Abstract
Peripheral mechanisms of self-tolerance often depend on the quiescent state of the immune system. To what degree such mechanisms can be engaged in the enhancement of allograft survival is unclear. In order to examine the role of the programmed death 1 (PD-1) pathway the maintenance of graft survival following blockade of costimulatory pathways, we utilized a single-antigen mismatch model of graft rejection where we could track the donor-specific cells as they developed endogenously and emerged from the thymus. We found that graft-specific T cells arising under physiologic developmental conditions at low frequency were actively deleted at the time of transplantation under combined CD28/CD40L blockade. However, this deletion was incomplete, and donor-specific cells that failed to undergo deletion upregulated expression of PD-1. Furthermore, blockade of PD-1 signaling on these cells via in vivo treatment with anti-PD-1 mAb resulted in rapid expansion of donor-specific T cells and graft loss. These results suggest that the PD-1 pathway was engaged in the continued regulation of the low-frequency graft-specific immune response and thus in maintenance of graft survival.
Keywords: Transplantation, Tolerance, Costimulation, T cells
Introduction
Following transplantation of tissues from allogeneic hosts, large numbers of recipient T cells are activated, clonally expand, and differentiate into effectors, which mediate rapid rejection. To generate effective responses T cells must receive signals delivered via the T-cell receptor (TCR), but also require co-stimulatory signals (1). The concept of blocking costimulatory pathways to prevent rejection or promote tolerance has captivated the interest of the transplant research community and shows great promise in clinical trials. Given the explosion of knowledge of the many related costimulatory receptors and ligands, there are many new opportunities for investigation. Yet, two of the originally discovered pathways, CD28 and CD40, remain among the most pivotal identified for the execution of rejection. Short-term blockade of the CD28 pathway with the CTLA4-Ig fusion protein and/or the CD40 pathway with monoclonal antibodies against CD154 or CD40 can potently inhibit rejection and induce long-term graft acceptance many rodent and non-human primate transplant models (2-11). While the mechanisms by which short-term blockade of these pathways results in peripheral tolerance remain incompletely defined, there is evidence that both peripheral deletion of donor-reactive T cells and the development of regulatory mechanisms can be involved. However, relatively few studies have directly tracked the functional status and fate of donor-reactive T cells during tolerance induction in vivo.
Recent studies in viral infection and transplant models have shown that PD-1 (CD279), another member of the CD28 family of costimulatory molecules, can play an important role in peripheral tolerance induction and the exhaustion of antigen-specific T cells. PD-1 is a negative regulator of T cell function that is inducibly expressed on CD4+ T cells, CD8+ T cells, NK cells, B cells, and activated monocytes (12). In T cells, its expression is upregulated early following TCR signaling, but it has been shown by a number of investigators to be expressed on tolerant and exhausted cells in models of autoimmunity and chronic viral infection, respectively (13-23). Ligation by either PD-L1 or PD-L2 transduces a negative signal when transmitted simultaneously with TCR signaling, but does not produce this effect in the absence of TCR engagement (12). Therefore, PD-1 serves to downmodulate T cell activation only in the presence of antigen. This negative regulatory function is accomplished through the cytoplasmic domain of the molecule, which recruits intracellular phosphatases SHP-1 and SHP-2 (12). The recruitment of these phosphatases to the membrane leads to the dephosphorylation of several key signaling intermediates and thus the attenuation of TCR-mediated signals.
The role of PD-1 as a negative regulator of T cell responses has been investigated in models of chronic viral infection, autoimmunity, and most recently, transplantation. Studies from chronic LCMV infection in mice revealed that functionally exhausted Ag-specific T cells expressed high levels of surface PD-1, and that inhibiting signaling through this receptor reversed the exhausted phenotype and restored production of IFN-g and cytolytic function (13). Moreover, blockade of PD-1 or PD-L1 in vivo not only restored T cell function, but also led to viral clearance. Recent reports examining HIV-, HCV-, and HBV-infected human patients revealed high levels of PD-1 expression on Ag-specific T cells during the course of chronic viral infection (15-19) and that blockade of PD-1 could restore the function of viral reactive T cells in vitro (16-18), suggesting that PD-1 may be a potential therapeutic target for chronic viral infections. Studies in murine autoimmune models have provided further evidence that PD-1 is a critical component of peripheral tolerance. For example, genetic silencing or blockade of the PD-1/PD-L1 pathway was shown to accelerate progression and severity of both autoimmune diabetes and experimental autoimmune encephalomyelitis (21, 22, 24), and to reverse established tolerance in the NOD model of type 1 diabetes by promoting expansion and cytokine production by Ag-specific T cells (20).
Recent studies have examined the role of the PD-1 pathway in transplantation tolerance, primarily by blocking the pathway and demonstrating acceleration of rejection of murine skin or cardiac allografts or worsening of GVHD (25-28). The generation of a PD-L1 fusion protein allowed supra-physiological stimulation of PD-1-expressing T cells and has been shown to promote allograft survival in murine models of cardiac, islet, and corneal transplantation (29-31). Recently, Najafian and colleagues demonstrated that, while dispensable for the induction of mixed allogeneic chimerism, the PD-1 pathway is critical for the maintenance of peripheral tolerance in a murine cardiac allograft model (32). However, precise analysis of the level and kinetics of PD-1 expression on donor-reactive T cells, and their functional phenotype before and after PD-1 blockade, has been hampered by the lack of suitable models in which graft-specific T cells can be tracked throughout the course of the anti-donor immune response. An important study by Sayegh and colleagues made use of CD4+ T cell receptor transgenic cells specific for I-Abm12, and thus was able to demonstrate increased Ag-specific T cell proliferation, decreased apoptosis, and Th1 differentiation following PD-L1 blockade (27). This study highlights the distinct advantages and opportunities in using TCR transgenic model systems to explore the impact of various costimulatory pathways, be they positive or negative, on transplantation tolerance.
In order to explore the role of PD-1-dependent mechanisms in the maintenance of transplantation tolerance, we designed a model system in which readily trackable numbers of donor-reactive T cells were continuously produced in vivo. This system afforded the number, phenotype and functional properties of graft-specific CD8+ T cells to be systematically tracked over the course of several weeks during transplant acceptance or rejection. As in our previous studies using an adoptive transfer approach (33, 34), we observed that when the initial precursor frequency of donor-reactive T cells is high, recipient mice were refractory to tolerance induction using transient CD28/CD40 blockade. However, when the initial precursor frequency of donor-reactive T cells was low, long-term graft acceptance was uniformly achieved. Moreover, we observed that while transient costimulation blockade led to the deletion of a majority of graft-specific T cells when stimulated at low frequency, a population of PD-1+ donor-reactive T cells persisted in recipients bearing surviving donor skin grafts. Importantly, interruption of the PD-1 pathway with either anti-PD-1 or anti-PD-L1 mAbs led to a rapid expansion of graft-specific T cells concurrent with their re-acquisition of effector function and the precipitation of graft rejection. Thus, our data demonstrate the synergism of blocking positive costimulatory pathways and signaling through negative regulatory pathways in maintaining allograft survival in the face of newly emerging low-frequency thymic emigrants. We conclude that the PD-1 pathway plays a pivotal role in maintaining peripheral tolerance by actively suppressing the proliferation and effector function of low frequencies of remaining donor-specific CD8+ T cells.
Methods
Mice
Adult 6- to 8-wk old B6.SJL-PtprcaPep3b/BoyJ (CD45.1+) mice were purchased from Jackson Laboratory (Bar Harbor, ME). OT-I Rag−/− TCR transgenic mice were purchased from Jackson Labs and were bred to Thy1.1+ B6 mice at Emory University. mOVA.B6 mice (35) were a gift of Dr. Marc Jenkins and were maintained at Emory University DAR. All experiments were conducted in accordance with institutional oversight and guidelines for animal care and use.
Skin grafting
Full thickness skin grafts (∼1 cm2) were transplanted on the dorsal thorax of recipient mice and secured with a Band-Aid (Johnson & Johnson, Arlington, TX) for 5 days. Graft survival was then followed by daily visual inspection. Rejection was defined as the complete loss of viable epidermal graft tissue.
OT-I B6 chimera generation
Low frequency OT-I B6 chimeras were generated by pre-treating recipient mice on day - 1 with 15-20mg/kg busulfan (Busulfex; Orphan Medical, Minnetonka, MN). On day 0, bone marrow from syngeneic and OT-I (Rag−/−, Thy1.1+, CD45.2+) TCR transgenic donors was harvested and mixed at a ratio of 4:1 or 5:1 prior to i.v. injection of 15-20×106 cells/recipient. A single 500 mg dose of each hamster anti-murine CD40L Ab (MR-1) and human CTLA4.Ig were included at day 0 to prevent anti-CD45.2 responses (36). OT-I donor chimerism (Thy1.1+, CD8+, Vα2+ and CD45.2+) was monitored in peripheral blood.
Antibody treatment
Antibody was administered intraperitoneally. Where indicated, recipients of skin grafts received treatment with 500 µg each of hamster anti-mouse CD40L mAb (MR-1, Bioexpress) and human CTLA-4 Ig (Bristol-Meyers Squibb) administered i.p. on the day of transplantation (day 0) as well as on post-transplant days 2, 4, and 6. Rat anti-mouse PD-L1 (10F.9G2) or rat IgG2b isotype control at 200mg on days 0, 3, 6 and 9 (13). 500mg of hamster anti-mouse aPD-1 (J43) (37), or control hamster Ig on day 0, then 250mg on days 2, 4, 6 and 8. Anti-CD25 (PC.61) (Bioexpress) was administered at 500 ug/mouse/day on days 0, 2, 4, and 6.
Flow cytometry
Surface staining, intracellular staining and CFSE analysis was carried out as previously described (38) using murine conjugated mAbs CD8 APC/Alexa405 (5H10) (Caltag), Thy1.1 PerCP, CD45.2 FITC/APC-Alexa750, Vα2 PE, PD-1 FITC (J43), CTLA-4-PE TNF-α PE (MP6-XT22), IFN-γ PE-Cy7/PE (XMG1.2) (Pharmingen). BrdU staining was carried out with a kit per manufacturer's instructions (Pharmingen). Data acquisition was performed on an LSRII flow cytometer (Beckton Dickinson) and analyzed using FlowJo software (Treestar, San Carlos, CA).
BrdU labeling for in vivo proliferation
Following PD-1 blockade, mice were pulsed with 1 mg i.p. of 5-bromo-2-deoxyuridine (BrdU) once per day for two days, then sacrificed on the third day. BrdU incorporation was detected using the APC-BrdU Flow Kit according to the manufactures protocol (BD Biosciences, San Jose, CA).
