Abstract
Trabectedin is a promising anticancer agent, but dose-limiting hepatotoxicity was observed during phase I/II clinical trials. Dexamethasone (DEX) has been shown to significantly reduce trabectedin-mediated hepatotoxicity. The current study was designed to assess the capability of sandwich-cultured primary rat hepatocytes (SCRH) to predict the hepato-protective effect of DEX against trabectedin-mediated cytotoxicity. The role of multidrug resistance-associated protein 2 (Mrp2; Abcc2) in trabectedin hepatic disposition also was examined. In SCRH from wild-type Wistar rats, cytotoxicity was observed after 24-hr continuous exposure to trabectedin. SCRH pretreated with additional DEX (1 µM) exhibited a 2–3-fold decrease in toxicity at 100 nM and 1000 nM trabectedin. Unexpectedly, toxicity in SCRH from Mrp2-deficient (TR−) compared to wild-type Wistar rats was markedly reduced. Depletion of glutathione from SCRH using buthionine sulfoximine (BSO) mitigated trabectedin toxicity associated with 100 nM and 1000 nM trabectedin. Western blot analysis demonstrated increased levels of CYP3A1/2 and Mrp2 in SCRH pretreated with DEX; interestingly, Mrp4 expression was increased in SCRH after BSO exposure. Trabectedin biliary recovery in isolated perfused livers from TR− rats was decreased by ~75% compared to wild-type livers. In conclusion, SCRH represent a useful in vitro model to predict the hepatotoxicity of trabectedin observed in vivo. The protection by DEX against trabectedin-mediated cytotoxicity may be attributed, in part, to enhanced Mrp2 biliary excretion and increased metabolism by CYP3A1/2. Decreased trabectedin toxicity in SCRH from TR− rats, and in SCRH pretreated with BSO, may be due to increased basolateral excretion of trabectedin by Mrp3 and/or Mrp4.
Keywords: Trabectedin, Dexamethasone, Sandwich-cultured primary rat hepatocytes, Multidrug resistance-associated proteins
Introduction
Trabectedin (Ecteinascidin 743, Yondelis™) is a DNA alkylating agent extracted from the marine tunicate Ecteinascidia turbinate that exhibits potent antineoplastic activity against a variety of tumor cells at nanomolar concentrations (Izbicka et al., 1998). Trabectedin is currently under phase II/III evaluation in advanced gastrointestinal stromal tumors, pretreated soft tissue sarcoma, ovarian cancer and breast cancer (Laverdiere et al., 2003; Blay et al., 2004; Yovine et al., 2004; Zelek et al., 2006).
Trabectedin is metabolized primarily by CYP 3A4; 2C9, 2C19, 2D6 and 2E1, and to a lesser extent conjugated by the phase II enzymes uridine diphosphoglucuronosyl transferase (UGT) and glutathione-S-transferase (GST) (Reid et al., 2002; Brandon et al., 2006). Extensive metabolism of trabectedin was demonstrated in a mass balance study conducted in patients with advanced cancer (Beumer et al., 2007b). Following i.v. [14C]trabectedin administration to patients, ~55.5% and ~5.9% of the dose was recovered in feces and urine, respectively, suggesting that trabectedin and its metabolites undergo biliary excretion (Beumer et al., 2005). However, the involvement of transport proteins in hepatobiliary disposition of trabectedin remains to be elucidated.
During phase I/II clinical trials, the most prevalent adverse effect associated with trabectedin treatment was dose-limiting hepatotoxicity secondary to increases in blood alkaline phosphatase and/or bilirubin levels (Delaloge et al., 2001; Ryan et al., 2001; Taamma et al., 2001). An in vitro cytotoxicity study using HepG2 cells showed increased trabectedin-mediated toxicity after 1-hr preincubation with inhibitors of cytochrome P450 (CYP) enzymes involved in trabectedin metabolism; CYP inducers and phase II enzyme inhibitors did not significantly affect trabectedin-mediated toxicity, suggesting that trabectedin metabolites may be less toxic compared to the parent compound (Brandon et al., 2005). Pretreatment of female Fisher rats with DEX has been shown to ameliorate the hepatotoxic effects of trabectedin while coadministration of DEX with trabectedin did not reduce toxicity (Donald et al., 2003). Consistent with the protective effect of DEX on trabectedin-mediated toxicity in rats, DEX premedication markedly reduced trabectedin-mediated hepatotoxicity in patients with advanced sarcoma (Grosso et al., 2006). However, this hepato-protective effect of DEX could not be mimicked in vitro using primary rat hepatocytes cultured on a fibronectin matrix (Donald et al., 2004).
Significant induction of Mrp2 mRNA and protein by DEX treatment has been reported in primary rat and mouse hepatocytes (Kast et al., 2002; Luttringer et al., 2002; Turncliff et al., 2004). Mrp2, a transport protein localized on the hepatic canalicular plasma membrane, is responsible for the biliary excretion of organic anions as well as numerous drugs including pravastatin, vincristine and etoposide (Cui et al., 1999; Kawabe et al., 1999; Konig et al., 1999; Sasaki et al., 2002). Animals with hereditary conjugated hyperbilirubinemia [Mrp2-deficient rats (TR−) and Eisai hyperbilirubinemic rats (EHBR)] have been used extensively to examine the substrate specificity and in vivo function of Mrp2 (Westley et al., 2006; Zamek-Gliszczynski et al., 2006). Patients with Dubin-Johnson syndrome lack MRP2 and hence suffer from hereditary conjugated hyperbilirubinemia (Paulusma et al., 1997). In the absence of Mrp2/MRP2, rats and humans exhibit increased expression of basolateral Mrp3/MRP3 as a compensatory mechanism that enables the excretion of Mrp2/MRP2 substrates into the systemic circulation, thus avoiding excessive accumulation of organic anions in the hepatocyte (Kiuchi et al., 1998; Hirohashi et al., 1999; Johnson et al., 2006; Nies and Keppler, 2007).
Drug-induced liver injury has emerged as the most common reason for the withdrawal of Food and Drug Administration-approved drugs from the market (Kaplowitz, 2005; FDA, 2007). Covalent adduct formation, immunotoxicity, and disruption of cellular bioenergetics have been considered previously as mechanisms underlying hepatotoxicity, but more recent data suggest that hepatic transport proteins may be an important site of toxic interactions (Fattinger et al., 2001; Funk et al., 2001; Leslie et al., 2007). The objective of this study was to evaluate the capability of sandwich-cultured primary rat hepatocytes (SCRH), which more closely mimic the metabolic and transport capabilities of the intact liver, to predict the hepato-protective effect of DEX against trabectedin-mediated hepatotoxicity. An additional objective was to examine the role of Mrp2 in trabectedin hepatobiliary disposition.
Materials and Methods
Materials
Trabectedin (Ecteinascidin 743, Yondelis™, NSC 648766) was supplied by the National Cancer Institute, Developmental Therapeutics Program Repository (http://dtp.nci.nih.gov/branches/dscb/repo_open.html) and was prepared as a 10 mM stock solution in DMSO. DEX and BSO were purchased from Sigma Chemical Co. (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM), human recombinant insulin, modified essential media non-essential amino acids, L-glutamine, penicillin and streptomycin were purchased from GIBCO (Invitrogen Corporation, Grand Island, NY). ITS+ (insulin, transferin, and selenium) was purchased from BD Biosciences (Bedford, MA).
Animals
Male wild-type Wistar rats (220–300 g; Charles River Laboratories, Raleigh, NC) and male TR− Wistar rats (220–300 g; in-house breeding colony originally obtained from Dr. Mary Vore, University of Kentucky, Lexington, KY) were used for hepatocyte isolation and as liver donors for isolated perfused liver experiments. Male wild-type Wistar rats (>400 g) were used as blood donors for isolated perfused liver experiments. Rats were anesthetized with ketamine/xylazine (60/12 mg/kg intraperitoneally) before surgical manipulation. The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill approved all procedures.
Hepatocyte Isolation and Culture
Hepatocytes were isolated from male Wistar or TR− rats (220–300 g) by a modification of the two-step collagenase digestion method as described previously (Liu et al., 1998). Hepatocyte viability was >85% as determined by trypan blue exclusion. Hepatocytes were plated at a density of ~1.5×106 cells/well in 6-well plates coated with 0.1 ml of rat tail collagen Type I solution (1.5 mg/ml, pH 7.4) in 1.5 ml of plating medium (phenol red-free DMEM containing 5% fetal bovine serum, 0.1 mM MEM nonessential amino acids, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 4 µg/ml human recombinant insulin and 1 µM DEX), and allowed to attach for 2–3 hr at 37□ in a humidified incubator with 95%O2/5%CO2. After attachment, medium was replaced to remove dead/unattached cells. To achieve a sandwich configuration, medium was aspirated and cells were overlaid with 0.1 ml of rat tail collagen Type I solution (1.5 mg/ml, pH 7.4) at 24 hr (day 1). Collagen was allowed to gel for 1 hr in a 37□ humidified incubator 95%O2/5% CO2, and then culture medium was added (1.5 ml/well; phenol red-free DMEM supplemented with 0.1 mM MEM nonessential amino acids, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1% ITS+ and 0.1 µM DEX). Subsequently, the culture medium was changed daily until the conduct of experiments.
DEX and BSO Pretreatment in SCRH
From day 2 to day4, additional DEX (1 µM) or BSO (500 µM) was added to the culture medium containing 0.1 µM DEX. On day 3, SCRH were incubated for 24 hr in culture medium containing a range of trabectedin concentrations (1–1000 nM) to compare trabectedin-mediated cytotoxicity between SCRH pretreated with vehicle (0.1 µM DEX) and additional DEX (1 µM) or BSO (500 µM). DEX and trabectedin were prepared in DMSO, and BSO was solubilized in water. Stock solutions were diluted in culture medium for treatment. The final concentration of DMSO was <0.2% and had no effect on untreated controls.
Determination of Cell Viability Using LDH Release
LDH leakage into the culture medium was measured at the end of the 24 hr exposure using the Cytotoxicity Detection kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions. The degree of LDH release was expressed as a percentage of the maximum cellular LDH release, which was measured by adding 2% Triton X-100 to SCRH.
Western Blot Analysis of Mrp2, 3, 4 and CYP3A1/2 Proteins
On day 4, the medium was removed and cells were washed twice with ice-cold phosphate-buffered saline (PBS). Subsequently, cells were lysed with 400 µl of lysis buffer [1 mM EDTA, 1% sodium dodecyl sulfate (SDS), pH 8, with Complete™ protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany)] in each well of a 6-well plate. Lysates were stored at −20□ until western blot analysis. The whole cell lysates were thawed on ice and protein concentrations were determined using the BCA protein assay (Pierce, Rockford, IL). Protein samples (25 µg/well) were separated by SDS-polyacrylamide gel electrophoresis, and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Blots were blocked with Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk for 1 hr at room temperature. Subsequently, the membrane was incubated with appropriate primary antibodies for 2 hr at room temperature or over-night at 4□ and then rinsed three times at 10-min intervals with TBS-T. Mrp2, 3 and 4 were detected using monoclonal antibodies M2III-6, M3II-9 and M4I-10, respectively, at a 1:1000 dilution (Alexis Biochemicals, San Diego, CA). CYP3A1/2 protein expression was determined with a polyclonal antibody against rat CYP3A1/2 at a 1:1500 dilution (Xenotech, Kansas City, KS). Protein loading was normalized to β-actin expression using a β-actin antibody at a dilution of 1:5000 (C4; Chemicon, San Fransisco, CA). Immunoreactive protein bands were detected by chemilumiscence using a Bio-Rad VersaDoc imaging system and densitometry analysis was achieved using the Quantity One software package v.4.1 (Bio-Rad Laboratories, Hercules, CA).
Isolated Perfused Liver Study
Recirculating isolated perfused liver (IPL) studies were performed with male Wistar wild-type and TR− rat livers as described previously (Brouwer and Thurman, 1996). Briefly, livers were perfused in situ with oxygenated Krebs-Henseleit bicarbonate buffer (pH 7.4) after cannulation of the bile duct and portal vein. Livers were removed from the body cavity and placed into a humidified perfusion chamber at 37□. Perfusion was continued with oxygenated buffer containing 20% (v/v) heparinized male rat blood at a flow rate of 20 ml/min. Livers were allowed to equilibrate for 10–15 min prior to bolus administration of 24 nmol of trabectedin (preliminary recovery studies in the perfusion system without the liver indicated that the actual dose delivered to the IPL was decreased by 50% due to nonspecific binding/degradation in blood-containing perfusate). Liver viability was assessed by monitoring portal pressure (<15 cm of H2O), observing gross morphology, and measuring bile flow in perfused livers from control and TR− rats (>0.8 and >0.3 µl/min/g liver, respectively). Taurocholate (0.5 µmol/min, in saline) was infused to maintain bile flow. Bile was collected in toto every 10 min, and the volume was determined gravimetrically (specific gravity 1.0). Perfusate samples (0.5 ml) were immediately centrifuged following collection to obtain plasma for analysis.
Analytical Methods
Bile and perfusate samples were analyzed by liquid chromatography with detection by tandem mass spectrometry (Applied Biosystems API 4000 triple quadrupole with TurboIonSpray interface, MDS Sciex, Concord, ON, Canada). Trabectedin and cimetidine (internal standard) were eluted from an Aquasil C18 column (dp=5µm, 2.1 × 50 mm, Thermo-Electron, Waltham, MA) using a mobile phase gradient (A: water with 0.1% formic acid, B: methanol with 0.1% formic acid); 0–2.5 min hold at 95% B, a linear gradient to 10% B from 2.5–3 min, 3–4 min hold at 10% B; flow rate was 0.75 mL/min (Shimadzu solvent delivery system, Columbia, MD). Trabectedin and cimetidine were detected in positive ion mode using multiple reaction monitoring; trabectedin: 744.2 → 495.2 m/z, cimetidine: 250.9 → 156.9 m/z. Trabectedin was quantified with calibration curves prepared in the appropriate matrix using analyte:internal standard (trabectedin:cimetidine) peak area ratios. The lower limit of detection was 1 nM for all analytes and standard curves ranged from 1 to 1000 nM.
Statistical Analysis
Statistical significance was assessed using two-way analysis of variance with Bonferroni’s post hoc test, except where the groups being compared had unequal variances, in which case log-transformed data were assessed. SCRH data were presented as the mean ± S.E.M. when at least three sets of independent experiments were performed in triplicate. In all cases, p < 0.05 was considered to be statistically significant.
Results
DEX effect on trabectedin-mediated cytotoxicity in SCRH from wild-type rats
Trabectedin-mediated cytotoxicity was defined as LDH release, relative to a Triton X-100 treated control, from hepatocytes into the culture medium. Following 2 hr of trabectedin exposure, LDH release was not increased relative to untreated SCRH (data not shown). However, cytotoxicity was observed after 24 hr of continuous trabectedin exposure to SCRH (Fig. 1). From day 2 to day 4, additional DEX (1 µM) was added to the culture medium containing 0.1 µM DEX to examine the effect of DEX on trabectedin-mediated cytotoxicity; SCRH were incubated with trabectedin for 24 hr starting on day 3. This pretreatment of SCRH with additional DEX (1 µM) decreased toxicity of trabectedin compared to vehicle-pretreated SCRH (Fig. 1).
Fig. 1.
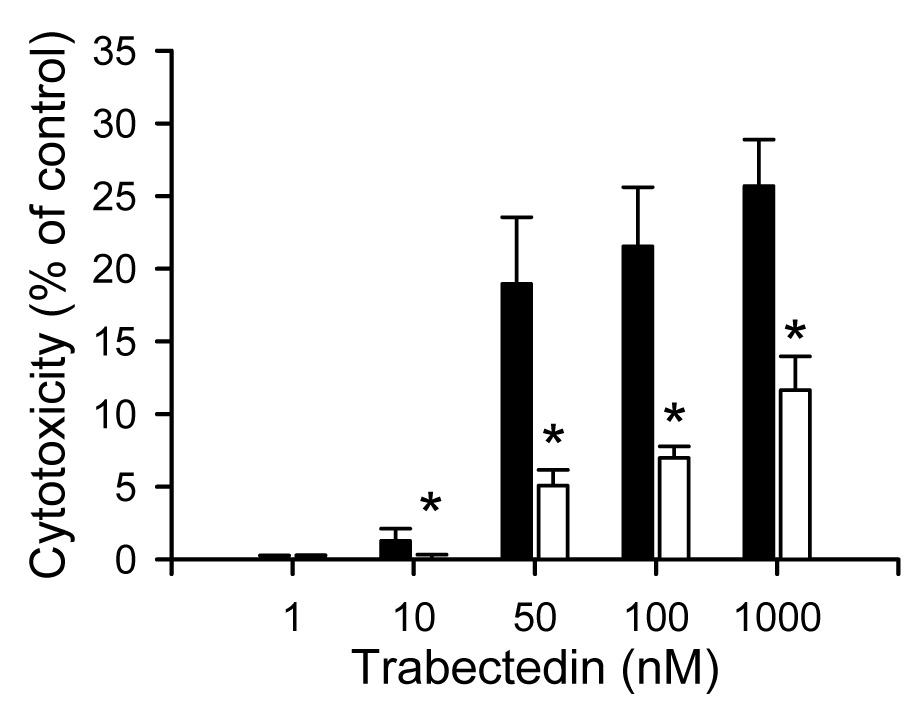
Effect of DEX on trabectedin-mediated cytotoxicity in SCRH isolated from wild-type Wistar rats. Cytotoxicity was determined by measuring LDH release into the culture medium after 24 hr trabectedin (1 – 1000 nM) exposure. Each bar represents the mean ± S.E.M. (n=3) for samples from vehicle- (0.1 µM DEX; solid bars) or additional DEX (1 µM; white bars); *, p < 0.05.
Trabectedin-mediated cytotoxicity in SCRH from TR− rats
Primary hepatocytes from Mrp2-deficient TR− rats were utilized to examine the involvement of Mrp2 in trabectedin-mediated cytotoxicity at 0.1 µM DEX. Unexpectedly, cytotoxicity caused by exposure to 50 nM and 100 nM trabectedin in TR− SCRH was reduced compared to that in wild-type SCRH (Fig. 2).
Fig. 2.
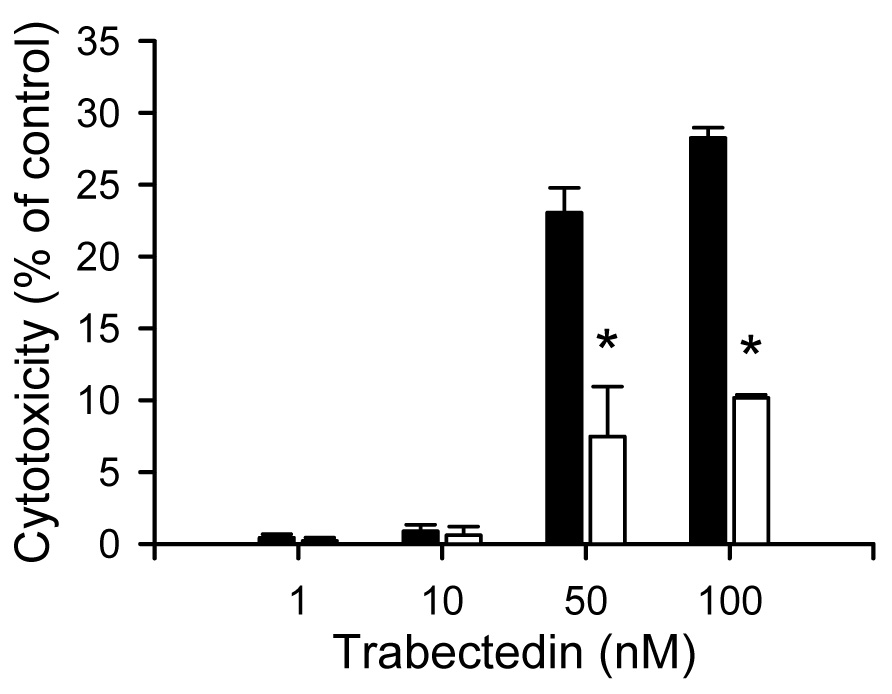
Trabectedin-mediated cytotoxicity in SCRH from wild-type and TR− rats. Cytotoxicity was determined by measuring LDH release into the culture medium after 24 hr trabectedin (1 – 100 nM) exposure. Each bar represents the mean ± S.E.M. (n=3) for samples from wild-type (solid bars) or TR− (white bars) SCRH; *, p < 0.05.
BSO effect on trabectedin-mediated cytotoxicity in wild-type and TR− SCRH
Elevated levels of glutathione in TR− compared to wild-type rat hepatocytes has been proposed as one mechanism that can protect the hepatocyte from drug-induced hepatotoxicity (Lu et al., 1996). To determine the effect of GSH depletion on trabectedin cytotoxicity, BSO (500 µM), an inhibitor of γ-glutamylcysteine synthetase which is the rate-liming step in GSH synthesis, was incubated for 24 hr prior to trabectedin exposure in SCRH from wild-type and TR− rats (Griffith and Meister, 1979). Interestingly, toxicity in wild-type SCRH pretreated with BSO was reduced compared to vehicle-pretreated wild-type SCRH (Fig. 3). Trabectedin-mediated cytotoxicity in BSO-pretreated TR− SCRH was not different from vehicle-pretreated TR− SCRH (data not shown).
Fig. 3.
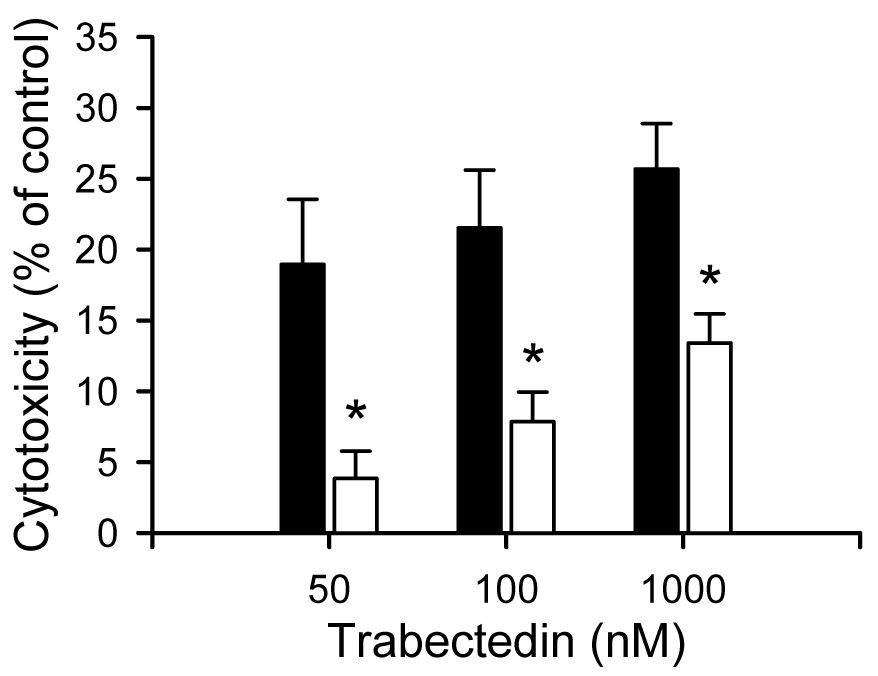
Effect of BSO on trabectedin-mediated cytotoxicity. Cytotoxicity was determined by measuring LDH release into the culture medium after 24 hr trabectedin (50, 100 and 1000 nM) exposure. BSO (500 µM) was added to the culture medium 24 h prior to trabectedin exposure. Each bar represents the mean ± S.E.M. (n=3) for samples from vehicle- (solid bars) or BSO-pretreated (white bars) SCRH; *, p < 0.05.
Alteration of Mrp2, 3, 4 and CYP3A1/2 protein expression by DEX and BSO
When SCRH were cultured with additional DEX (1µM), the fold increase in expression of CYP3A1/2 and Mrp2 was 5.0±2.5 and 2.5±0.6, respectively, compared to vehicle, whereas the fold increase in protein expression of Mrp3 and Mrp4 was 1.2±0.7 and 1.4±0.4, respectively (Fig. 4). Interestingly, in BSO-pretreated SCRH, the fold increase in Mrp4 expression was 3.1±0.7 whereas the fold increase in Mrp2, Mrp3 and CYP3A1/2 protein expression was 1.5±0.7, 1.9±0.4 and 1.2±0.9, respectively.
Fig. 4.
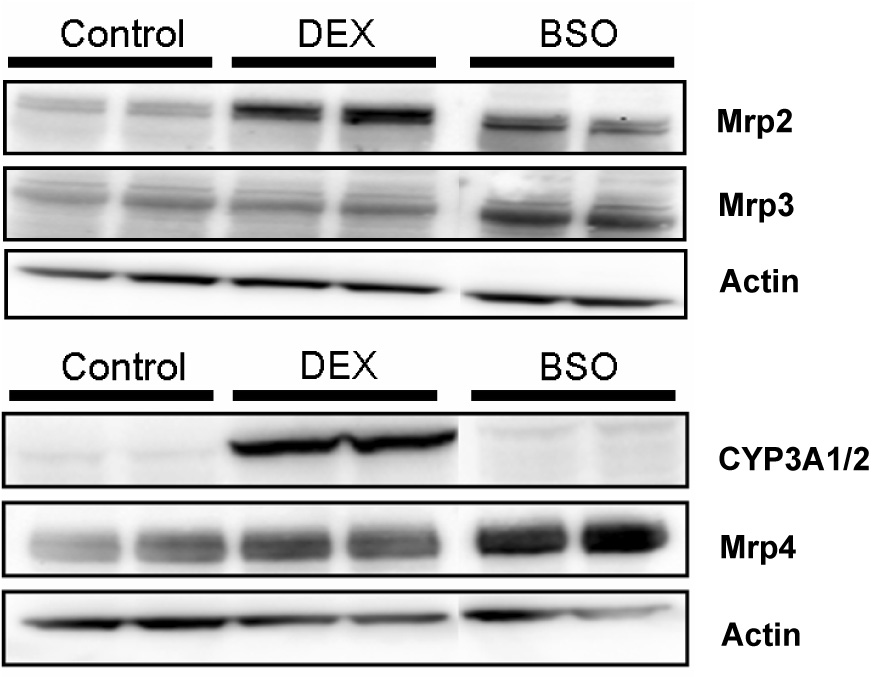
Representative Western blot from whole cell lysates for Mrp2, 3, 4 and CYP3A1/2 in SCRH pretreated with vehicle (0.1 µM DEX), additional DEX (1 µM) or BSO (500 µM) (25 µg protein/well; n=3).
Hepatobiliary disposition of trabectedin in IPLs
IPLs from wild-type and TR− rats were utilized to examine the involvement of Mrp2 in trabectedin hepatobiliary disposition. Trabectedin concentrations in perfusate were not different between livers from wild-type and TR− rats; trabectedin was rapidly extracted by the liver and concentrations were negligible after 20 min (Fig. 5.a). Biliary recovery of trabectedin represented less than 3 % of the dose in wild-type rat livers, consistent with extensive metabolism of trabectedin in rats as reported previously (Fig. 5.b) (Reid et al., 2002). Biliary recovery of trabectedin in IPLs from TR− rats was decreased by ~75% compared to wild-type IPLs.
Fig. 5.
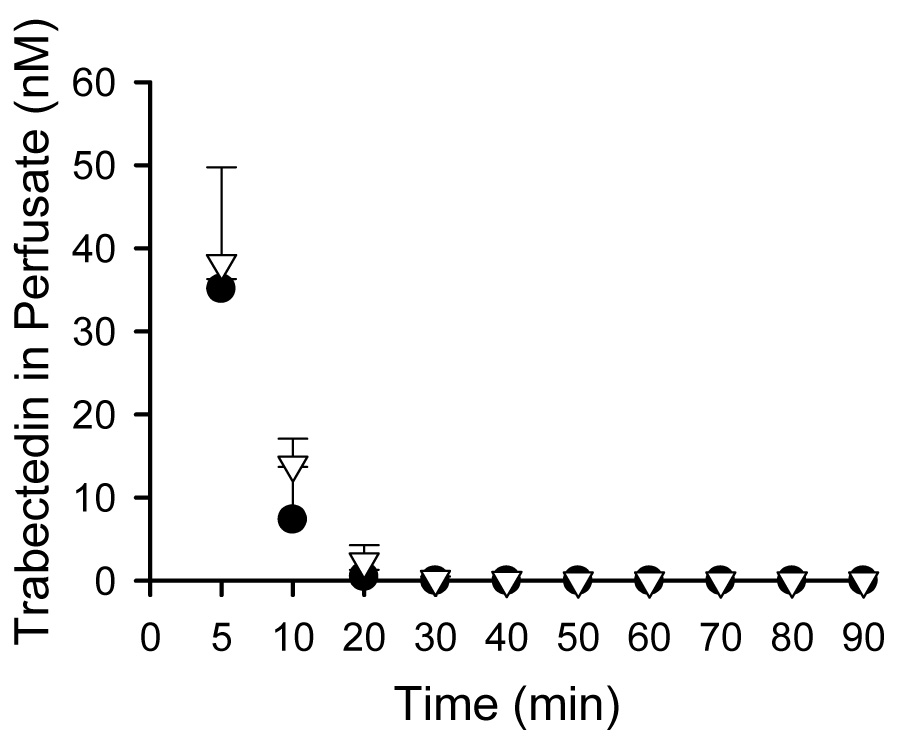
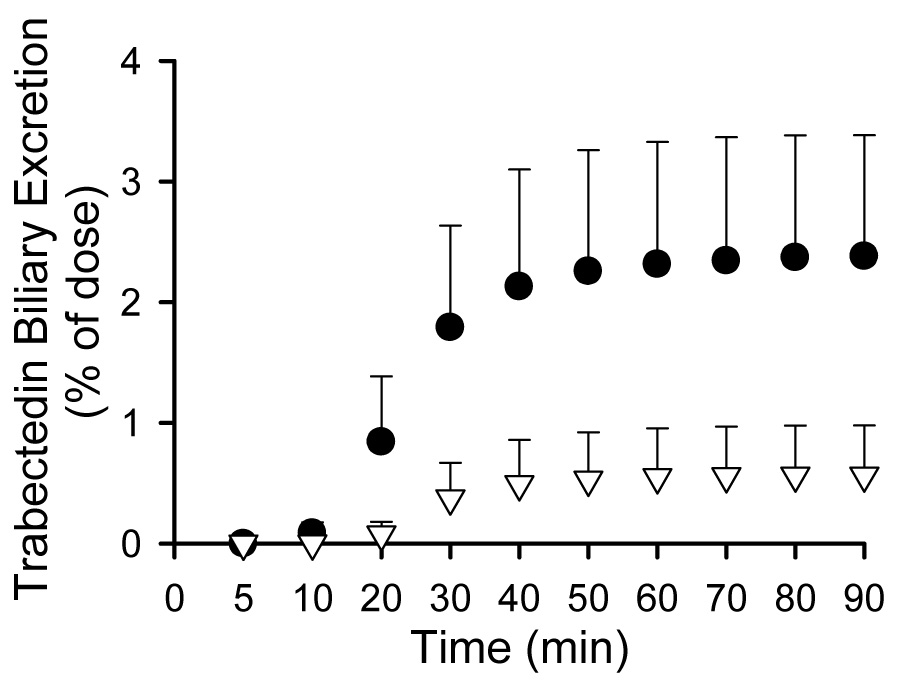
Trabectedin perfusate concentrations (Fig. 5.a) and cumulative biliary excretion (Fig. 5.b) in isolated perfused livers from wild-type (solid circle) and TR− (white triangle) rats. Data are presented as mean ± S.D., n= 3/group.
Discussion
SCRH were selected to investigate the hepato-protective effect of DEX against trabectedin-mediated cytotoxicity because they maintain metabolic enzyme activity and develop functional bile canalicular networks with proper localization of hepatic transport proteins (Liu et al., 1998; LeCluyse et al., 1999; Liu et al., 1999; Hoffmaster et al., 2004). Previous experiments in freshly isolated rat hepatocytes cultured in a conventional configuration were unable to replicate the hepato-protective effect of DEX against trabectedin-mediated cytotoxicity when rats were pretreated orally with 20 mg/kg/day of DEX 24 hr prior to hepatocyte isolation (Donald et al., 2004). When freshly isolated hepatocytes are cultured on a single collagen substratum (conventional configuration), hepatocytes do not form bile canalicular networks, do not re-establish cell polarity, and gradually lose expression of CYP enzymes (LeCluyse et al., 1996; George et al., 1997). The loss of CYP expression over time in culture of human hepatocytes varies for each isoform (George et al., 1997); mRNA levels of some P450s such as CYP1A2 and CYP2C9 did not change over time in culture whereas CYP2E1 and CYP3A4 expression declined gradually over time in culture. Therefore, induction of CYP3A protein by DEX prior to hepatocyte isolation may be lost during culture in a conventional configuration. In contrast, incubation of SCRH with additional DEX (1 µM) increased CYP3A1/2 protein levels compared to vehicle-treated SCRH (Fig. 4.) and may provide hepato-protection from trabectedin-mediated cytotoxicity.
Vectorial transport studies in polarized cell monolayers examining cellular accumulation of [14C] trabectedin in the absence and presence of P-gp inhibitors indicated that trabectedin is a substrate for P-gp (Beumer et al., 2007a). However, P-gp was only able to confer resistance to trabectedin when expressed at very high levels, suggesting that the intrinsic clearance of P-gp for trabectedin is low. Even though trabectedin seems to be more toxic than its metabolites based on previous cytotoxicity studies (Brandon et al., 2005), limited information is available on the interaction of other hepatic transport proteins with trabectedin.
The present studies are the first to demonstrate the involvement of Mrp2 in the biliary excretion of trabectedin in whole rat livers. Although the biliary excretion of unchanged trabectedin was low as a percentage of the total dose, the biliary excretion of trabectedin in IPLs from TR− rats was decreased ~75% compared to wild-type IPLs (Fig. 5.b). As shown in Fig. 4, induction of Mrp2 protein expression by additional DEX (1µM) in SCRH may provide hepato-protection from trabectedin-mediated cytotoxicity by increasing the biliary excretion of parent compound and/or derived metabolites.
Interestingly, TR− SCRH were more resistant to trabectedin-mediated cytotoxicity relative to wild-type controls, despite the increased potential for trabectedin accumulation in TR− hepatocytes due to impaired biliary excretion. This observation could be explained by modulation of the expression/activity of hepatic basolateral efflux transport proteins and metabolic enzymes in TR− rats. Mrp2-deficient rats are well-known to exhibit higher Mrp3 expression (Hirohashi et al., 1998; Ogawa et al., 2000; Johnson et al., 2006). Mrp3 mediates hepatic basolateral efflux of substrates from hepatocytes to the blood, and is thought to compensate for impaired biliary excretion of organic anions during Mrp2 deficiency (Hirohashi et al., 2000; Xiong et al., 2000). In addition, the expression and activity of several phase I and II metabolic enzymes that may be involved in trabectedin detoxification are modulated in TR− rats. For example, the total P450 content and activity of CYP1A1/2 and CYP2B1/2 were significantly higher in TR− compared to wild-type rats, although testosterone 6β-hydroxylation mediated by CYP3A1/2 was lower in TR− compared to wild-type rats (Newton et al., 2005; Silva et al., 2005). TR− rats exhibit up-regulated UGT1a (Johnson et al., 2006). Since Mrp2 plays an important role in GSH biliary excretion, impaired function of Mrp2 in TR− rats has been attributed to higher hepatocellular concentrations of GSH relative to wild-type rats (Lu et al., 1996). The important role of GSH in detoxification of the reactive metabolite of acetaminophen, N-acetyl-p-benzoquinoneimine, was demonstrated recently in TR− rats (Silva et al., 2005); TR− rats were more resistant to acetaminophen hepatotoxicity compared to wild-type rats, but this protection against acetaminophen hepatotoxicity in TR− rats was completely reversed by BSO treatment. Previous studies in cultured rat hepatocytes have documented that 200 µM BSO reduced GSH levels by 50% after 5 hr (Sinbandhit-Tricot et al., 2003). Interestingly, in the present study, pretreatment of SCRH with BSO (500 µM) for 48 hr decreased trabectedin–mediated cytotoxicity relative to vehicle-pretreated SCRH.
A novel finding in the current study was induction of Mrp4 in SCRH associated with BSO treatment. MRP4 is a basolateral protein involved in the transport of xenobiotics and endogenous substrates such as cyclic nucleotides (cAMP and cGMP) and bile acids (van Aubel et al., 2002; Reid et al., 2003; Rius et al., 2003; Tian et al., 2006). MRP4 mediates the efflux of GSH across the basolateral membrane of hepatocytes into blood by cotransport with monoanionic bile acids (Rius et al., 2003). BSO may play a role as a GSH-depleting agent not only by inhibiting synthesis, but also by enhancing hepatic efflux of GSH out of the hepatocyte by increasing the expression of Mrp4. Whether upregulation of Mrp4 by BSO is a direct effect, or an indirect response associated with oxidative stress due to GSH depletion, requires further investigation. MRP4 mRNA and protein expression were significantly up-regulated in liver samples from patients with cholestasis, indicating a potential role of MRP4 in protecting the liver by decreasing the intracellular accumulation of detergent-like bile acids (Gradhand et al., 2007). Hence, the protective role of BSO in trabectedin-mediated cytotoxicity may be due to increased efflux of trabectedin or toxic metabolites by Mrp4, although the affinity of trabectedin for Mrp4 is unknown.
In conclusion, the hepato-protective effect of DEX on trabectedin toxicity may be due to increased metabolism by CYP3A1/2 as well as enhanced biliary excretion by Mrp2. Although the interaction of trabectedin and/or derived metabolites with basolateral transport proteins such as Mrp3 and 4 remains to be elucidated, the unexpected attenuation of trabectedin toxicity in SCRH from TR− rats, and in SCRH pretreated with BSO, may be associated with increased basolateral excretion of trabectedin /metabolites by Mrp3 and/or Mrp4.
Acknowledgements
This research was funded by NIH CA106101 and GM41935. Elaine M. Leslie was the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR). The analytical expertise of Dr. Arlene Bridges is gratefully acknowledged.
Abbreviations
- ABC
ATP binding cassette
- BSO
buthionine sulfoximine
- CYP
cytochrome P450
- DEX
dexamethasone
- DMEM
Dulbecco’s modified Eagle’s medium
- EHBR
Eisai hyperbilirubinemic rats
- GSH
glutathione
- GST
glutathione-S-transferase
- LDH
lactate dehydrogenase
- MRP
multidrug resistance-associated protein
- P-gp
P-glycoprotein
- SCRH
sandwich-cultured primary rat hepatocytes
- UGT
uridine diphosphoglucuronosyl transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beumer JH, Buckle T, Ouwehand M, Franke NE, Lopez-Lazaro L, Schellens JH, Beijnen JH, van Tellingen O. Trabectedin (ET-743, Yondelis) is a substrate for P-glycoprotein, but only high expression of P-glycoprotein confers the multidrug resistance phenotype. Invest New Drugs. 2007a;25:1–7. doi: 10.1007/s10637-006-7773-9. [DOI] [PubMed] [Google Scholar]
- Beumer JH, Rademaker-Lakhai JM, Rosing H, Hillebrand MJ, Bosch TM, Lopez-Lazaro L, Schellens JH, Beijnen JH. Metabolism of trabectedin (ET-743, Yondelis) in patients with advanced cancer. Cancer Chemother Pharmacol. 2007b;59:825–837. doi: 10.1007/s00280-006-0342-2. [DOI] [PubMed] [Google Scholar]
- Beumer JH, Rademaker-Lakhai JM, Rosing H, Lopez-Lazaro L, Beijnen JH, Schellens JH. Trabectedin (Yondelis, formerly ET-743), a mass balance study in patients with advanced cancer. Invest New Drugs. 2005;23:429–436. doi: 10.1007/s10637-005-2902-4. [DOI] [PubMed] [Google Scholar]
- Blay JY, Le Cesne A, Verweij J, Scurr M, Seynaeve C, Bonvalot S, Hogendoorn P, Jimeno J, Evrard V, van Glabbeke M, Judson I. A phase II study of ET-743/trabectedin ('Yondelis') for patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2004;40:1327–1331. doi: 10.1016/j.ejca.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Brandon EF, Meijerman I, Klijn JS, den Arend D, Sparidans RW, Lazaro LL, Beijnen JH, Schellens JH. In-vitro cytotoxicity of ET-743 (Trabectedin, Yondelis), a marine anti-cancer drug, in the Hep G2 cell line: influence of cytochrome P450 and phase II inhibition, and cytochrome P450 induction. Anticancer Drugs. 2005;16:935–943. doi: 10.1097/01.cad.0000180121.16407.38. [DOI] [PubMed] [Google Scholar]
- Brandon EF, Sparidans RW, Guijt KJ, Lowenthal S, Meijerman I, Beijnen JH, Schellens JH. In vitro characterization of the human biotransformation and CYP reaction phenotype of ET-743 (Yondelis, Trabectidin), a novel marine anticancer drug. Invest New Drugs. 2006;24:3–14. doi: 10.1007/s10637-005-4538-9. [DOI] [PubMed] [Google Scholar]
- Brouwer KL, Thurman RG. Isolated perfused liver. Pharm Biotechnol. 1996;8:161–192. doi: 10.1007/978-1-4899-1863-5_10. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Delaloge S, Yovine A, Taamma A, Riofrio M, Brain E, Raymond E, Cottu P, Goldwasser F, Jimeno J, Misset JL, Marty M, Cvitkovic E. Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients--preliminary evidence of activity. J Clin Oncol. 2001;19:1248–1255. doi: 10.1200/JCO.2001.19.5.1248. [DOI] [PubMed] [Google Scholar]
- Donald S, Verschoyle RD, Greaves P, Gant TW, Colombo T, Zaffaroni M, Frapolli R, Zucchetti M, D'Incalci M, Meco D, Riccardi R, Lopez-Lazaro L, Jimeno J, Gescher AJ. Complete protection by high-dose dexamethasone against the hepatotoxicity of the novel antitumor drug yondelis (ET-743) in the rat. Cancer Res. 2003;63:5902–5908. [PubMed] [Google Scholar]
- Donald S, Verschoyle RD, Greaves P, Orr S, Jimeno J, Gescher AJ. Comparison of four modulators of drug metabolism as protectants against the hepatotoxicity of the novel antitumor drug yondelis (ET-743) in the female rat and in hepatocytes in vitro. Cancer Chemother Pharmacol. 2004;53:305–312. doi: 10.1007/s00280-003-0744-3. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- FDA. Drug induced liver toxicity. 2007
- Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001;167:83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- George J, Goodwin B, Liddle C, Tapner M, Farrell GC. Time-dependent expression of cytochrome P450 genes in primary cultures of well-differentiated human hepatocytes. J Lab Clin Med. 1997;129:638–648. doi: 10.1016/s0022-2143(97)90199-2. [DOI] [PubMed] [Google Scholar]
- Gradhand U, Lang T, Schaeffeler E, Glaeser H, Tegude H, Klein K, Fritz P, Jedlitschky G, Kroemer HK, Bachmakov I, Anwald B, Kerb R, Zanger UM, Eichelbaum M, Schwab M, Fromm MF. Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J. 2007 doi: 10.1038/sj.tpj.6500451. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- Grosso F, Dileo P, Sanfilippo R, Stacchiotti S, Bertulli R, Piovesan C, Jimeno J, D'Incalci M, Gescher A, Casali PG. Steroid premedication markedly reduces liver and bone marrow toxicity of trabectedin in advanced sarcoma. Eur J Cancer. 2006;42:1484–1490. doi: 10.1016/j.ejca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Chu XY, Tamai I, Tsuji A, Sugiyama Y. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2) J Pharmacol Exp Ther. 2000;292:265–270. [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Ito K, Ogawa K, Kume K, Shimizu T, Sugiyama Y. