9C). Furthermore, addition of Mn2+ and PMA together did not result in an increase in stimulation over Mn2+ alone. Therefore, agents which alter integrin function without modulating affinity do not affect matrix assembly in this system.
Figure 9.
Matrix assembly in response to PMA treatment. pFN was incubated with CHOα5 cells along with 100 nM PMA (A) or no PMA (B) for 4 h followed by immunofluorescence staining of FN fibrils. (C) FN(syn−) was incubated with CHOα5 cells for 16 h. An additional 4-h incubation was then performed in the presence of 100 nM PMA followed by detection of FN fibrils by immunofluorescence. Bar, 10 μm.
DISCUSSION
These analyses provide new insights into the process of FN assembly into a fibrillar matrix. First, FN assembly into DOC-insoluble matrix begins with a slow initiation phase followed by a more rapid accumulation phase. This biphasic process is very similar to the nucleation and growth phases during assembly of linear polymers such as actin filaments. Second, Mn2+ activation of integrins does not increase the amount of FN binding during the initiation phase but instead increases the rate of subsequent matrix growth, suggesting that a higher level of integrin activation is required during the later stages of the assembly process. Third, α5β1-mediated assembly is dependent on the presence of the synergy site and, as we showed previously, also requires the RGD sequence. Fourth, integrins activated by external agents such as antibodies or Mn2+ are able to assemble FN without the need for a synergy site. Together, our results demonstrate that FN assembly proceeds through at least two different stages and each stage has different requirements for integrin activation state and FN sequences. Initially, integrins bind sufficient FN for fibril formation to begin. Later in the process, the level of integrin activation determines the rate of accumulation of a dense, DOC-insoluble matrix. The synergy site is required in this second phase where contact with the receptor may serve to enhance integrin activity.
Ligand binding induces a conformational change in the extracellular domain of integrin receptors, and this change is linked to an alteration in receptor activity (reviewed in Ginsberg et al., 1992; Humphries, 1996; Mould, 1996). Mn2+ also alters the conformation of integrins (Bazzoni et al., 1995; Humphries, 1996) and promotes high levels of ligand binding (Gailit and Ruoslahti, 1988; Mould et al., 1995). Bazzoni et al. (1995) have shown that the anti-β1 antibody 9EG7 recognizes an epitope which is induced on the integrin upon binding to ligand or stimulation with Mn2+. This observation suggests that Mn2+ increases the rate of conversion of FN to DOC-insoluble matrix and enhances assembly of FN(syn−) by stabilizing a more active α5β1 integrin conformation. Although Mn2+ can significantly increase the rate of FN assembly, the effect does not occur immediately. With both native FN and FN(syn−), enhancement of matrix assembly only occurred after fibril formation had been initiated. One interpretation of these results is that initiation of assembly has different integrin activation requirements than accumulation into DOC-insoluble matrix. Mn2+ may induce an integrin conformation particularly favorable for this later stage. Mn2+ is probably not acting by allowing α5β1 interactions with the mutant synergy site since synergy site activity is dependent on the arginine residue (Aota et al., 1994) which in our mutation has been changed to a glutamate.
FN(syn−) assembly differs from that of FN(RGD−). A minimal matrix consisting of short sparse fibrils of FN(syn−) could be detected with both CHOα5 cells as well as At-T20α5 cells (our unpublished observations). The initiation step was slowed considerably and progression beyond that phase was prevented. FN(RGD−), in contrast, was not assembled (Sechler et al., 1996). In addition, Mn2+ was able to stimulate FN(syn−) assembly but had no effect on FN(RGD−). Therefore, under certain conditions it may be possible to make a matrix without a synergy site but not without an RGD sequence. A dual requirement for both the synergy site and the RGD sequence in matrix assembly is also consistent with the results of Nagai et al. (1991) who showed that monoclonal anti-FN antibodies that map near the RGD or synergy sites inhibited the formation of a FN matrix.
Not all agents that stimulate cell adhesion can affect matrix assembly. Treatment of CHOα5 cells with PMA did not alter the assembly of either FN or FN(syn−) matrix. In contrast, PMA did increase α5β1-mediated adhesion to FN fragments lacking a synergy site (Danen et al., 1995). Adhesion to these fragments was maximal when cells were treated with a combination of activating antibodies, Mn2+, and PMA. Therefore, the requirements for cell adhesion are different from the requirements for FN matrix assembly. PMA was not able to induce exposure of the 9EG7 epitope in α5β1 integrin, indicating that the conformational change in response to Mn2+ stimulation is not induced by PMA (Bazzoni et al., 1995). PMA has been shown to affect the binding of 125I-labeled FN to fibroblasts with an established FN matrix (Somers and Mosher, 1993) which may be representative of the later stages of assembly. Our data show that PMA does not influence the early events of assembly initiation and accumulation but do not rule out the possibility that it could modulate later stages of fibril formation.
