Abstract
Due to the intimate interactions between histones and DNA, the characterization of histones has become the focus of great attention. A series of mass spectrometry-based technologies have been dedicated to the characterization and quantitation of different histone forms. This review focuses on the discussion of mass spectrometry-based strategies used for the characterization of histones and their post-translational modifications.
Keywords: histone, mass spectrometry, post-translational modification
Histones are a group of highly conserved proteins throughout eukaryotic evolution [1]. The core histones (H4, H3, H2A and H2B) form an octamer, in which a H2A–H2B dimer associates on each side of the H3–H4 tetramer through an interaction between the C-terminal halves of H2B and H4 [2]. The negatively charged DNA superhelix wraps around the histone octamer to form repeating nucleosome cores, which further assemble into higher order chromatin fibers stabilized by the linker histones (H1 and H5) and linker DNA [3]. The histone fold regions in core histones are highly conserved α-helices, which are connected by two loops. The third histone structural motif comprises the flexible and irregular histone tails, which are extended and reach outside of the nucleosomal unit to interact with DNA [4].
Challenge of molecular characterization: post-translational modifications & sequence variations
As a building block of the nucleosome, histones play an important role in chromatin assembly and affect the interactions between DNA and other chromatin-associated proteins. Histones regulate gene transcription through their diverse post-translational modifications (PTMs), which include lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, lysine ubiquitination, lysine sumoylation, and poly-ADP-ribosylation of glutamic acid [5-8]. Among all the PTMs, histone acetylation and methylation have been most extensively studied. Histone hyperacetylation has long been implicated in associated with transcriptional activation [9]. Phosphorylation is linked with the activation of genes and condensation of chromatin during mitosis [10]. Methylation, on the other hand, is linked with both active and repressed promoters [11-14]. Histone modifications may also act in concert, as suggested in the histone code hypothesis [6,8,15,16]. For example, the methylation of K4 and K79 on H3 in yeast is mediated by ubiquitination of K123 on H2B [17]. In addition, H3 acetylation and phosphorylation are synergistically coupled in response to epidermal growth factor stimulation. Phsophorylation of H3 S10 increases the activity of histone acetyl-transferase Gcn5 for subsequent H3 K14 acetylation [10,18,19].
The reversible acetylation and deacetylation of histones are critical in controlling chromatin remodeling [20]. Lysines on the N-terminal tails of histones (H4: K5, K8, K12 and K16; H3: K9, K14, K18 and K23) are subject to acetylation [21]. Acetylation of histones occurs at the ε-amine group of a lysine residue, removing positive charges and thus reducing the electrostatic affinity of histones to the negatively charged DNA. Acetylation induces a relaxed chromatin structure, which enables RNA polymerase and transcription factors greater access to promoter regions. In contrast to histone acetylation, deacetylation of histones induces chromatin condensation. The hypoacetylated state of chromatin may be related to aberrant gene expression through transcription repression [22].
Histone methylation is a key regulator of gene expression and heterochromatin function [23]. This modification has been found mostly within the N-terminal domains of H3 and H4. For instance, H4 is predominantly methylated at K20 [24-27]. H4 K20 methylation is associated with DNA damage response and is necessary for the recruitment of the checkpoint protein Crb2 [23]. K4 and K9 on H3 are commonly methylated in higher eukaryotes. K4 methylation accumulates in transcriptionally active regions, whereas K9 methylation accumulates in gene-silencing regions [11,15,28,29].
In addition to histone acetylation and methylation, important progress has been made in the study of other covalent modifications. While all histones are subject to phosphorylation, linker histones contain a relatively high content of phosphorylation. In bovine thymus tissue, every mole of histone H1 may incorporate up to 1.3 moles of phosphate [30]. Different types of histone phosphorylation are suggested to be correlated with different levels of chromatin organization: H1 and H3 phosphorylation play roles in chromosome condensation; and H2A phosphorylation is associated with heterochromatin condensation [31,32]. Furthermore, histone phosphorylation is utilized as a tool of transcriptional regulation by eukaryotes. As an example, S10 phosphorylation of H3 works synergistically with H3 K9 acetylation to prevent H3 K9 methylation and subsequent gene silencing [11,33,34].
Histone PTMs have been reported to occur predominantly on the N-terminal tails. However, the importance of histone modifications beyond N-terminal domains has recently become the focus of attention [35]. The histone core domain may be highly modified, and these PTMs are also associated with gene activities [5,36]. For example, K59 methylation on H4 is related to gene silencing, and K91 acetylation on H4 is important for regulating chromatin assembly in yeast [35]. Methylation of H3 K79 is linked with transcription activation and euchromatin protection [23]. In addition to the core domain, perturbation on C-termini can influence chromatin without destroying the integral DNA–histone interactions. For instance, phosphorylation of H2A.X (histone H2A, family member X) at S129 in yeast and S139 in mammals is responsible for the regulation of DNA double-stranded break repair [37,38], as well as being linked to many other genetic activities [38-41]. Histone ubiquitination on H2A at K119 and H2B at K120 is associated with gene activation. The newly observed K123 ubiquitination of H2B is necessary for subsequent H3 K4 methylation in yeast [42,43]. The acetylation and deacetylation of K115 and K122 on H3 is linked to DNA metabolism regulating the formation of heterochromatin [44]. A unique methylation site was identified on mouse H3 K122, the function of which has not been well characterized to date [45].
Since histone PTMs are closely related to gene transcription, many diverse disorders can result from dysregulation. Aberrant acetylation and deacetylation of histones are associated with severe human disorders, including leukemia, epithelial cancers, Huntington's disease, fragile X syndrome and Rubinstein–Taybi syndrome [46]. It has been demonstrated that phosphorylation and acetylation of the C-terminal regulatory domain of histones are involved in the activity of the tumorsuppressor protein p53 [47-49]. Histone deacetylase (HDAC) inhibitors can increase histone acetylation, resulting in decreased proliferation, induced differentiation and programmed cell death, especially in transformed cells. Thus, HDAC inhibitors have become a potential therapeutic target for the treatment of cancer [50-54].
Although PTMs are purported to play a major role in gene regulation, recent research suggests that minor changes in the primary sequences of highly conserved histones may be the main determinant for chromatin structure and gene regulation [17,55]. Nucleosomes may recruit different histone variants to mark chromatin regions. These marked regions influence gene expression, antisilencing, heterochromatinization, formation of specialized regions of chromatin, DNA repair and meiotic events [56-61]. Histone variants appear to connect different chromatin regulatory mechanisms, such as histone PTMs or ATP-dependent chromatin remodeling [36,62,63].
Histone variants were discovered in 1969 and have gained increasing interest due to their crucial genetic function [64]. A simple protein known as archaeal histone protein was suggested to be the common ancestor of all the histones [65,66]. Subtypes of histones H1, H2A, H2B and H3 (which differ in amino acid sequence) may have risen either during embryogenesis or during the maturation of certain specialized cells [7]. Following three evolutionary events, H2A and H2B diverged faster than H3 and H4. In addition, H2A and H3 have evolved more than their partners, H2B and H4. Linker histone H1 is regarded as an evolutionary hybri,d and its variants have been long recognized [7,67-69]. The National Human Genome Research Institute (NHGRI) histone database records all the known subtypes of histones in different organisms [201].
Traditional approaches
Microsequencing & antibody-based approaches
Traditional methods to characterize the PTMs of histones are mainly based on microsequencing and immunoassay. While protein microsequencing provides accurate sequence information and has demonstrated success in the identification of histone PTMs [70-72], it requires a large amount of purified samples and only 20–25 residues can be routinely characterized. Other widely used immunoassay methods include: immunoprecipitation, immunofluorenscence and western blotting [73,74]. These methods are highly sensitivite, but are affected by antibody specificity due to cross-reactivity and adjacent concomitant histone modifications. Furthermore, there are a limited (but growing) number of such antibodies that are commercially available, of which the majority target N-terminus modifications [18,75-77].
Hydroxyapatite chromatography
In eukaryotic cells, the DNA is packaged into a proteinaceous structure (chromosomes) through arrays of nucleosomes [78]. Hydroxyapatite chromatography (HAP) is a technique for purifying of these chromosomal proteins by fractionating according to their DNA binding properties [79]. The separation is based on the binding of hydroxyapatite to DNA in chromatin. Proteins are selectively eluted from the immobilized chromatin with NaCl in phosphate buffer. The HAP dissociation method enables chromosomal proteins to be fractionated with high recovery and without nucleic acid contamination. HAP is suitable for large-scale preparation of histones, nonhistone proteins, histone oligmers and/or DNA [79-83].
Mass spectrometry-based proteomics
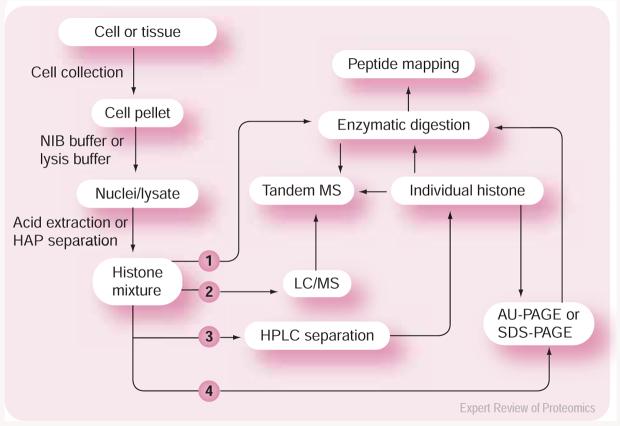
Mass spectrometry (MS) is a physicochemical analysis technique that determines the mass-to-charge ratios of gas-phase ions. MS forms the basis of many strategies for the proteomic characterization of proteins and protein mixtures. It has been successfully used by several groups in the analysis of histones [84-93]. figure 1 summarizes the general experimental design that modern mass spectromists use most frequently for histone analysis using MS-based methodologies. Liquid chromatography (LC) MS is a sensitive, accurate and fast method that can be used for intact protein analysis. Peptide mass finger-printing (PMF) is an efficient method to map histone PTMs using mass correlation. When it is necessary to locate a PTM or obtain sequence information, experiments by tandem MS (MS/MS) can be performed.
Figure 1. General flowchart of an experimental design for histone characterization.
(1) Shotgun proteomics. (2) Top-down proteomics. (3) Combined top-down and bottom-upproteomics. (4) Bottom-upproteomics.
AU: Acetic acid urea; HPLC: High-performance LC; LC: Liquid chromatography; MS: Mass spectrometry; NIB: Nuclear isolation buffer; HAP: Hydroxyapatite chromatography;PAGE: Polyacrylamide gel electrophoresis; SDS: Sodium dodecyl sulfate.
Reverse-phase, high-performance liquid chromatography mass spectrometry
Complex protein mixtures require separation prior to MS analysis. The separated proteins may be collected and analyzed independently offline or the eluted fractions can be infused directly into the mass spectrometer (online). Reverse-phase (RP), high-performance LC (HPLC) is widely used in LC/MS due to solvent compatibility with electrospray ionization (ESI). Online LC/MS has been employed as a fast screening approach to directly characterize the PTMs of histones because it greatly simplifies sample preparation and data interpretation [84,85,88,94]. The approach has great merit and has been used by numerous groups for the characterization of histones.
Zhang reported the use of a microbore C4 column for the separation of histones [88]. The LC was effective at separating the major core histone variants. In addition, the mass spectra revealed the degree of histone acetylation and methylation upon each variant. The work of the Gurley group pioneered histone separation by use of RP-LC (CN, C8, C18) [89]. Nucleoproteins, including histones, were fractionated from whole nuclei or chromosomes by use of a μBondapak CN RP column. A μBondapak C18 Radial-Pak column demonstrated the best resolving power for histone variants, whereas the Zorbax C8 column worked best for nonhistone proteins. Lindner also made significant advances in the science of histone separation. They used the Nucleosil 300-5 C4 column to efficiently separate several H1 subtypes (H4, two H2B variants, two H2A variants and two H3 subtypes) as well as the high-mobility group proteins (HMG-1 and -2) [90]. Su and colleagues recently demonstrated the use of LC/MS using a custom-manufactured C18 column for an improved separation of histones. The column was coupled to an ESI time-of-flight (TOF) mass spectrometer for the routine and fast screening analysis of histones, which can identify the changes of modified isoforms and/or histone variants between different cell lines or after drug treatment [95]. Consequently, a complete characterization of both core and linker histones was achieved and the results suggest that histones H2A and H2B are present as both modified forms and sequence variants. In similar studies, Bonenfant and colleagues applied C18 RP-LC/MS to the study of histones derived from Jurkat cells [94], and more recently, Naldi and colleagues baseline separated eight histone forms using a C4 Jupiter column [96].
Hydrophilic-interaction LC & offline hydrophilic interaction LC/MS
The compatibility of RP-HPLC with ESI-MS makes it an ideal first choice for LC/MS separation. However, the similarity of the highly basic histone variants limits the effectiveness of RP-HPLC. Hydrophilic-interaction LC (HILIC) is a powerful technique that has proved to be extremely well suited for histone separation. Lindner optimized HILIC to attain excellent resolution of core and linker histones [97-100]. In their work, the protein mixture was fractionated on a weak cation-exchange column using an increasing sodium perchlorate gradient in a methanephosphonic acid-triethylamine buffer (pH 3.0) in the presence of organic solvent. The combined use of HILIC and RP-HPLC enabled them to isolate various H2A variants, acetylated forms of H2A and H4 and phosphorylated H1 isoforms with high purity, whose identities were determined by using offline MS detection. The complete separation of these forms is a prerequisite for studying their biological functions. Sarg and colleagues employed HILIC to simultaneously separate and identify Lys20 methylation states from acetylated H4 isoforms. The separation enabled the following determinations [26,27]:
H4 hyperacetylation (tri- and tetraacetylation) blocked trimethylation of H4 lysine 20
Trimethylated K20 of H4 in mammalian tissues was related to aging
The HILIC technique is not without disadvantages. The high salt concentrations used in the mobile phase render HILIC incompatible with online MS analysis. The need to do sample clean-up and collect fractions offline makes this method time consuming, labor intensive and subject to sample loss.
HP capillary electrophoresis-MS
HP capillary electrophoresis (HPCE) is another means to separate histone proteins. Its many advantages include:
Low sample requirement (nanoliters or less) as compared with traditional HPLC
Better resolution of histones than by sodium dodecyl sulfate (SDS), acetic acid urea (AU) or AU-Triton (AUT) gel eletrophoresis
Ease of automation
High efficiency due to short separation time.
The technique is especially well suited for resolving acetylated and phosphorylated forms. Gurley, Lindner and Mizzen separately demonstrated HPCE methods to efficiently separate histones [101-107].
The combination of HPCE and MS opens exciting new prospects for histone investigation. Aguilar and colleagues first applied online CE-MS to characterize histones from calf thymus [108]. The use of a hydroxypropylmethylcellulose-coated column significantly improved the separation of basic histones. A simple dialysis step prior to CE separation and the use of co-axial sheath liquid flow between the CE and ESI-MS interface made the online CE-ESI-MS possible [108-111]. Despite the potential power of the approach, CE-MS of histones is difficult due to:
Interaction between positively charged proteins and ionized silanols
Reliable CE interface with ESI-MS
Interference from the background electrolyte
Despite these limitations, the utility of CE-MS for histone protein characterization remains extremely promising.
Acetic acid-urea polya crylamide gel electrophoresis
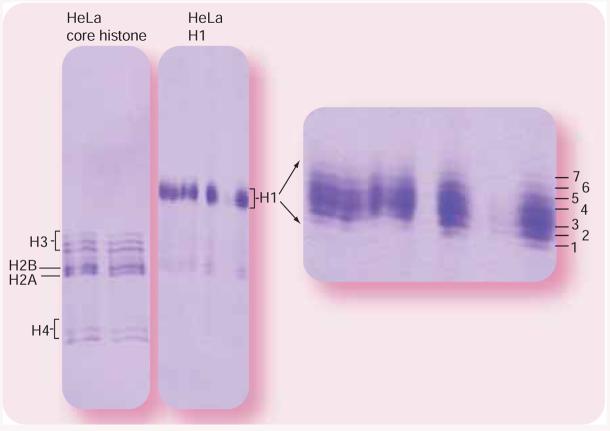
Histones are highly basic. Under acidic (e.g., acetic acid, pH 3–4) conditions, histones are positively charged both on the unprotected N-termini and the amino group of the lysine side chain. The charge of histones is altered by acetylation of lysine and phosphorylation of serine, threonine and tyrosine. Traditional SDS gel electrophoresis fails to separate these modified isoforms because its separation is based predominantly on size. However, AU gel electrophoresis, described by Panyim and Chalkley in 1969, is able to separate very similar basic proteins based on differences in size and effective charge [112]. At acidic pH, basic histones with a high isoelectric point have a net positive charge. Any removal or addition of these net positive charges on the relative small histones (e.g., H4, 102 amino acids) will cause a significant increase or decrease in effective mobility. Using AU polyacrylamide gel electrophoresis (PAGE), acetylated histone H4 isoforms are well separated based on the numbers of lysine acetylations. The more challenging separation of proteins H2A, H2B and H3 with partial acetylation can be achieved by addition of a nonionic detergent such as Triton® X-100 (AUT-PAGE). figure 2 illustrates an example of AU-PAGE separation for core (especially acetylated H3 and H4) and linker histones. AU-PAGE is an excellent platform for purification of isoforms that will then be characterized by MS. Although AUT-PAGE results in better separation for different histone forms [90,113-115], the addition of the detergent, Triton® X-100, reduced the effectiveness of subsequent in-gel digestion and MS detection. Therefore, AU-PAGE is the preferred separation method prior to MS analysis.
Figure 2. Acetic acid-urea polyacrylamide gel electrophoresis (AU-PAGE) of histones derived from HeLa cells.
Histones were fractionated into the core and linker histones prior to AU-PAGE separation.
Peptide mass fingerprinting
PMF, also known as peptide mass mapping (PMM), is a methodology that is used to identify proteins after digestion by comparing the masses of the observed peptides with those of a theoretical protein digest. In PMF, intact proteins are in-gel or in-solution digested using appropriate proteolytic enzymes. The peptide masses are then determined by MS analysis and searched against theoretical protein digests generated from an appropriate protein database. Matches between the observed and theoretical peptides/proteins are scored. The highest scoring protein is then taken to be the protein undergoing characterization.
PMF is a very efficient means of identification and characterization of PTMs. Each PTM introduces a specific mass shift relative to theoretical mass of an unmodified protein (table 1). The location and number of modifications can be determined based on the mass shift of the respective peptide. For example, PMF was used to identify methylation on H3 K79 using histones purified from human cultured cells, calf thymus, chicken erythrocytes and Saccharomyces cerevisiae [87,116-118]. The approach has also been used by other groups to characterize histone PTMs [86,119].
Table 1.
Common histone post-translational modifications.
| Modifications | Mass shift (Da) | Modified residues |
|---|---|---|
| Acetylation | 42.0106 | K, N-terminal |
| Methylation | 14.0156 | K, R |
| Phosphorylation | 79.9663 | S, T, Y |
| Oxidation | 15.9949 | M |
| Ubiquitination | 114.0429* | K |
When digestedby trypsin, the ubiquitin side chain leaves the dipeptide GlyGly.
Fourier transfer-ion cyclotron resonance (FT-ICR) is a massselective analyzer that offers the highest mass resolving power (>106) and mass accuracy (<1 ppm) [120,121]. FT-ICR-MS is increasingly being applied to solve complex proteomic problems [122]. High mass accuracy is especially important for histone PTM characterization. For example, histone lysine acetylation and trimethylation introduce mass shifts of 42.0106 and 42.0470 Da, respectively. In order to distinguish between these two modifications, a mass accuracy of 3.6 ppm for a protein with a molecular weight of 10 kDa would be required. FT-ICR-MS can routinely achieve the required mass accuracy. Using the power of FT-ICR combined with PMF, Zhang conducted a survey of histone modification in bovine thymus tissue [85]. The survey uncovered 20 novel PTMs, including K59 methylation and K91 acetylation [5,35,85,123].