Statistical Analyses
Statistical significance for skin graft survival was determined using the log-rank test. All other statistical comparisons were performed using the Mann-Whitney non-parametric test in Graphpad Prism software.
Results
Transient blockade of the CD28 and CD40 pathway induces long-term skin graft acceptance when the precursor frequency of donor-reactive T cells is low
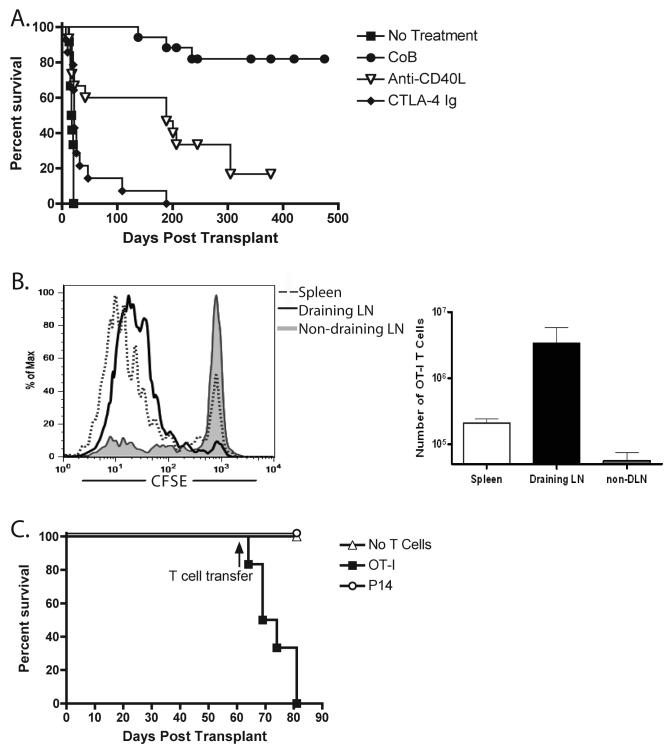
To study the fate and function of donor-reactive T cells in settings of transplant acceptance or rejection, we used a model in which skin from transgenic B6 donor mice that ubiquitously express membrane-bound chicken ovalbumin (mOVA) under the control of the β-actin promoter was transplanted to wild-type B6 recipients. mOVA skin grafts transplanted onto B6 mice are readily rejected in a CD8+ and CD4+ T cell-dependent fashion (35). In our experiments, untreated recipients rapidly rejected their grafts (MST 18 d). Short-term blockade of the CD28 pathway via CTLA4-Ig had a minimal effect on skin graft survival (MST 22 d) while anti-CD154 significantly extended survival (MST 189 d). As in other models (39), combined blockade of these pathways induced long-term graft survival (MST >200d) (Fig. 1A)
Figure 1. Long-term graft survival in costimulation blockade-treated recipients was maintained despite the presence of actively presented antigen.
(A) mOVA skin grafts were placed onto B6 recipients, which were then treated on day 0 and 2 with 0.25 mg anti-CD40L (MR-1), 0.25 mg CTLA-4 Ig or the combination of both (CoB) (n ≥ 10 mice per group). Treatment with CoB resulted in long-term graft survival with an MST of >500 days (. (B) 5×106 CFSE-labeled OT-I T cells were transferred i.v. to mOVA skin grafted B6 recipients (healed >60 days) (n=3 mice per group). 48 hours after transfer, OT-I accumulation and proliferation were assessed in the spleen, draining inguinal LN, or non-draining contralateral inguinal LN. Dividing OT-I T cells were detected in the spleen and draining LN, but not in the contralateral non-draining LN. (C) 5×106 CFSE labeled OT-I T cells or irrelevant P14 T cells were transferred i.v. to mOVA skin grafted B6 recipients (healed >60 days) (n=5 mice per group). Data demonstrate that adoptive transfer of naïve OT-I, but not P14, T cells precipitated graft rejection in mOVA skin graft recipients.
We considered several non-mutually exclusive mechanisms by which donor-reactive T cells might be controlled. These included peripheral deletion, functional inactivation (via anergy or exhaustion), peripheral regulation, and finally, a loss of graft immunogenicity resulting in immunologic ignorance. We performed two sets of experiments to address the issue of ignorance. First, we adoptively transferred CFSE-labeled OVA-specific CD8+ OT-I transgenic T cells into mice with surviving mOVA skin grafts (>60 days) after short-term costimulation blockade. OT-I T cells are specific for OVA257-264 in the context of Kb, an epitope which has be shown to be constitutively presented by mOVA skin (35). We assessed T cell division (CFSE dilution) in the draining lymph node, the contralateral node and in the spleen 48 hours after transfer. Graft-specific T cells underwent a burst of proliferation in the draining node and spleen but not in the non-draining node (Figure 1B), demonstrating that donor antigens are presented in an immunostimulatory form in mice bearing surviving grafts. As a second evaluation of the immunogenicity of the long-term surviving grafts, we performed similar adoptive transfer experiments, but assessed the survival of the grafts after transfer of OT-I T cells. Mice with long-term surviving mOVA grafts received either 5×106 OT-I T cells, a similar number of P14 TCR-Tg T cells (which recognize a peptide derived from the lymphocytic choriomeningitis virus) or no T cells. While grafts in mice that received no T cells or irrelevant P14 T cells continued to survive, the mice that received OT-I T cells rapidly rejected their mOVA grafts (Figure 1C). These results provided additional evidence that graft survival was not primarily due to immunologic ignorance.
Because the precursor frequency of T cells specific for OVA in wild-type mice is very low (< 1/106) and essentially undetectable under tolerogenic conditions, we developed a model in which physiologic frequencies of donor-reactive T cells could be tracked over time. To do this we created bone marrow chimeric mice. After conditioning with minimally myelosuppressive doses of busulfan, Thy1.2+, CD45.1+ B6 mice received mixed bone marrow from WT B6 donors and B6, RAG-deficient, Thy1.1+ CD45.2+ OT-I TCR transgenic donors (hereafter referred to as OT-I chimeras) to achieve consistent and stable levels of OT-I T cells allowing us to specifically target levels similar to observed allo-reactive frequencies (0.1-10%) (40, He, 2004 #35, 41). The use of the Thy1.1 and CD45.1 congenic markers as well as anti-mouse Vα2 or Kb/SIINFEKL tetramer permitted us four independent cell surface markers to precisely track graft-specific T cells at low frequencies. Six to eight weeks after chimerism induction, the levels of peripheral OT-I T cells stabilized (Fig 2A), and at this time mOVA skin grafts were applied. Based on preliminary experiments, mice were grouped according to the frequency of OT-I T cells in peripheral blood. The groups contained mice in which OT-I represented > 5%, 3-5%, or 0.1-3% of the total CD8+ T cell compartment. As expected, in untreated recipients from all three groups the grafts were promptly rejected (not shown, MST 14d).. However, in recipients that received transient blockade of the CD28 and CD40 pathways, graft survival was influenced by the initial frequency of donor-reactive T cells. Recipients harboring a high initial frequency of OT-I T cells (>5% of CD8+ compartment) consistently rejected mOVA skin grafts despite costimulation blockade (MST 14 days). In contrast, mOVA grafts transplanted to animals with lower frequencies (0.1-3%) of OT-I T cells went on to show long-term graft survival after transient costimulation blockade (MST >200d). Animals bearing between 3 and 5% OT-I chimerism demonstrated an intermediate phenotype in which the grafts were either rejected within the first 3 weeks or went on to long-term survival (Figure 2B). These results support previous findings suggesting that graft-specific precursor frequency is an important predictor of costimulation blockade resistant rejection (33, 34).
Figure 2.
Treatment of low but not high frequency OT-I chimeras with costimulation blockade following mOVA skin graft placement resulted in long-term graft survival. (A) B6 mice were treated with busulfan and mixed donor bone marrow derived from WT B6 and OT-I TCR tg mice as described in Materials and Methods. The three left panels depict the gating strategy to identify OVA257-264/Kb-specific OT-I T cells as they develop in these recipients, and the right panel depicts the kinetics of the appearance of CD8+ Thy1.1+ Vα2+ OT-I T cells in the peripheral blood following bone marrow transplant. Each line represents an individual mouse. (B) mOVA skin grafts were placed onto OT-I chimeric mice harboring the indicated frequencies of peripheral blood OT-I T cells (0.1-1% n=6, 1-3% n=5, 3-5% n=8, 5-12% n=8). Mice were treated days 0 and 2 with CTLA-4 Ig and αCD40L. Results indicated that recipients bearing >3% peripheral OT-I T cells rapidly rejected their grafts in the presence of costimulation blockade, mice bearing <3% peripheral OT-I cells enjoyed long-term graft survival. (C) Peripheral blood frequencies of OT-I (Thy1.1+) T cells after skin grafting are depicted (n ≥ 8 mice/group). The frequencies of OT-I T cells in mice with an initial OT-I frequency of <3% declined following mOVA skin graft and costimulation blockade. (E) Frequencies of donor-BM derived non-T cell chimerism after skin grafting are depicted (n ≥ 8 mice/group). Constant levels of non-T cell donor chimerism were observed in these animals.
Long-term graft acceptance is associated with both deletional and non-deletional peripheral mechanisms
We next investigated the fate of graft-specific OT-I T cells under conditions of persistent graft survival. OT-I chimeras that received a syngeneic B6 skin graft, or that received no skin graft (not shown), exhibited no change in peripheral OT-I frequencies. Recipients with high initial frequencies of OT-I T cells rejected mOVA grafts despite costimulation blockade and did not show evidence of OT-I deletion. In contrast, when OT-I chimeras with initial precursor frequencies of OT-I T cells in the 1-3% range were treated with costimulation blockade and received mOVA skin grafts, the number of OT-I T cells in peripheral blood significantly declined over the first 3-4 weeks after transplant, but were not completely deleted (Fig. 2C). These findings are consistent with previous observations that transient costimulation blockade can promote peripheral deletion, (42, Fehr, 2005 #39, 43, 44) over a period of weeks (38, 45). However, by using newer, more sensitive polychromatic flow cytometric techniques we observed that a residual population of OT-I T cells could readily be detected (0.35±0.06% of CD8+ compartment) >80 days post-transplant (Fig. 2C). Overall donor bone marrow chimerism in these recipients was unchanged, as the level of non-OT-I CD45.2+ (CD8−) donor-derived cells was maintained at approximately 1-2% for the term of the experiment, similar to control treatment groups (Fig. 2D). Therefore, the observed reduction in peripheral OT-I T cells was not due to waning levels of overall donor bone marrow chimerism. Thus, these findings suggest that ongoing peripheral deletion is one mechanism for controlling graft specific CD8+ T cells.
PD-1 expression is associated with maintained graft survival and functional impairment of donor-reactive CD8+ T cells in low frequency OT-I chimeras treated with costimulation blockade
Given that recipients with a low frequency of donor-reactive T cell precursors still bore surviving grafts as well as significant numbers of donor-reactive T cells in periphery, we sought to determine the mechanisms controlling the function of this residual population and preventing rejection. First, we performed a phenotypic analysis of the OT-I T cells in recipients possessing long-term surviving mOVA grafts to screen for potentially upregulated inhibitory molecules known to be associated with exhaustion or anergy in T cells stimulated at initial low frequency, including CTLA-4, BTLA, and PD-1. For comparison, we analyzed OT-I T cells in mice that received a syngeneic B6 skin graft or in mice harboring a high frequency of OT-I T cells which had rejected an mOVA graft despite costimulation blockade (Figure 3A). We found a marked increase in the cell surface expression of PD-1 on residual OT-I T cells stimulated at an initial low frequency in the presence of a surviving mOVA graft relative to controls at day 45 post-transplant (Fig. 3B). These cells also stained positive for Vα2 and with the Kb-SIINFEKL tetramer, demonstrating that the residual T cells are in fact donor-reactive and that TCR expression is not overtly altered (not shown). Additional phenotypic analysis revealed no evidence for increased FoxP3 expression or other regulatory markers such as GITR, CD25 (not shown) or CTLA-4 (Figure 3C).
Figure 3. Maintained graft survival was associated with PD-1 expression on donor-reactive T cells.
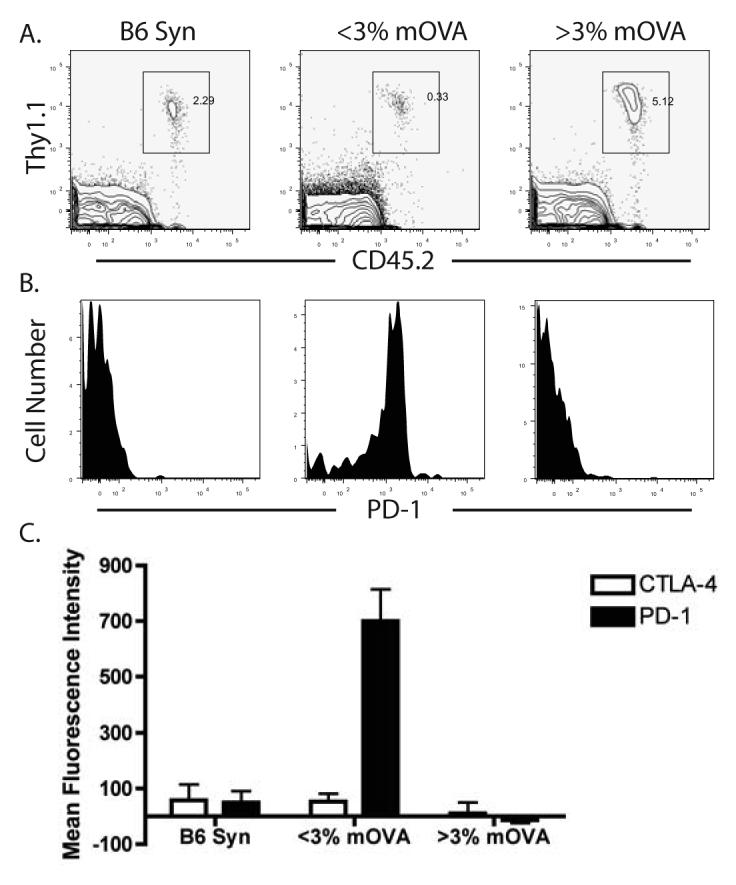
The phenotype of OT-I T cells was analyzed in B6 OT-I chimeras that received either a syngeneic B6 graft or an mOVA graft. (A) The frequencies of Thy1.1+ CD45.2+ (OT-I) T cells in recipients of syngeneic or mOVA skin grafts. Plots are gated on CD8+ lymphocytes. (B) The level of PD-1 expression OT-I T cells from these mice was determined. Plots shown are gated OT-I T cells, and demonstrate that high PD-1 expression is observed on T cells from low frequency OT-I chimeras receiving an mOVA skin graft and costimulation blockade. (C) Peripheral OT-I T cells from OT-I chimeric mice receiving syngeneic B6 skin grafts (n=5), low frequency OT-I chimeric mice receiving mOVA skin grafts (n=16), and high frequency OT-I chimeric mice receiving mOVA skin grafts (n=3) were analyzed for the presence of PD-1 and CTLA-4. The mean fluorescence intensity of these markers on CD8+ Thy1.1+ Vα2+ cells reveal that while CTLA-4 was not upregulated in any of the groups, low frequency OT-I chimeras receiving mOVA skin grafts consistently expressed PD-1 (p<0.05).