We have recently proposed a model for FN matrix assembly in which FN is converted from an inactive form in solution to an active form at the cell surface (Sechler et al., 1996). In this model, FN is activated for assembly by binding to integin receptors, thus exposing sites required for the FN–FN interactions needed for fibril formation. Consistent with this model, Ugarova et al. (1995) have demonstrated the presence of reversible conformational states within FN fragments containing the cell-binding domain. Different epitopes were exposed depending on whether the fragment was in a “compact” or “extended” form. This type of regulation is not unique to FN. The focal adhesion protein vinculin, for example, has been shown to undergo changes in its conformation which serve to expose ligand-binding sites (reviewed in Jockusch and Rudiger, 1996). The results presented in this report show that, along with FN, integrins also play a dynamic role in multiple stages of matrix assembly. During initiation, integrin-FN binding and receptor clustering provide a nucleus of activated FNs (Figure 10A). As in other polymerization reactions, this phase is slow. Ligation of FN by α5β1 may induce the appropriate conformational changes within the integrin to stabilize the interaction and allow accumulation of additional FN dimers and conversion to DOC-insoluble matrix (Figure 10B). This step can be accelerated by stimulating the integrins with Mn2+. In the absence of the synergy site, the RGD region alone is sufficient for some initial binding but the assembly process becomes stalled, which explains the extended lag phase observed with the mutant protein. Mn2+ apparently induces a conformation that further activates α5β1 and allows the assembly of FN(syn−) to proceed (Figure 10C).
Figure 10.
Model for Mn2+ activation of α5β1 integrin and FN matrix assembly. (A) FN binding to α5β1 results in receptor clustering and unfolding of the FN molecule, exposing FN-FN binding sites required for initiation of matrix assembly. (B) A different α5β1 conformation is induced, thus allowing the formation of FN fibrils. Mn2+ can increase the rate of conversion from conformation A to conformation B. (C) In the absence of the synergy region of FN, conformation B is not induced and matrix assembly does not proceed. Mn2+ stimulation of α5β1 results in an activated receptor that can support FN assembly in the absence of the synergy site.
Until recently, only α5β1 integrin had been reported to function in initiating the formation of a FN matrix. However, α5-null and β1-null cells assemble a FN matrix, indicating that other receptors must be able to compensate for the loss of α5β1 (Yang et al., 1993; Wennerberg et al., 1996; Yang and Hynes, 1996). αIIbβ3 and αvβ3 integins have been shown to initiate and sustain FN fibril polymerization in transfected cell lines (Wu et al., 1995, 1996; this report). However, these integrins require exogenous activation by antibodies to achieve maximal matrix incorporation. FN assembly by antibody-activated β3 integrins is similar to FN(syn−) assembly by Mn2+-stimulated α5β1. Neither process requires the synergy site but both are dependent on exogenous activators. Apparently, the unique interaction between the synergy-RGD sites of FN and α5β1 integrin is designed to be particularly favorable for the polymerization of fibrils.
We have shown that the ability of α5β1 integrin to support FN matrix assembly is dependent on the presence of both the RGD sequence and the synergy site. Mn2+ activation of α5β1 speeds up the conversion from DOC-soluble to -insoluble matrix and abrogates the need for a synergy site. The Mn2+ effect is not seen until the formation of fibrils has been initiated, indicating that the affinity requirements of FN-α5β1 interactions change during the later stages of assembly.
ACKNOWLEDGMENTS
We thank Drs. Ken Yamada and Rudy Juliano for their kind gifts of anti-integrin antibodies and Dr. Mark Ginsberg for generously providing CHO αvβ3 cells and LIBS6-activating antibody. We are grateful to Jen Luczak and Mike Fitzgerald for excellent technical assistance and to Dr. Saw Kyin of the Departmental DNA Synthesis/Sequencing facility for preparation of oligonucleotides. This research was supported by National Institutes of Health grant CA44627 (to J.E.S.). J.L.S. was supported by a postdoctoral fellowship from the New Jersey Commission on Cancer Research and S.A.C. is an American Heart Association-Genentech Clinician Scientist Awardee.
Footnotes
Abbreviations used: DOC, deoxycholate; FN, fibronectin; FN(RGD−), recombinant FN lacking the RGD cell-binding sequence; FN(syn−), recombinant FN with mutation of the synergy site; pFN, plasma fibronectin; recFN, recombinant FN; PMA, phorbol 12-myristate 13-acetate.