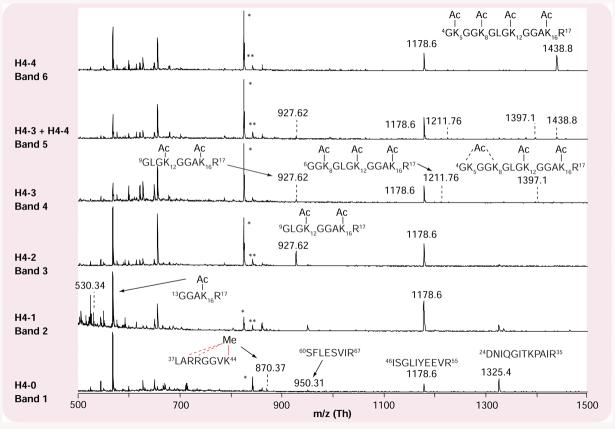
figure 3 illustrates an example of the application of PMF to characterize histone H4 acetylation in yeast, S. cerevisiae. In this example, the histone mixture purified with acid extraction was separated by AU-PAGE. Different gel bands corresponding to acetylated H4 isoforms were excised and in-gel digested with trypsin preceding matrix-assisted laser desorption/ionization (MALDI) TOF-MS analysis. The results indicate that yeast histone H4 possesses a similar acetylation pattern in the N-terminal region to that of mammalian histone H4 (K16 acetylated initially followed by K12 and then K5 and/or K8). Unlike mammalian H4, yeast H4 was not observed with the predominant dimethylation of K20.
Figure 3. Application of peptide mass fingerprinting to characterize histone H4 acetylation in yeast Saccharomyces cerevisiae.
Histones purified by acid extraction were separated by acetic acid-urea polyacrylamide gel electrophoresis. The gel bands corresponding to acetylated H4 isoforms were excised and in-gel digested with trypsin proceeding matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. The results indicate that yeast histone H4 possesses a similar acetylation pattern in the N-terminal region to that of mammalian histone H4, where K16 was predominantly acetylated first, followed by K12 and then K5 and/or K8.
*Interferences due to Coomassie blue. **Interferences due to trypsin.
Ac: Acetyl; Me: Methyl; m/z: Mass-to-charge ratio.
Direct absolute quantitation by MS is problematic due to the difference in the ionization efficiency of peptides with different sequences. However, changes in the relative abundance of peptides with the same (or very similar) sequences may be determined in a semiquantitative manner by direct examination of the mass spectra. For this reason, PMF is a simple and effective means of quickly and reliably obtaining relative quantitative data. The approach is particularly well suited for assaying distributions and/or changes in PTMs for a specific digested fragment. The approach has been referred to as a MS western blot [124]. The distribution of novel methylations at H3 K79 was determined in this manner [118,124], along with a thorough comparison of methylation at lysines 4, 9 and 79 and their relation to acetylation of H3 in the N-terminal region [124].
Tandem MS
Despite the power and simplicity of the PMF approach, it is unable to differentiate overlapping isobaric peptides of different sequences. In such cases, MS/MS is required to obtain an unambiguous identification and accurate quantitation. MS/MS involves at least two stages of mass analysis, in which the first stage is to isolate the ions of interest and the second stage is to determine the mass-to-charge ratio of product ions after fragmentation. The peptide/protein sequence can then be inferred from the pattern of fragmentation. The commonly used techniques to fragment peptides include collision-induced dissociation (CID) or collision-activated dissociation (CAD), post-source decay (PSD), infrared multiphoton dissociation (IRMPD) and electron capture/transfer dissociation (ECD/ETD) [125-129].
MS/MS by CID has proved to be a successful approach in the identification of histone PTMs, such as glycosylation [130], phosphorylation [131], acylation [132], acetylation [87,133] and methylation [87,134]. figure 4 illustrates one example of such application. A triacetylated peptide of yeast histone H4 in the N-terminal region was identified by the use of MS/MS, and validated the results of PMF described in figure 3. In addition to histone characterization, MS/MS is routinely applied to global proteomic studies, also known as shotgun proteomics. For example, nano-LC/MS/MS was used for global profiling of perchloric acid soluble proteins in breast cancer cells [135]. Over 150 unique proteins were identified, including the linker histones and HMG proteins.
Figure 4.

Application of tandem mass spectrometry to the identification of triacetylation of H4 peptide in yeast Saccharomyces cerevisiae.
By monitoring the relative abundances of precursor or fragment ions generated during MS/MS experiments, relative quantitative changes of corresponding isoforms can also be inferred. Nano-LC/MS/MS is the ideal approach when the sample is a complex mixture and the purification of individual proteins is challenging. Nano-LC/MS/MS has been applied to qualitative and quantitative anaylsis of histone PTMs [91]. The approach revealed 32 modifications, including acetylation, methylation and ubiquitination, for all four human histones, of which seven were purported to be novel PTMs. Dose-dependent changes in histone acetylation were quantitatively determined in response to treatment of the cells with the HDAC inhibitor, PXD101.
CID fragmentation of the peptide produces ions that not only indicate the peptides sequence, but also the identity of the PTMs. Neutral losses and immonium ions are common fragmentation products that have great diagnostic significance. For example, the loss of 59 Da is observed for trimetylated lysine residues. For low mass-accuracy instruments, this neutralloss is useful to differentiate between trimetylation and acetylation [136,137]. Likewise, the immonioum ion, observed at 98 Th is characteristic of lysine monomethylation, and the immonium ion at 84 Th is found in the presence of lysine dimethylation and/or trimethylation. Lysine acetylation generates a diagnostic ion at 143 Th and 126 Th. The latter ion results from the loss of NH3 from the former immunion ion and is reported to be more specific for lysine acetylation and nine-times more sensitive [129,136-141].
The newest family of ion-dissociation techniques, ECD, was first discovered by Fred McLafferty in 1998 [125]. In ECD, protonated peptides/proteins capture free electrons that, in turn, induce fragmentation of the protein backbone. The many diagnostic fragments and the intact labile PTMs make ECD advantageous in the study of PTMs of intact proteins. Medzihradszky and his coworkers employed ECD-FT-ICR-MS to characterize histone H2B variants in tetrahymena and studied their PTMs from intact proteins [142]. They also performed enzymatic digestions for the intact proteins and used classical peptide HPLC/ESI-LC/MS/MS to identify H2B PTMs, which showed complementary information with intact protein MS and MS/MS analysis. A more complete map of the PTMs can be obtained by combining these two methods. The only limitation of the technique is its relatively low efficiency for fragmentation, and thus requires a higher amount of sample.
The requirement of a dense population of near-thermal electrons for fragmentation in ECD limits its use exclusively in FT-ICR, since thermal electrons are not trapped in other instruments with radiofrequency electrostatic fields, such as quadrupole ion trap and quadrupole TOF [129]. To circumvent this limitation of ECD, Hunt and coworkers developed ETD, which uses an anion to transfer electrons to multiply protonated proteins/peptides and induces fragmentation of the backbone in a pathway similar to ECD [129]. They demonstrated the successful application of this method to characterize histone H3.1 PTMs and the identification of a new member of the H2A gene family by online coupling with chromatographic elution of protein mixtures [143].
Top-down proteomics has also been successfully applied to the characterization and quantitation of histone forms and PTMs. Excellent examples have been reported by Kelleher and coworkers [144-147]. Different gene families of histone H2A, H2B and H3 variants were identified by use of high mass-accuracy FT-ICR-MS along with their changes in relative expression and modifications during cell cycling [144,145,147]. The same group employed FT-ICR-MS to quantitate the modified forms or positional isomers of histone H4 using the relative abundances of their fragment ions following HPLC and HILIC fractionation [146]. A novel approach, integrating a quadrupole FT-MS with top-down fragmentation using ECD and shotgun annotation of histone modification, was developed to characterize histone H4 and its multiple PTMs by this group [148]. The shotgun annotation approach enables complete and automated characterization of human histones containing two to six PTMs by considering PTMs during database searching. However, top-down proteomic approaches require prepurified histone fractions (such as H4, H2A, H2B and H3) in a large amount. Thus, a rapid characterization in a single run would be impractical, as is necessary in the analysis of clinical samples.
Characterization of low-abundance modifications (phosphorylation & ubiqutination)
Successful MS characterization of histones begins with careful sample preparation. The histones must first be purified and the desired isoforms enriched or derivatized as necessary. Phosphorylation presents a challenge for MS detection due to its much lower abundance than acetylation and methylation. Immobilized metal affinity chromatography (IMAC) has been demonstrated by Hunt and coworkers as an effective way to enrich these low-abundance peptides [149]. They combined IMAC and nano-LC/MS/MS to detect 19 phosphorylation sites on six H1 isoforms derived from asynchronously grown HeLa cells. The same group used stable isotopic labeling, IMAC and MS/MS to measure relative levels of site-specific phosphorylation on H1 [149]. Propionic anhydride derivatization was used to label and block trypsin cleavage of unmodified lysines. This reagent creates longer peptides that are more hydrophobic and betterretained on the RP column. Cation-exchange chromatography was also employed as a means to separate the phosphorylated isoforms and determine the order of sequential H1 phosphorylation [150]. Identification of phosphorylation on serine and threonine residues was facilitated by the presence of corresponding neutral loss of phosphoric acid (98 Da in positive ion mode and 80 Da in negative mode) and data-dependent MS/MS/MS experiments [151-152].
Ubiquitination of proteins is a frequent event in eukaryotic cells used to regulate protein abundance. Ubiqutination serves as a tag for protein destruction by the proteosome [153]. This makes ubiquitination an extremely important PTM, but also one that is difficult to monitor due to its rapid turnover. Kirkpatrick and coworkers developed an approach for the purification and identification of ubquinitated proteins, including histones [154]. In their study, HEK293 human cells were stably transfected with co-expressing epitope-tagged His (6X)–ubiquitin and green fluorescent protein (GFP). Nickel-affinity chromatography was employed to purify ubiquitinated proteins. Following in-solution tryptic digestion, peptide mixture was analyzed by nano-LC/MS/MS using a quadrupole ion-trap mass spectrometer (LCQ). Trypsin cleaves ubiquitin from the lysine, leaving behind its two C-terminal glycines. Fragmentation of peptides that contain the modified K-GG yielded a characteristic 114 Da neutral loss; resulting from fragmentation of the GG [155]. Using this approach,21 ubiquitinated proteins (including ubiquitin-conjugating enzymes and histone) were identified [154].
Stable isotope labeling
Precise quantification of modification changes is important in understanding the effect of histone PTMs on gene transcription. Waterborg and colleagues described pulse, pulse-chase and steady-state acetylation labeling methods to study the kinetics of histone acetylation after treatment with HDAC inhibitor trichostain A in plant alfalfa [156]. Pulse-chase method introduces radioactive acetyl-coenzyme A, produced by radioactive acetate, into permeabilized cells for a short period of time (the pulse time). The residual acetate is immediately washed away to start the chase condition. The acetylation turnover rates are then determined by measuring the specific radioactivity of eachhistone over the period of chase. One requirement of this method is that acetate uptake is not rate limiting, and conversion from acetate into acetyl coenzyme A is rapid. A major limitation of this approach is that acidification of the cytoplasm can affect the subsequent chase conditions. The rate is also affected by the length of pulse labeling. Another approach to measure the turnoverrate of acetylation is by use of continuous acetylation labeling under steady-state conditions. Both approaches determine the turnover rate by measuring the specific radioactivity of histones, which usually takes several days and requires special handling of the radioactive materials.
Chromatin immunoprecipitation assay combined with quantitative real-time PCR is also used to measure acetylation turnover rates at selected genes and DNA domains [157]. This approach relies on the specificity of the antibodies of known modification sites, which may be affected by many factors, such as adjacent multiple modifications and experimental bias.
The advent of stable isotope labeling that utilizes 18O-, 15N- and 13C-labeling as well as isotope-coded affinity tag, either by chemical derivatization or metabolic incorporation, enables MS to measure protein abundances quantitatively at intact protein and their proteolytic peptides [158-166]. These approaches are powerful because they measure relative abundance between unlabeled protein/peptide and its corresponding internal standard labeled with the heavy stable isotope. While these approaches are quite attractive, they produce complex data sets and labeling efficiency must be carefully controlled.
Smith and colleagues introduced a method to directly measure endogenous acetylation levels of H4 by use of stable isotope labeling and MS/MS [167,168]. In their report, histone H4 was incubated with deuterated acetic anhydride so that unmodified lysine residues would be in vitro labeled with a deuterated acetyl group [167]. Thus, any lysine residues acetylated in vivo had an unlabeled acetyl group (42 Da) and those modified in vitro (unacetylated in vivo) had a labeled acetyl group (45 Da). In vitro acetylation of lysines also prevents trypsin cleavage at lysine residues, and thus a peptide encompassing amino acids 4–17 in histone H4 would be generated containing all four possible sites of acetylation in the N-terminal region. The resulting tryptic digest contains isotopic clusters with mass shifts of 3 Da (or multiples thereof). The endogenous acetylation levels can then be determined by CID-MS/MS of these isotopic species followed by calculation of the ratios of the labeled and unlabeled productions.
Using this approach, Smith determined the percentage of yeast histone H4 in vivo acetylated isoforms through the isotope patterns of the molecular ions, which had 12% nonacetylation, 36% monoacetylation, 28% diacetylation, 13% triacetylation and 12% tetra-acetylation. By calculating the ratio of the production intensities, the level of residue specific acetylations was also determined, which had 80% K16 acetylation, followed by 54% K12 acetylation, 32% K5 acetylation and 24% K8 acetylation. In addition, the average level of in vivo acetylation per molecule in fragment 4–17 was obtained based on the data from both MS and MS/MS experiments, as each molecule contained 1.8- and 1.9-acetylated lysine residues. This labeling approach can be applied to all protein samples (tissue or cells) for quantification of acetylation level changes. However, the mathematical calculation is complicated and over- or underderivatization will increase the uncertainty in the measurements. Furthermore, a high yield of fragment ions is required for this analysis.
More recently, the technique known as stable isotopic labeling with amino acids in cell culture (SILAC) is an efficient alternative to other isotopic labeling methods. SILAC has been attracting more and more interest in MS-based quantitative proteomics since its invention in 2002 [165,169]. Briefly, cells are grown in either a regular media or a media with a heavy isotope-labeled amino acid. In general, complete incorporation of labeled amino acids can be achizeved after four or five cell cycles. The differences in labeling efficiency between one sample and the other can thus be eliminated. Proteins can then be purified following the combination of the equal amounts of cells grown in heavy and light media, and the heavy:light protein mixture is subject to systematically proteomic analysis. Protein quantitation is performed by calculating the relative abundance of corresponding heavy:light peptide pairs. Using SILAC, no further differential effect is induced by the purification strategies, enzymatic digestion and MS response. SILAC coupled with MS may even enable the simultaneous identification and quantification of protein and/or peptide without the aid of MS/MS, and thus largely simplify data analysis [170,171].
Data analysis
While enrichment is important in reducing complexity, automated data analysis software is also required to interpret the resulting data. Due to the inherent complexity of the resulting data, automated software is crucial for analysis. For bottom-up proteomics, database search engines such as Mascot and SEQUEST are valuable tools for identifying proteins, and, in some cases, their sites of modifications [172-179]. The combinatorial nature of histone PTMs renders such characterization challenging. Specific algorithms have been designed primarily for the analysis of modifications. These include FindMod [180], SALSA [181-183], ProSight PTM [184,185] and algorithms for the multi-dimensional LC/MS/MS [186,187]. These approaches use both mass and/or the characteristic fragmentation of the modification and amino acid to uncover modification sites. These strategies are designed to localize a limited set of predicted modifications. It is still impractical to use these algorithms to search for the over 200 known protein modifications simultaneously [188]. Another strategy uses comparative MALDI-TOF and targeted MS/MS to identify unknown modifications [189]. This strategy begins with a comparative MALDI-TOF screening of a control and treated sample. Differences between the spectra are used to qualitatively assess potential modified peptides. The peptides are further interrogated by LC/MS/MS to identify and localize the modifications. MALDI-TOF was demonstrated through the analysis of acetylation and methylation of histone H4 derived from butyratetreated LLC-PK1 cells following AUT gel separation and in-gel digestion.
Conclusion
The PTMs of histones have been the focus of much attention due to the intimate interaction between histones and DNA. A series of MS-based technologies have been dedicated to the characterization and quantitation of histones and their post-translationally modified isoforms, where the ultimate goals are to understand the functions of different histone forms in the structural and functional alterations of chromatin, determine their roles in different types of cancer and evaluate the efficacy of epigenetic therapies such as HDAC inhibitors. This review focuses on the discussion of proteomic strategies that take advantage of various biological and analytical techniques based on MS for histone studies. In this post-genome area, novel methodologies are continually developed for the task of determining patterns of histone variants and their PTMs in order to better understand their roles in cellular regulation.
Expert commentary
MS is adding new insights into chromatin biology. The technique is capable of identifying new modifications with greater speed than can be achieved with indirect molecular biological approaches. Several groups have recently used MS at the principle means to identify several new modification sites. These discoveries have stimulated interest in the role that these modifications play in chromatin structure and gene expression.
Five-year view
Continued use and development of MS in chromatin research will lead to better insights into the synergy and cross-talk of histone modifications. Due to the complex nature of the histones, advances in separation technologies and MS are needed to sample quantitatively the full dynamic range of modifications present.
Key issues.
The analysis of histone variants and their modifications is challenging by any approach.
Mass spectrometry provides a direct measurement of histone modifications.
Successful proteomic strategies rely on good separations.
Stable isotope labeling is an effective strategy for quantitative analysis of modification changes.
Contributor Information
Xiaodan Su, The Ohio State Unviersity, Department of Molecular Virology Immunology & Medical Genetics, Human Cancer Genetics,100 West 18 Avenue, Columbus, OH, USA.
Chen Ren, The Ohio State Unviersity, Department of Pharmacy, 100 West 18 Avenue, Columbus, OH, USA.
Michael A Freitas, The Ohio State Unviersity, Department of Molecular Virology Immunology & Medical Genetics, Human Cancer Genetics, 100 West 18 Avenue, Columbus, OH, USA.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.DeLange RJ, Smith EL. Histones: structure and function. Annu. Rev. Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan V. Histone structure and the organization of the nucleosome. Annu. Rev. Biophys. Biomol. Struct. 1997;26:83–112. doi: 10.1146/annurev.biophys.26.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 4.Luger K, Richmond TJ. The histone tails of the nucleosome. Curr. Opin. Genet. Dev. 1998;8(2):140–146. doi: 10.1016/s0959-437x(98)80134-2. [DOI] [PubMed] [Google Scholar]
- 5.Freitas MA, Sklenar AR, Parthun MR. Application of mass spectrometry to the identification and quantification of histone post-translational modifications. J. Cell. Biochem. 2004;92(4):691–700. doi: 10.1002/jcb.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spotswood HT, Turner BM. An increasingly complex code. J. Clin. Invest. 2002;110(5):577–582. doi: 10.1172/JCI16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenberg I. Histones. Annu. Rev. Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- 8.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 9.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103(2):263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15(18):2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wysocka J, Sayegh J, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306(5694):279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbert GL, Daujat S, Snowden AW, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118(5):545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Richards EJ. Chromatin methylation: who's on first? Curr. Biol. 2002;12(20):R694–R695. doi: 10.1016/s0960-9822(02)01208-3. [DOI] [PubMed] [Google Scholar]
- 16 ••.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. Discussion of the complex combinatorial nature of histone modification. [DOI] [PubMed] [Google Scholar]
- 17 ••.Turner BM. Cellular memory and the histone code. Cell. 2002;111(3):285–291. doi: 10.1016/s0092-8674(02)01080-2. Discussion of the complex combinatorial nature of histone modification. [DOI] [PubMed] [Google Scholar]
- 18.Lo WS, Trievel RC, Rojas JR, et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell. 2000;5(6):917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 19.Mahadevan LC, Clayton AL, Hazzalin CA, Thomson S. Phosphorylation and acetylation of histone H3 at inducible genes: two controversies revisited. Novartis Found. Symp. 2004;259:102–111. discussion 111–104, 163–109. [PubMed] [Google Scholar]
- 20.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl Acad. Sci. USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziato AT, Hansen JC. Role of histone acetylation in the assembly and modulation of chromatin structures. Gene Expr. 2000;9(12):37–61. doi: 10.3727/000000001783992687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Zhao H. Gene expression analysis reveals that histone deacetylation sites may serve as partitions of chromatin gene expression domains. BMC Genomics. 2005;6(1):44–53. doi: 10.1186/1471-2164-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell. 2004;119(5):603–614. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Kourmouli N, Jeppesen P, Mahadevhaiah S, et al. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J. Cell. Sci. 2004;117(Pt 12):2491–2501. doi: 10.1242/jcs.01238. [DOI] [PubMed] [Google Scholar]
- 25.Talasz H, Lindner HH, Sarg B, Helliger W. Histone H4-lysine 20 monomethylation is increased in promoter and coding regions of active genes and correlates with hyperacetylation. J. Biol. Chem. 2005;280(46):38814–38822. doi: 10.1074/jbc.M505563200. [DOI] [PubMed] [Google Scholar]
- 26.Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J. Biol. Chem. 2002;277(42):39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- 27.Sarg B, Helliger W, Talasz H, Koutzamani E, Lindner HH. Histone H4 hyperacetylation precludes histone H4 lysine 20 trimethylation. J. Biol. Chem. 2004;279(51):53458–53464. doi: 10.1074/jbc.M409099200. [DOI] [PubMed] [Google Scholar]
- 28.Wysocka J, Swigut T, Milne TA, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121(6):859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Strahl BD, Ohba R, Cook RG, Allis CD. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in tetrahymena. Proc. Natl Acad. Sci. USA. 1999;96(26):14967–14972. doi: 10.1073/pnas.96.26.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabuchi H, Hashimoto E, Nakamura S, Yamamura H, Nishizuka Y. Phosphorylation of calf thymus H1 histone by muscle glycogen phosphorylase kinase. J. Biochem. (Tokyo) 1981;89(5):1433–1437. doi: 10.1093/oxfordjournals.jbchem.a133335. [DOI] [PubMed] [Google Scholar]
- 31.Mizzen CA, Dou Y, Liu Y, Cook RG, Gorovsky MA, Allis CD. Identification and mutation of phosphorylation sites in a linker histone. Phosphorylation of macronuclear H1 is not essential for viability in tetrahymena. J. Biol. Chem. 1999;274(21):14533–14536. doi: 10.1074/jbc.274.21.14533. [DOI] [PubMed] [Google Scholar]
- 32.Gurley LR, Walters RA, Barham SS, Deaven LL. Heterochromatin and histone phosphorylation. Exp. Cell. Res. 1978;111(2):373–383. doi: 10.1016/0014-4827(78)90182-9. [DOI] [PubMed] [Google Scholar]
- 33.Rea S, Eisenhaber F, O'Carroll D, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Lin Q, Yoon HG, et al. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol. Cell. Biol. 2002;22(16):5688–5697. doi: 10.1128/MCB.22.16.5688-5697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 •.Ye J, Ai X, Eugeni EE, et al. Histone H4 lysine 91 acetylation a core domain modification associated with chromatin assembly. Mol. Cell. 2005;18(1):123–130. doi: 10.1016/j.molcel.2005.02.031. The biology of histone modifications discoveredby mass spectrometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosgrove MS, Boeke JD, Wolberger C. Regulated nucleosome mobility and the histone code. Nat. Struct. Mol. Biol. 2004;11(11):1037–1043. doi: 10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- 37.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10(15):886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 38.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 39.Mahadevaiah SK, Turner JM, Baudat F, et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 2001;27(3):271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 40.Chen HT, Bhandoola A, Difilippantonio MJ, et al. Response to RAG-mediated VDJ cleavage by NBS1 and γ-H2AX. Science. 2000;290(5498):1962–1965. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen S, Casellas R, Reina-San-Martin B, et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414(6864):660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dover J, Schneider J, Tawiah-Boateng MA, et al. Methylation of histone H3by COMPASS requires ubiquitination of histone H2B by Rad6. J. Biol. Chem. 2002;277(32):28368–28371. doi: 10.1074/jbc.C200348200. [DOI] [PubMed] [Google Scholar]
- 43.Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418(6893):104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- 44.Hyland EM, Cosgrove MS, Molina H, et al. Insights into the role of histone H3 and histone H4 core modifiable residues in Saccharomyces cerevisiae. Mol. Cell. Biol. 2005;25(22):10060–10070. doi: 10.1128/MCB.25.22.10060-10070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cocklin RR, Wang M. Identification of methylation and acetylation sites on mouse histone H3 using matrix-assisted laser desorption/ionization time-of-flight and nanoelectrospray ionization tandem mass spectrometry. J. Protein Chem. 2003;22(4):327–334. doi: 10.1023/a:1025334006014. [DOI] [PubMed] [Google Scholar]
- 46.Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell. Mol. Life Sci. 2001;58(56):728–736. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hupp TR, Meek DW, Midgley CA, Lane DP. Regulation of the specific DNA binding function of p53. Cell. 1992;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Scolnick DM, Trievel RC, et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell Biol. 1999;19(2):1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi K, Herrera JE, Saito S, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12(18):2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 51.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 52.Marks PA, Richon VM, Kelly WK, Chiao JH, Miller T. Histone deacetylase inhibitors: development as cancer therapy. Novartis Found. Symp. 2004;259:269–281. [PubMed] [Google Scholar]
- 53.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl Cancer Inst. 2000;92(15):1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 54.Piekarz R, Bates S. A review of depsipeptide and other histone deacetylase inhibitors in clinical trials. Curr. Pharm. Des. 2004;10(19):2289–2298. doi: 10.2174/1381612043383980. [DOI] [PubMed] [Google Scholar]
- 55.Mizuguchi G, Vassilev A, Tsukiyama T, Nakatani Y, Wu C. ATP-dependent nucleosome remodeling and histone hyperacetylation synergistically facilitate transcription of chromatin. J. Biol. Chem. 2001;276(18):14773–14783. doi: 10.1074/jbc.M100125200. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad K, Henikoff S. Epigenetic consequences of nucleosome dynamics. Cell. 2002;111(3):281–284. doi: 10.1016/s0092-8674(02)01081-4. [DOI] [PubMed] [Google Scholar]
- 57.Ausio J, Abbott DW. The many tales of a tail: carboxyl-terminal tail heterogeneity specializes histone H2A variants for defined chromatin function. Biochemistry. 2002;41(19):5945–5949. doi: 10.1021/bi020059d. [DOI] [PubMed] [Google Scholar]
- 58.Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19(3):295–310. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- 59.Sarma K, Reinberg D. Histone variants meet their match. Nat. Rev. Mol. Cell. Biol. 2005;6(2):139–149. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- 60.Smith MM. Centromeres and variant histones: what, where, when and why? Curr. Opin. Cell. Biol. 2002;14(3):279–285. doi: 10.1016/s0955-0674(02)00331-9. [DOI] [PubMed] [Google Scholar]
- 61.Henikoff S, Furuyama T, Ahmad K. Histone variants, nucleosome assembly and epigenetic inheritance. Trends Genet. 2004;20(7):320–326. doi: 10.1016/j.tig.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Sif S. ATP-dependent nucleosome remodeling complexes: enzymes tailored to deal with chromatin. J. Cell. Biochem. 2004;91(6):1087–1098. doi: 10.1002/jcb.20005. [DOI] [PubMed] [Google Scholar]
- 63.Studitsky VM, Walter W, Kireeva M, Kashlev M, Felsenfeld G. Chromatin remodeling by RNA polymerases. Trends Biochem. Sci. 2004;29(3):127–135. doi: 10.1016/j.tibs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 64.van Holde KE. Chromatin. Series in Molecular Biology. Vol. 530. Springer-Verlag; NY, USA: 1988. [Google Scholar]
- 65.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat. Struct. Biol. 2003;10(11):882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- 66.Sandman K, Reeve JN. Structure and functional relationships of archaeal and eukaryal histones and nucleosomes. Arch. Microbiol. 2000;173(3):165–169. doi: 10.1007/s002039900122. [DOI] [PubMed] [Google Scholar]
- 67.Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S. The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur. J. Biochem. 2004;271(17):3459–3469. doi: 10.1111/j.1432-1033.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- 68.Khochbin S. Histone H1 diversity: bridging regulatory signals to linker histone function. Gene. 2001;271(1):1–12. doi: 10.1016/s0378-1119(01)00495-4. [DOI] [PubMed] [Google Scholar]
- 69.Brown DT. Histone variants: are they functionally heterogeneous? Genome Biol. 2001;2(7):1–6. doi: 10.1186/gb-2001-2-7-reviews0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han KK, Tetaert D, Debuire B, Dautrevaux M, Biserte G. Sequential Edman degredation. Biochimie. 1977;59(7):557–576. doi: 10.1016/s0300-9084(77)80166-1. [DOI] [PubMed] [Google Scholar]
- 71.Shively JE. The chemistry of protein sequence analysis. EXS. 2000;88:99–117. doi: 10.1007/978-3-0348-8458-7_7. [DOI] [PubMed] [Google Scholar]
- 72.Chicoine LG, Schulman IG, Richman R, Cook RG, Allis CD. Nonrandom utilization of acetylation sites in histones isolated from Tetrahymena. Evidence for functionally distinct H4 acetylation sites. J. Biol. Chem. 1986;261(3):1071–1076. [PubMed] [Google Scholar]
- 73.Crane-Robinson C, Hebbes TR, Clayton AL, Thorne AW. Chromosomal mapping of core histone acetylation by immunoselection. Methods. 1997;12(1):48–56. doi: 10.1006/meth.1997.0446. [DOI] [PubMed] [Google Scholar]
- 74.Suka N, Suka Y, Carmen AA, Wu J, Grunstein M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell. 2001;8(2):473–479. doi: 10.1016/s1097-2765(01)00301-x. [DOI] [PubMed] [Google Scholar]
- 75.Jiang G, Yang F, Sanchez C, Ehrlich M. Histone modification in constitutive heterochromatin versus unexpressed euchromatin in human cells. J. Cell. Biochem. 2004;93(2):286–300. doi: 10.1002/jcb.20146. [DOI] [PubMed] [Google Scholar]
- 76.Benson LJ, Gu Y, Yakovleva T, et al. Modifications of H3 and H4 during chromatin replication, nucleosome assembly, and histone exchange. J. Biol. Chem. 2006;281(14):9287–9296. doi: 10.1074/jbc.M512956200. [DOI] [PubMed] [Google Scholar]
- 77.Edmondson DG, Davie JK, Zhou J, Mirnikjoo B, Tatchell K, Dent SY. Site-specific loss of acetylation upon phosphorylation of histone H3. J. Biol. Chem. 2002;277(33):29496–29502. doi: 10.1074/jbc.M200651200. [DOI] [PubMed] [Google Scholar]
- 78.Luger K, Rechsteiner TJ, Flaus AJ, Waye MM, Richmond TJ. Characterization of nucleosome core particles containing histone proteins made in bacteria. J. Mol. Biol. 1997;272(3):301–311. doi: 10.1006/jmbi.1997.1235. [DOI] [PubMed] [Google Scholar]
- 79.Bloom KS, Anderson JN. Fractionation and characterization of chromosomal proteins by the hydroxyapatite dissociation method. J. Biol. Chem. 1978;253(12):4446–4450. [PubMed] [Google Scholar]
- 80.Bluthmann H, Mrozek S, Gierer A. Non-histone chromosomal proteins. Their isolation and role in determining specificity of transcription in vitro. Eur. J. Biochem. 1975;58(2):315–326. doi: 10.1111/j.1432-1033.1975.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 81.MacGillivray AJ, Rickwood D, Cameron A, et al. Methods for isolation and characterization of chromosomal non-histone proteins. Fractionation of chromatin on hydroxyapatite and characterization of the non-histone proteins by ion exchange chromatography and polyacrylamide gel electrophoresis. Methods Enzymol. 1975;40:160–171. doi: 10.1016/s0076-6879(75)40014-3. [DOI] [PubMed] [Google Scholar]
- 82.Rickwood D, MacGillivray AJ. Improved techniques for the fractionation of non-histone proteins of chromatin on hydroxyapatite. Eur. J. Biochem. 1975;51(2):593–601. doi: 10.1111/j.1432-1033.1975.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 83.Fischman GJ, Lambert MW, Studzinski GP. Purification and properties of a nuclear DNA endonuclease from HeLa S3 cells. Biochim. Biophys. Acta. 1979;567(2):464–471. doi: 10.1016/0005-2744(79)90132-3. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Freitas MA, Wickham J, et al. Differential expression of histone post-translational modifications in acute myeloid and chronic lymphocytic leukemia determined by high-pressure liquid chromatography and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15(1):77–86. doi: 10.1016/j.jasms.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 85 ••.Zhang L, Eugeni EE, Parthun MR, Freitas MA. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma. 2003;112(2):77–86. doi: 10.1007/s00412-003-0244-6. The discovery of over 20 modificaitons by mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 86.Zhang K, Williams KE, Huang L, et al. Histone acetylation and deacetylation: identification of acetylation and methylation sites of HeLa histone H4 by mass spectrometry. Mol. Cell. Proteomics. 2002;1(7):500–508. doi: 10.1074/mcp.m200031-mcp200. [DOI] [PubMed] [Google Scholar]
- 87.Zhang K, Tang H, Huang L, et al. Identification of acetylation and methylation sites of histone H3 from chicken erythrocytes by high-accuracy matrix-assisted laser desorption ionization-time-of-flight, matrix-assisted laser desorption ionization-postsource decay, and nanoelectrospray ionization tandem mass spectrometry. Anal. Biochem. 2002;306(2):259–269. doi: 10.1006/abio.2002.5719. [DOI] [PubMed] [Google Scholar]
- 88.Zhang K, Tang H. Analysis of core histones by liquid chromatography-mass spectrometry and peptide mapping. J. Chromatogr. B. 2003;783(1):173–179. doi: 10.1016/s1570-0232(02)00631-1. [DOI] [PubMed] [Google Scholar]
- 89.Jackson PS, Gurley LR. Analysis of nucleoproteins by direct injection of dissolved nuclei or chromosomes into a high-performance liquid chromatographic system. J. Chromatogr. 1985;326:199–216. doi: 10.1016/s0021-9673(01)87446-x. [DOI] [PubMed] [Google Scholar]
- 90.Lindner H, Helliger W, Puschendorf B. Separation of friend erythroleukaemic cell histones and high-mobility-group proteins by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1988;450(3):309–316. doi: 10.1016/s0021-9673(01)83585-8. [DOI] [PubMed] [Google Scholar]
- 91.Beck HC, Nielsen EC, Matthiesen R, et al. Quantitative proteomic analysis of post-translational modifications of human histones. Mol. Cell Proteomics. 2006;5(7):1314–1325. doi: 10.1074/mcp.M600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 92.Du YC, Gu S, Zhou J, et al. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca2+/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell Proteomics. 2006;5(6):1033–1044. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- 93.Villar-Garea A, Imhof A. The analysis of histone modifications. Biochim. Biophys. Acta. 2006;1764(12):1932–1939. doi: 10.1016/j.bbapap.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Bonenfant D, Coulot M, Towbin H, Schindler P, van Oostrum J. Characterization of histones H2A and H2B variants and their post-translational modifications by mass spectrometry. Mol. Cell. Proteomics. 2005;5(3):541–552. doi: 10.1074/mcp.M500288-MCP200. [DOI] [PubMed] [Google Scholar]
- 95.Su X, Jacob NK, Amunugama R, et al. Liquid chromatography mass spectrometry profiling of histones. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007 doi: 10.1016/j.jchromb.2006.12.037. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naldi M, Andrisano V, Fiori J, et al. Histone proteins determined in a human colon cancer by highperformance liquid chromatography and mass spectrometry. J. Chromatogr. A. 2006;1129(1):73–81. doi: 10.1016/j.chroma.2006.06.100. [DOI] [PubMed] [Google Scholar]
- 97.Lindner H, Sarg B, Meraner C, Helliger W. Separation of acetylated core histones by hydrophilic-interaction liquid chromatography. J. Chromatogr. A. 1996;743(1):137–144. doi: 10.1016/0021-9673(96)00131-8. [DOI] [PubMed] [Google Scholar]
- 98.Lindner H, Sarg B, Helliger W. Application of hydrophilic-interaction liquid chromatography to the separation of phosphorylated H1 histones. J. Chromatogr. A. 1997;782(1):55–62. doi: 10.1016/s0021-9673(97)00468-8. [DOI] [PubMed] [Google Scholar]
- 99.Sarg B, Helliger W, Hoertnagl B, Puschendorf B, Lindner H. The N-terminally acetylated form of mammalian histone H1(o), but not that of avian histone H5, increases with age. Arch Biochem. Biophys. 1999;372(2):333–339. doi: 10.1006/abbi.1999.1503. [DOI] [PubMed] [Google Scholar]
- 100.Sarg B, Green A, Soderkvist P, Helliger W, Rundquist I, Lindner HH. Characterization of sequence variations in human histone H1.2 and H1.4 subtypes. FEBS. J. 2005;272(14):3673–3683. doi: 10.1111/j.1742-4658.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 101.Gurley LR, London JE, Valdez JG. High-performance capillary electrophoresis of histones. J. Chromatogr. 1991;559(12):431–443. doi: 10.1016/0021-9673(91)80091-t. [DOI] [PubMed] [Google Scholar]
- 102.Lindner H, Helliger W, Dirschlmayer A, Jaquemar M, Puschendorf B. High-performance capillary electrophoresis of core histones and their acetylated modified derivatives. Biochem. J. 1992;283(Pt 2):467–471. doi: 10.1042/bj2830467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lindner H, Sarg B, Helliger W. Capillary electrophoresis analysis of histones, histone variants, and their post-translationally modified forms: a review. J. Capill. Electrophor. Microchip. Technol. 2003;8(34):59–67. [PubMed] [Google Scholar]
- 104.Lindner H, Wesierska-Gadek J, Helliger W, Puschendorf B, Sauermann G. Identification of ADP-ribosylated histones by the combined use of high-performance liquid chromatography and electrophoresis. J. Chromatogr. 1989;472(1):243–249. doi: 10.1016/s0021-9673(00)94110-4. [DOI] [PubMed] [Google Scholar]
- 105.Lindner H, Wurm M, Dirschlmayer A, Sarg B, Helliger W. Application of high-performance capillary electrophoresis to the analysis of H1 histones. Electrophoresis. 1993;14(56):480–485. doi: 10.1002/elps.1150140174. [DOI] [PubMed] [Google Scholar]
- 106.Lindner H, Helliger W, Sarg B, Meraner C. Effect of buffer composition on the migration order and separation of histone H1 subtypes. Electrophoresis. 1995;16(4):604–610. doi: 10.1002/elps.1150160197. [DOI] [PubMed] [Google Scholar]
- 107.Mizzen CA, McLachlan DR. Capillary electrophoresis of histone H1 variants at neutral pH in dynamically modified fused-silica tubing. Electrophoresis. 2000;21(12):2359–2367. doi: 10.1002/1522-2683(20000701)21:12<2359::AID-ELPS2359>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 108.Aguilar C, Hofte AJ, Tjaden UR, van der Greef J. Analysis of histones by on-line capillary zone electrophoresis-electrospray ionisation mass spectrometry. J. Chromatogr. A. 2001;926(1):57–67. doi: 10.1016/s0021-9673(01)00962-1. [DOI] [PubMed] [Google Scholar]
- 109.Hernandez-Borges J, Neususs C, Cifuentes A, Pelzing M. On-line capillary electrophoresis-mass spectrometry for the analysis of biomolecules. Electrophoresis. 2004;25(14):2257–2281. doi: 10.1002/elps.200405954. [DOI] [PubMed] [Google Scholar]
- 110.Simpson DC, Smith RD. Combining capillary electrophoresis with mass spectrometry for applications in proteomics. Electrophoresis. 2005;26(78):1291–1305. doi: 10.1002/elps.200410132. [DOI] [PubMed] [Google Scholar]
- 111.Schmitt-Kopplin P, Frommberger M. Capillary electrophoresis-mass spectrometry: 15 years of developments and applications. Electrophoresis. 2003;24(2223):3837–3867. doi: 10.1002/elps.200305659. [DOI] [PubMed] [Google Scholar]
- 112.Panyim S, Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch. Biochem. Biophys. 1969;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- 113.Bonner WM, West MH, Stedman JD. Two-dimensional gel analysis of histones in acid extracts of nuclei, cells, and tissues. Eur. J. Biochem. 1980;109(1):17–23. doi: 10.1111/j.1432-1033.1980.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 114.Waterborg JH. Existence of two histone H3 variants in dicotyledonous plants and correlation between their acetylation and plant genome size. Plant Mol. Biol. 1992;18(2):181–187. doi: 10.1007/BF00034947. [DOI] [PubMed] [Google Scholar]
- 115.Waterborg JH, Winicov I, Harrington RE. Histone variants and acetylated species from the alfalfa plant Medicago sativa. Arch. Biochem. Biophys. 1987;256(1):167–178. doi: 10.1016/0003-9861(87)90435-8. [DOI] [PubMed] [Google Scholar]
- 116.Feng Q, Wang H, Ng HH, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr. Biol. 2002;12(12):1052–1058. doi: 10.1016/s0960-9822(02)00901-6. [DOI] [PubMed] [Google Scholar]
- 117 •.Ng HH, Feng Q, Wang H, et al. Lysine methylation within the globular domain of histone H3by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 2002;16(12):1518–1527. doi: 10.1101/gad.1001502. The biology of histone modifications discovered by mass spectrometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109(6):745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 119.Ren C, Zhang L, Freitas MA, Ghoshal K, Parthun MR, Jacob ST. Peptide mass mapping of acetylated isoforms of histone H4 from mouse lymphosarcoma cells treated with histone deacetylase (HDACs) inhibitors. J. Am. Soc. Mass Spectrom. 2005;16(10):1641–1653. doi: 10.1016/j.jasms.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Comisarov MB, Marshall AG. Fourier transform ion cyclotron resonance spectroscopy. Chem. Phys. Lett. 1974;25:282–283. [Google Scholar]
- 121.Marshall AG, Comisarow MB. Fourier transform methods in spectroscopy. J. Chem. Educ. 1975;52:638–641. [Google Scholar]
- 122.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom. Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 123.Zhang LW, Freitas MA. Comparison of peptide mass mapping and electron capture dissociation as assays for histone posttranslational modifications. Int. J. Mass Spectrom. 2004;234(13):213–225. [Google Scholar]
- 124.Zhang K, Siino JS, Jones PR, Yau PM, Bradbury EM. A mass spectrometric “western blot” to evaluate the correlations between histone methylation and histone acetylation. Proteomics. 2004;4(12):3765–3775. doi: 10.1002/pmic.200400819. [DOI] [PubMed] [Google Scholar]
- 125.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 1998;120(13):3265–3266. [Google Scholar]
- 126.Cerda BA, Horn DM, Breuker K, Carpenter BK, McLafferty FW. Electron capture dissociation of multiply-charged oxygenated cations. A nonergodic process. Eur. J. Mass Spectrom. 1999;5(5):335–338. [Google Scholar]
- 127.McLafferty FW, Horn DM, Breuker K, et al. Electron capture dissociation of gaseous multiply charged ions by Fourier-transform ion cyclotron resonance. J. Am. Soc. Mass Spectrom. 2001;12(3):245–249. doi: 10.1016/S1044-0305(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 128.Zubarev RA, Horn DM, Fridriksson EK, et al. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000;72(3):563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 129.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl Acad. Sci. USA. 2004;101(26):9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reid GE, Stephenson JL, Jr, McLuckey SA. Tandem mass spectrometry of ribonuclease A and B: N-linked glycosylation site analysis of whole protein ions. Anal. Chem. 2002;74(3):577–583. doi: 10.1021/ac015618l. [DOI] [PubMed] [Google Scholar]
- 131.Taniguchi H, Suzuki M, Manenti S, Titani K. A mass spectrometric study on the in vivo posttranslational modification of GAP-43. J. Biol. Chem. 1994;269(36):22481–22484. [PubMed] [Google Scholar]
- 132.Johnson RS, Ohguro H, Palczewski K, Hurley JB, Walsh KA, Neubert TA. Heterogeneous N-acylation is a tissue- and species-specific posttranslational modification. J. Biol. Chem. 1994;269(33):21067–21071. [PubMed] [Google Scholar]
- 133.Michael SM, Chien BM, Lubman DM. Detection of electrospray ionization using a quadrupole ion trap storage/reflectron time-of-flight mass spectrometer. Anal. Chem. 1993;65:2614–2620. [Google Scholar]
- 134.Vishwanathan K, Tackett RL, Stewart JT, Bartlett MG. Determination of arginine and methylated arginines in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000;748(1):157–166. doi: 10.1016/s0378-4347(00)00399-6. [DOI] [PubMed] [Google Scholar]
- 135.Zougman A, Wisniewski JR. Beyond linker histones and high mobility group proteins: global profiling of perchloric acid soluble proteins. J. Proteome Res. 2006;5(4):925–934. doi: 10.1021/pr050415p. [DOI] [PubMed] [Google Scholar]
- 136.Hirota J, Satomi Y, Yoshikawa K, Takao T. Epsilon -N,N,N-trimethyllysine-specific ions in matrix-assisted laser desorption/ionization-tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17(5):371–376. doi: 10.1002/rcm.924. [DOI] [PubMed] [Google Scholar]
- 137.Zhang K, Yau PM, Chandrasekhar B, et al. Differentiation between peptides containing acetylated or tri-methylated lysines by mass spectrometry: an application for determining lysine 9 acetylation and methylation of histone H3. Proteomics. 2004;4(1):1–10. doi: 10.1002/pmic.200300503. [DOI] [PubMed] [Google Scholar]
- 138.Medzihradszky KF, Darula Z, Perlson E, et al. O-sulfonation of serine and threonine: mass spectrometric detection and characterization of a new posttranslational modification in diverse proteins throughout the eukaryotes. Mol. Cell. Proteomics. 2004;3(5):429–440. doi: 10.1074/mcp.M300140-MCP200. [DOI] [PubMed] [Google Scholar]
- 139.Borchers C, Parker CE, Deterding LJ, Tomer KB. Preliminary comparison of precursor scans and liquid chromatography-tandem mass spectrometry on a hybrid quadrupole time-of-flight mass spectrometer. J. Chromatogr. A. 1999;854(12):119–130. doi: 10.1016/s0021-9673(99)00479-3. [DOI] [PubMed] [Google Scholar]
- 140.Kim JY, Kim KW, Kwon HJ, Lee DW, Yoo JS. Probing lysine acetylation with a modification-specific marker ion using high-performance liquid chromatography/electrospray-mass spectrometry with collision-induced dissociation. Anal. Chem. 2002;74(21):5443–5449. doi: 10.1021/ac0256080. [DOI] [PubMed] [Google Scholar]
- 141.Burlingame AL, Zhang X, Chalkley RJ. Mass spectrometric analysis of histone posttranslational modifications. Methods. 2005;36(4):383–394. doi: 10.1016/j.ymeth.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 142.Medzihradszky KF, Zhang X, Chalkley RJ, et al. Characterization of Tetrahymena histone H2B variants and posttranslational populations by electron capture dissociation (ECD) Fourier transform ion cyclotron mass spectrometry (FT-ICR MS) Mol. Cell Proteomics. 2004;3(9):872–886. doi: 10.1074/mcp.M400041-MCP200. [DOI] [PubMed] [Google Scholar]
- 143.Coon JJ, Ueberheide B, Syka JE, et al. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc. Natl Acad. Sci. USA. 2005;102(27):9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Siuti N, Roth MJ, Mizzen CA, Kelleher NL, Pesavento JJ. Gene-specific characterization of human histone H2B by electron capture dissociation. J. Proteome Res. 2006;5(2):233–239. doi: 10.1021/pr050268v. [DOI] [PubMed] [Google Scholar]
- 145 •.Thomas CE, Kelleher NL, Mizzen CA. Mass spectrometric characterization of human histone H3: a bird's eye view. J. Proteome Res. 2006;5(2):240–247. doi: 10.1021/pr050266a. A top-dowmmass spectrometry study. [DOI] [PubMed] [Google Scholar]
- 146.Pesavento JJ, Mizzen CA, Kelleher NL. Quantitative analysis of modified proteins and their positional isomers by tandem mass spectrometry: human histone H4. Anal. Chem. 2006;78(13):4271–4280. doi: 10.1021/ac0600050. [DOI] [PubMed] [Google Scholar]
- 147.Boyne MT, II, Pesavento JJ, Mizzen CA, Kelleher NL. Precise characterization of human histones in the H2A gene family by top down mass spectrometry. J. Proteome Res. 2006;5(2):248–253. doi: 10.1021/pr050269n. [DOI] [PubMed] [Google Scholar]
- 148.Pesavento JJ, Kim YB, Taylor GK, Kelleher NL. Shotgun annotation of histone modifications: a new approach for streamlined characterization of proteins by top down mass spectrometry. J. Am. Chem. Soc. 2004;126(11):3386–3387. doi: 10.1021/ja039748i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garcia BA, Busby SA, Barber CM, Shabanowitz J, Allis CD, Hunt DF. Characterization of phosphorylation sites on histone H1 isoforms by tandem mass spectrometry. J. Proteome Res. 2004;3(6):1219–1227. doi: 10.1021/pr0498887. [DOI] [PubMed] [Google Scholar]
- 150.Garcia BA, Joshi S, Thomas CE, et al. Comprehensive phosphoprotein analysis of linker histone H1 from Tetrahymena thermophila. Mol. Cell Proteomics. 2006;5(9):1593–1609. doi: 10.1074/mcp.M600086-MCP200. [DOI] [PubMed] [Google Scholar]
- 151.Kruger R, Hung CW, Edelson-Averbukh M, Lehmann WD. Iodoacetamide-alkylated methionine can mimic neutral loss of phosphoric acid from phosphopeptides as exemplified by nano-electrospray ionization quadrupole time-of-flight parent ion scanning. Rapid Commun. Mass Spectrom. 2005;19(12):1709–1716. doi: 10.1002/rcm.1976. [DOI] [PubMed] [Google Scholar]
- 152.Barber CM, Turner FB, Wang Y, et al. The enhancement of histone H4 and H2A serine 1 phosphorylation during mitosis and S-phase is evolutionarily conserved. Chromosoma. 2004;112(7):360–371. doi: 10.1007/s00412-004-0281-9. [DOI] [PubMed] [Google Scholar]
- 153.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 154.Kirkpatrick DS, Weldon SF, Tsaprailis G, Liebler DC, Gandolfi AJ. Proteomic identification of ubiquitinated proteins from human cells expressing His-tagged ubiquitin. Proteomics. 2005;5(8):2104–2111. doi: 10.1002/pmic.200401089. [DOI] [PubMed] [Google Scholar]
- 155.Warren MR, Parker CE, Mocanu V, Klapper D, Borchers CH. Electrospray ionization tandem mass spectrometry of model peptides reveals diagnostic fragment ions for protein ubiquitination. Rapid Commun. Mass Spectrom. 2005;19(4):429–437. doi: 10.1002/rcm.1798. [DOI] [PubMed] [Google Scholar]
- 156.Waterborg JH, Kapros T. Kinetic analysis of histone acetylation turnover and trichostatin A induced hyper- and hypoacetylation in alfalfa. Biochem.Cell Biol. 2002;80(3):279–293. doi: 10.1139/o02-021. [DOI] [PubMed] [Google Scholar]
- 157.Katan-Khaykovich Y, Struhl K. Dynamics of global histone acetylation and deacetylation in vivo: rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes Dev. 2002;16(6):743–752. doi: 10.1101/gad.967302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu H, Pan S, Gu S, Bradbury EM, Chen X. Amino acid residue specific stable isotope labeling for quantitative proteomics. Rapid Commun. Mass Spectrom. 2002;16(22):2115–2123. doi: 10.1002/rcm.831. [DOI] [PubMed] [Google Scholar]
- 159.Pasa-Tolic L, Jensen PK, Anderson GA, et al. High throughput proteome-wide precision measurements of protein expression using mass spectrometry. J. Am. Chem. Soc. 1999;121(34):7949–7950. [Google Scholar]
- 160.Berhane BT, Limbach PA. Stable isotope labeling for matrix-assisted laser desorption/ionization mass spectrometry and post-source decay analysis of ribonucleic acids. J. Mass Spectrom. 2003;38(8):872–878. doi: 10.1002/jms.504. [DOI] [PubMed] [Google Scholar]
- 161.Oda Y, Huang K, Cross FR, Cowburn D, Chait BT. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl Acad. Sci. USA. 1999;96(12):6591–6596. doi: 10.1073/pnas.96.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu H, Hunter TC, Pan S, Yau PM, Bradbury EM, Chen X. Residue-specific mass signatures for the efficient detection of protein modifications by mass spectrometry. Anal. Chem. 2002;74(7):1687–1694. doi: 10.1021/ac010853p. [DOI] [PubMed] [Google Scholar]
- 163.Gu S, Pan S, Bradbury EM, Chen X. Use of deuterium-labeled lysine for efficient protein identification and peptide de novo sequencing. Anal. Chem. 2002;74(22):5774–5785. doi: 10.1021/ac0204350. [DOI] [PubMed] [Google Scholar]
- 164.Gu S, Pan S, Bradbury EM, Chen X. Precise peptide sequencing and protein quantification in the human proteome through in vivo lysine-specific mass tagging. J. Am. Soc. Mass Spectrom. 2003;14(1):1–7. doi: 10.1016/S1044-0305(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 165.Pan S, Gu S, Bradbury EM, Chen X. Single peptide-based protein identification in human proteome through MALDI-TOF MS coupled with amino acids coded mass tagging. Anal. Chem. 2003;75(6):1316–1324. doi: 10.1021/ac020482s. [DOI] [PubMed] [Google Scholar]
- 166.Yan W, Chen SS. Mass spectrometry-based quantitative proteomic profiling. Brief Funct. Genomic. Proteomic. 2005;4(1):27–38. doi: 10.1093/bfgp/4.1.27. [DOI] [PubMed] [Google Scholar]
- 167 •.Smith CM, Gafken PR, Zhang Z, Gottschling DE, Smith JB, Smith DL. Mass spectrometric quantification of acetylation at specific lysines within the amino-terminal tail of histone H4. Anal. Biochem. 2003;316(1):23–33. doi: 10.1016/s0003-2697(03)00032-0. Quantitative mass spectrometry study. [DOI] [PubMed] [Google Scholar]
- 168.Smith CM. Quantification of acetylation at proximal lysine residues using isotopic labeling and tandem mass spectrometry. Methods. 2005;36(4):395–403. doi: 10.1016/j.ymeth.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 169.Ong SE, Blagoev B, Kratchmarova I, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics. 2002;1(5):376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 170.Su X, Zhang L, Lucas DM, et al. Histone H4 acetylation dynamics determined by stable isotope labeling with amino acids in cell culture and mass spectrometry. Anal. Biochem. 2007;363(1):22–34. doi: 10.1016/j.ab.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang L, Su X, Liu S, et al. Histone H4 N-terminal acetylation in Kasumi-1 cells treated with depsipeptide determined by acetic acid-urea polyacrylamide gel electrophoresis, amino acid coded mass tagging, and mass spectrometry. J. Proteome Res. 2007;6(1):81–88. doi: 10.1021/pr060139u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bafna V, Edwards N. SCOPE: a probabilistic model for scoring tandem mass spectra against a peptide database. Bioinformatics. 2001;17:S13–S21. doi: 10.1093/bioinformatics/17.suppl_1.s13. [DOI] [PubMed] [Google Scholar]
- 173.Colinge J, Masselot A, Giron M, Dessingy T, Magnin J. OLAV: towards high-throughput tandem mass spectrometry data identification. Proteomics. 2003;3:1454–1463. doi: 10.1002/pmic.200300485. [DOI] [PubMed] [Google Scholar]
- 174.Eng JK, McCormack AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 175.Field HI, Fenyȯ D, Beavis RC. RADARS, a bioinformatics solution that automates proteome mass spectral analysis, optimises protein identification, and archives data in a relational database. Proteomics. 2002;2:36–47. [PubMed] [Google Scholar]
- 176.Mann M, Wilm M. Error tolerant identification of peptides in sequence databases by peptide sequence tags. Anal. Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 177.Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence database using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 178.Sadygov RG, Liu H, Yates JR. Statistical models for protein validation using tandem mass spectral data and protein amino acid sequence databases. Anal. Chem. 2004;76:1664–1671. doi: 10.1021/ac035112y. [DOI] [PubMed] [Google Scholar]
- 179.Zhang N, Aebersold R, Schwikowshi B. ProbID: a probabilistic algorithm to identify peptides through sequence database searching using tandem mass spectral data. Proteomics. 2002;2:1406–1412. doi: 10.1002/1615-9861(200210)2:10<1406::AID-PROT1406>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 180.Wilkins MR, Gasteiger E, Gooley AA, et al. High-throughput mass spectrometric discovery of protein post-translational modifications. J. Mol. Biol. 1999;289(3):645–657. doi: 10.1006/jmbi.1999.2794. [DOI] [PubMed] [Google Scholar]
- 181.Hansen BT, Jones JA, Mason DE, Liebler DC. SALSA: a pattern recognition algorithm to detect electrophile-adducted peptides by automated evaluation of CID spectra in LC-MS-MS analyses. Anal. Chem. 2001;73(8):1676–1683. doi: 10.1021/ac001172h. [DOI] [PubMed] [Google Scholar]
- 182.Liebler DC. Proteomic approaches to characterize protein modifications: new tools to study the effects of environmental exposures. Environ. Health Perspect. 2002;110(Suppl1):3–9. doi: 10.1289/ehp.02110s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liebler DC, Hansen BT, Davey SW, Tiscareno L, Mason DE. Peptide sequence motif analysis of tandem MS data with the SALSA algorithm. Anal. Chem. 2002;74(1):203–210. doi: 10.1021/ac0155512. [DOI] [PubMed] [Google Scholar]
- 184.LeDuc RD, Taylor GK, Kim YB, et al. ProSight PTM: an integrated environment for protein identification and characterization by top-down mass spectrometry. Nucleic Acids Res. 2004;32(Web Server issue):W340–W345. doi: 10.1093/nar/gkh447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Taylor GK, Kim YB, Forbes AJ, Meng F, McCarthy R, Kelleher NL. Web and database software for identification of intact proteins using “top down” mass spectrometry. Anal. Chem. 2003;75(16):4081–4086. doi: 10.1021/ac0341721. [DOI] [PubMed] [Google Scholar]
- 186.MacCoss MJ, McDonald WH, Saraf A, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc. Natl Acad. Sci. USA. 2002;99(12):7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.MacCoss MJ, Wu CC, Liu H, Sadygov R, Yates JR., III A correlation algorithm for the automated quantitative analysis of shotgun proteomics data. Anal. Chem. 2003;75(24):6912–6921. doi: 10.1021/ac034790h. [DOI] [PubMed] [Google Scholar]
- 188.Gatlin CL, Eng JK, Cross ST, Detter JC, Yates JR., III Automated identification of amino acid sequence variations in proteins by HPLC/microspray tandem mass spectrometry. Anal. Chem. 2000;72(4):757–763. doi: 10.1021/ac991025n. [DOI] [PubMed] [Google Scholar]
- 189.Person MD, Monks TJ, Lau SS. An integrated approach to identifying chemically induced posttranslational modifications using comparative MALDI-MS and targeted HPLC-ESI-MS/MS. Chem. Res. Toxicol. 2003;16(5):598–608. doi: 10.1021/tx020109f. [DOI] [PubMed] [Google Scholar]
Website
- 201.NHGRI Histone Database. http://research.nhgri.nih.gov/histones.