In vivo PD-1 blockade leads to graft loss and expansion of the donor-reactive T cell population
Because the OT-I chimera model afforded us the opportunity to identify and track graft-specific T cells at low but biologically and clinically relevant frequencies, we were able to assess the functionality of the PD-1 expressing donor-reactive T cells. To examine whether the PD-1 pathway plays an important role in maintaining graft survival, we administered monoclonal antibodies to interrupt the PD-1 pathway in vivo. OT-I chimeric mice bearing surviving mOVA skin grafts for greater than three months after costimulation blockade were given either a blocking anti-PD-1 (J43) or anti-PD-L1 (10F.9G2) (13, 37, 46). In vivo blockade of the PD-1 receptor or its ligand PD-L1 induced rapid graft rejection in >75% of the OT-I chimeric mice (Fig. 4A). In contrast, recipients treated with isotype control antibodies showed continued graft survival (Fig. 4A). Furthermore, recipients treated with a depleting anti-CD25 monoclonal antibody (PC.61) did not undergo rejection of their grafts, which suggests that regulation via CD25+ Treg is not a dominant mechanism in this model of peripheral tolerance.
Figure 4. In vivo PD-1 blockade precipitated graft loss and induced donor-reactive T cell expansion.
Low frequency (<3%) OT-I chimeric animals with mOVA skin grafts surviving >90 days were treated with indicated antibody at day 0 (>120 days post transplant). Anti-PD-1 (J43) or hamster IgG isotype control were given as 500 µg on day 0 and 250 µg every other day; rat anti-mouse PD-L1 (10F.9G2) or rat IgG2b isotype control was given at 200 µg every third day. Both regimens were discontinued after 2 weeks. Anti-CD25 was given at 500 µg on days 0, 2, 4, and 6. n ≥ 8 mice per group. (A), Treatment with anti-PD-1 or anti-PDL-1, but not isotype control or anti-CD25, precipitated graft rejection in recipients of long-surviving skin grafts. (B) The frequency of OT-I CD8+ T cells in low frequency OT-I chimeric animals increased following in vivo PD-1 blockade (day 120). (C), Representative histograms of PD-1 expression on CD8+ Thy1.1+ Vα2+ T cells prior to (day 97) and post in vivo PD-L1 blockade (day 150). PD-1 is down regulated on CD8+ Thy1.1+ Vα2+ T cells following anti-PDL-1 treatment and subsequent graft loss, but is maintained on CD8+ Thy1.1+ Vα2+ T cells in recipients treated with rat IgG2b isotype control.
In addition to its profound effect on graft survival, blockade of the PD-1 pathway led to a change in the functional phenotype of donor-reactive T cells. Treatment with anti-PD-L1 resulted in an increased frequency of donor-reactive T cells in the peripheral blood of recipients at the time of graft rejection (Fig. 4B). At day 97, after graft rejection had occurred, Thy1.1+ CD45.2+ CD8+ T cells were analyzed for their surface expression of PD-1. Results showed that treatment with anti-PD-L1, but not isotype control antibody, resulted in the loss of surface expression of PD-1 (Fig. 4C), suggesting that these cells may have undergone a reversion of their exhausted status.
Tolerized donor-reactive T cells regain proliferative capacity and effector function following PD-1 blockade
To determine whether donor-reactive T cells treated with anti-PD-L1 exhibited increased proliferative potential and effector function on a per-cell basis, we analyzed in vivo cell cycling and ex vivo cytokine production by donor-reactive T cells. First, we observed that following PD-1 blockade, but not after treatment with an isotype control, the graft-specific T cell population exhibited a significant increase in the frequency of BrdU+ cells (Fig. 5A), demonstrating the re-acquisition of proliferative potential of these cells following the cessation of ligation of PD-1. Secondly, ex vivo restimulation with cognate peptide showed that >50% of donor-reactive OT-I T cells made IFN-γ following in vivo PD-L1 blockade, relative to <15% of donor-reactive cells from mice that received isotype control (Fig. 5B). Analysis of absolute numbers of these IFN-γ-secreting effectors revealed a >5 fold increase in graft-specific effector cells eight days after PD-1 blockade over isotype control (Fig. 5B). In conclusion, these results support the hypothesis that PD-1 expression on graft-specific T cells leads to repressed proliferative capacity as well as effector function.
Figure 5. OT-I T cells regained proliferative capacity and cytokine effector function post PD-1 blockade.
Low frequency OT-I chimeric animals with surviving mOVA skin grafts for >60 days were treated with anti-PD-1 or control hamster Ig. At days 6 and 7 post-PD-1 blockade, mice were given 1mg BrdU in PBS i.p. (n=3-4 mice per group). At day 8 following treatment, spleen and LN were harvested and interrogated for (A) BrdU uptake or (B) the ability to make IFN-γ after 5 hours of SIINFEKL restimulation. Results indicated that low frequency OT-I chimeras treated with anti-PD-1 exhibited an increased frequency of BrdU+ cycling cells (A, p=0.0073) and IFN-γ+ cells (B, p=0.0373), as compared to mice receiving hamster isotype control antibody.
Discussion
Previous studies have defined a critical role for donor-reactive T cell precursor frequency in susceptibility to tolerance induction via blockade of the CD28 and CD40 costimulatory pathways (33, 34). However, the mechanisms by which donor-reactive T cells stimulated at low frequency become susceptible to tolerance induction are not well understood. In order to explore the mechanisms underlying the induction and maintenance of transplantation tolerance at low frequency, we used a model system in which transgenic donor-reactive T cells were continuously produced in vivo. This system allowed us to systematically track the number, phenotype and functional properties of endogenously generated graft-specific CD8+ T cells over the course of graft acceptance or rejection in order to more closely investigate the mechanisms of tolerance induction at low precursor frequency. Using this system, we confirmed our previous observation that when the initial precursor frequency of donor-reactive T cells was high (>4%), recipient mice were refractory to tolerance induction using transient CD28/CD40 blockade. However, when the initial precursor frequency of donor-reactive T cells was low (<3%), long-term graft acceptance was achieved.
The observed long-term graft survival in low frequency OT-I chimeras was somewhat unexpected given the transient course of antibody therapy and the expected influx of newly emergent OT-I T cells into the periphery, which are derived from the bone marrow of OT-I chimeric mice. This is in contrast to the situation observed in adoptive transfer experiments, wherein all of the donor-reactive T cells are present and can be tolerized during the initial antibody-administration period. Iwakoshi et al. utilized a similar TCR transgenic synchimeric system with an anti-Kb TCR transgenic (KB5) and found that as anti-CD40L antibody levels waned graft rejection ensued, suggesting that persistent treatment was necessary for graft acceptance in this system (43). Interestingly, the level of TCR tg T cell chimerism in this model was approximately twice the level observed in our low frequency OT-I chimeras, further suggesting that low donor-reactive precursor frequency is critical for the ability of peripheral tolerance to be maintained in the absence of active costimulation blockade. It should be noted that the half-life of hamster anti-CD154 (MR-1) has been calculated as approximately 12 days (47, 48)} and the human Ig-fusion protein CTLA-4.Ig is estimated to be less than 4 days in mice (49, 50). This is clinically relevant in that patients that are weaned off of immunosuppression often experience episodes of acute rejection. Our results suggest that patients with organs with a high degree of MHC matching, and thus lower precursor frequencies of allo-reactive T cells potentially emerging from the thymus, may be more likely to experience continued graft survival via mechanisms of peripheral tolerance (such as increased PD-1 expression) following discontinuation of immunosuppressive therapy.
The mechanisms underlying the observation that low-frequency OT-I chimeras could be tolerized by CD28/CD40 pathway blockade were two-fold: first, transient costimulation blockade led to the deletion of a majority of graft-specific T cells when stimulated at low frequency, and second, a detectable population of functionally quiescent PD-1+ donor-reactive T cells persisted in recipients bearing surviving donor skin grafts. The observed deletion of graft-specific cells stimulated at low frequency in the presence of costimulation blockade is consistent our previous report demonstrating that although low-frequency donor-reactive T cells were capable of dividing in the presence of costimulation blockade, they exhibited decreased accumulation and increased expression of markers of apoptosis, suggestive of enhanced cell death under these conditions (33). We speculate that low frequency T cells may exhibit increased death rates in the presence of costimulatory blockade because they may be required to undergo more rounds of division on a per cell basis in order to achieve a threshold quantity of effectors necessary to precipitate graft rejection (33).
We addressed the role of classical regulatory T cells in maintaining tolerance in this model by depleting CD25+ cells from the periphery (Figure 4A) and by adoptively transferring naïve OT-I T cells into recipients with long-term surviving grafts to test for the presence of functional regulation (Figure 1C). Evidence of regulation was not observed in either of these experiments. However, potential caveats include the fact that a number of CD25− regulatory T cell populations have recently been described (51, 52); thus the possibility that both CD25-negative Tregs and PD-1 dependent mechanisms are required for tolerance induction cannot be ruled out. Interestingly, Najafian and colleagues recently described a population of CD8+ PD-1+ Treg that were capable of inhibiting CD4+ but not CD8+ allospecific responses (52). These cells required PD-1 for their suppressive function. While we did not observe suppression of naïve CD8+ T cells in the adoptive transfer model, the possibility remains that the CD8+ PD-1+ cells may possess regulatory function under different conditions or towards other cell populations.
As stated above, the second mechanism we observed underlying the survival of skin grafts in recipients with a low frequency of donor-reactive T cells was a detectable population of functionally inactive PD-1+ donor-reactive T cells that persisted in recipients bearing surviving donor skin grafts. The upregulation of PD-1 on the surface of antigen-specific T cells is purported to be linked to the degree of antigen exposure of the cells (13, 22). Thus, we speculate that cells stimulated at low frequency exhibit increased PD-1 expression due to increased exposure to donor antigen:MHC complexes. This increased antigenic exposure may arise from the fact that low frequency donor-reactive T cells would encounter reduced competition for antigen. Indeed, we observed that after rejection occurred in recipients treated with anti-PD-L1 and antigen had been eliminated, surface PD-1 expression was down-regulated on the OT-I population. This finding further suggests that PD-1 expression is exquisitely dependent on the presence of antigen, as has been suggested in studies in models of autoimmunity and viral pathology (13, 22).
The ability to track donor-reactive T cells in this model allowed us to further demonstrate that interruption of the PD-1 pathway with either anti-PD-1 or anti-PD-L1 mAbs not only precipitated graft loss but also led to a rapid expansion of the graft-specific T cell population concurrent with their re-acquisition of effector function. These data suggest that expression of PD-1 is critically important for the attenuation of signals through the TCR that would otherwise allow these cells to respond once the costimulation blockade had waned. Our results suggest that perturbation of the provision of PD-1 signals allows the T cell to respond, presumably with the provision of sufficient costimulation. Therefore, our data highlight the ability of blocking positive costimulatory pathways and engaging negative regulatory pathways to syngerize in maintaining allograft survival. We conclude that the PD-1 pathway plays a pivotal role in maintaining peripheral tolerance by actively suppressing the proliferation and effector function of low frequencies of remaining donor-specific CD8+ T cells.
Based on the data presented here, we conclude that PD-1 dependent cell intrinsic mechanisms of peripheral tolerance are indeed active in maintaining allograft survival in much the same way T cells specific for nominal self-antigens are protected to prevent autoimmunity, and virus-specific T cells can be silenced to limit immunopathology in the setting of chronic viral infection. Recent work has suggested that blockade of PD-1 could be beneficial in control of persistent pathogens; however, this and other work in autoimmunity models suggest caution before clinical trials are begun. Clinically, for transplant recipients, monitoring of PD-1 expression could be a biomarker for a tolerogenic state. Conceivably, this could facilitate studies of treatment withdrawal for patients expressing high levels of PD-1 on donor-reactive peripheral T cells. Additionally, reagents designed to specifically engage this negative regulatory pathway both peri-operatively as well as in the maintenance phase after transplantation, might aid in promoting graft survival.
Footnotes
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei R, Gibson ML, Zheng X, Myrdal S, Gordon D, Bailey T, Bolling SF, Thompson CB. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson TC, Alexander DZ, Corbascio M, Hendrix R, Ritchie SC, Linsley PS, Faherty D, Larsen CP. Analysis of the B7 costimulatory pathway in allograft rejection. Transplantation. 1997;63:1463–1469. doi: 10.1097/00007890-199705270-00016. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 5.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J. Exp. Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenschow D, Zeng Y, Thistlethwaite J, Montag A, Brady W, Gibson M, Linsley P, Bluestone J. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, Rees P, Hendrix R, Price K, Kenyon NS, Hagerty D, Townsend R, Hollenbaugh D, Pearson TC, Larsen CP. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 8.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, Jonker M. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 9.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, Boon L, Jonker M. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation. 2005;79:1623–1626. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 10.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH, Jr., Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 11.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 13.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 17.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8(+) T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 19.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 23.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr., Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr., Sayegh MH, Najafian N. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 27.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, Yagita H, Azuma M, Turka LA, Sayegh MH. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 28.Tao R, Wang L, Han R, Wang T, Ye Q, Honjo T, Murphy TL, Murphy KM, Hancock WW. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175:5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 29.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76:994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 30.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe T, Duong T, Smith T, Gutierrez-Ramos JC, Rottman JB, Coyle AJ, Hancock WW. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 31.Watson MP, George AJ, Larkin DF. Differential effects of costimulatory pathway modulation on corneal allograft survival. Invest Ophthalmol Vis Sci. 2006;47:3417–3422. doi: 10.1167/iovs.05-1597. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180:7203–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Exner BG, Chilton PM, Schanie C, Ildstad ST. CD45 congenic bone marrow transplantation: evidence for T cell-mediated immunity. Stem Cells. 2004;22:1039–1048. doi: 10.1634/stemcells.22-6-1039. [DOI] [PubMed] [Google Scholar]
- 37.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 38.Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, Rees PA, Cheung MC, Mittelstaedt S, Bingaman AW, Archer DR, Pearson TC, Waller EK, Larsen CP. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 39.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 40.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 41.He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, Heeger PS. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- 42.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, Zhao G, Sykes M. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwakoshi NN, Markees TG, Turgeon N, Thornley T, Cuthbert A, Leif J, Phillips NE, Mordes JP, Greiner DL, Rossini AA. Skin allograft maintenance in a new synchimeric model system of tolerance. J Immunol. 2001;167:6623–6630. doi: 10.4049/jimmunol.167.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25- T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 45.Ito H, Kurtz J, Shaffer J, Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol. 2001;166:2970–2981. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- 46.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 47.Kalled SL, Cutler AH, Ferrant JL. Long-term anti-CD154 dosing in nephritic mice is required to maintain survival and inhibit mediators of renal fibrosis. Lupus. 2001;10:9–22. doi: 10.1191/096120301668384751. [DOI] [PubMed] [Google Scholar]
- 48.Pearson T, Markees TG, Wicker LS, Serreze DV, Peterson LB, Mordes JP, Rossini AA, Greiner DL. NOD congenic mice genetically protected from autoimmune diabetes remain resistant to transplantation tolerance induction. Diabetes. 2003;52:321–326. doi: 10.2337/diabetes.52.2.321. [DOI] [PubMed] [Google Scholar]
- 49.Bolling SF, Lin H, Turka LA. The time course of CTLAIg effect on cardiac allograft rejection. J Surg Res. 1996;63:320–323. doi: 10.1006/jsre.1996.0268. [DOI] [PubMed] [Google Scholar]
- 50.Linsley PS, Wallace PM, Johnson J, Gibson M, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 51.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izawa A, Yamaura K, Albin MJ, Jurewicz M, Tanaka K, Clarkson MR, Ueno T, Habicht A, Freeman GJ, Yagita H, Abdi R, Pearson T, Greiner DL, Sayegh MH, Najafian N. A novel alloantigen-specific CD8+PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J Immunol. 2007;179:786–796. doi: 10.4049/jimmunol.179.2.786. [DOI] [PubMed] [Google Scholar]