REFERENCES
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- Akiyama SK, Yamada SS, Chen W-T, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali IU, Mautner VM, Lanza RP, Hynes RO. Restoration of a normal morphology, adhesion, and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977;11:115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Aota S, Nomiau M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Aota S, Toshihiko T, Yamada KM. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266:15938–15943. [PubMed] [Google Scholar]
- Arroyo AG, Sanchez-Mateos P, Campanero MR, I. Martin-Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta 1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Shih D-T, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Bowditch RD, Halloran CE, Aota S, Obara M, Plow EF, Yamada KM, Ginsberg MH. Integrin αIIIbβ3 (platelet GPIIIb-IIIa) recognizes multiple sites in fibronectin. J Biol Chem. 1991;266:23323–23328. [PubMed] [Google Scholar]
- Bowditch RD, Hariharan M, Tominna EF, Smith JW, Yamada KM, Getzoff ED, Ginsberg MH. Identification of a novel integrin binding site in fibronectin: differential utilization by β3 integrins. J Biol Chem. 1994;269:10856–10863. [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985;228:1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Monoclonal antibodies to distinctive epitopes on the α and β subunits of the fibronectin receptor. Exp Cell Res. 1988;177:303–318. doi: 10.1016/0014-4827(88)90464-8. [DOI] [PubMed] [Google Scholar]
- Danen EJ, Aota S, vanKraats AA, Yamada KM, Ruiter DJ, vanMuijen GNP. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin α5β1. J Biol Chem. 1995;270:21612–21618. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989;108:1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Georges-Labouesse E, Hirsch E. Genetic analysis of integrin function in mice. Curr Opin Cell Biol. 1996;8:641–646. doi: 10.1016/s0955-0674(96)80105-0. [DOI] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Stimulation of integrin-mediated adhesion of T-lymphocytes and monocytes: two mechanisms with divergent biological consequences. J Exp Med. 1994;179:1307–1316. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger AL, III, Du X, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of the Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out integrin signaling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19191. [PubMed] [Google Scholar]
- Humphries MJ. Integrin activation: the link between ligand binding and signal transduction. Curr Opin Cell Biol. 1996;8:632–640. doi: 10.1016/s0955-0674(96)80104-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Rudiger M. Crosstalk between cell adhesion molecules: vinculin as a paradigm for regulation by conformation. Trends Cell Biol. 1996;6:311–315. doi: 10.1016/0962-8924(96)10022-2. [DOI] [PubMed] [Google Scholar]
- Kovach NL, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to beta 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- Morla A, Rouslahti E. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421–429. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF. Assembly of fibronectin into extracellular matrix. Curr Opin Struct Biol. 1993;3:214–222. [Google Scholar]
- Mould AP. Getting integrins into shape: recent insights into how integrin activity is regulated by conformational changes. J Cell Sci. 1996;109:2613–2618. doi: 10.1242/jcs.109.11.2613. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama S, Humphries MJ. Regulation of integrin α5β1-fibronectin interactions by divalent cations. J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, Yamada KM. Monoclonal antibody characterization of two distant sites required for function in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991;114:1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole TE, Loftus JC, Du X, Glass AA, Ruggeri ZM, Shattil SJ, Plow EP, Ginsberg MH. Affinity modulation of the alpha IIb beta 3 integrin (platelet GPIIb-IIIa) is an intrinsic property of the receptor. Cell Regul. 1990;1:883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–240. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, LaChance RM, Broekelmann TJ, Kennedy CJR, Wagner EA, Carter WG, McDonald JA. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989;108:2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Takada Y, Schwarzbauer JE. Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J Cell Biol. 1996;134:575–585. doi: 10.1083/jcb.134.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Schwarzbauer JE. Coordinate regulation of fibronectin fibril assembly and actin stress fiber formation. Cell Adhes Commun. 1997;4:413–424. doi: 10.3109/15419069709004458. [DOI] [PubMed] [Google Scholar]
- Somers CE, Mosher DE. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem. 1993;268:22277–22280. [PubMed] [Google Scholar]
- Ugarova TP, Zamarron C, Veklich Y, Bowditch RD, Ginsberg MH, Weisel JW, Plow EF. Conformational transitions in the cell binding domain of fibronectin. Biochemistry. 1995;34:4457–4466. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Hughes PE, Ginsberg MH, McDonald JA. Identification of a new biological function for the integrin avb3: initiation of fibronectin matrix assembly. Cell Adhes Commun. 1996;4:149–158. doi: 10.3109/15419069609014219. [DOI] [PubMed] [Google Scholar]
- Wu C, Kevins V, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are critical steps in the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Yamada SS, Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci USA. 1976;73:1271–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in α5β1 integrin can be replaced by αv integrins. Mol Biol Cell. 1996;7:1737–1748. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]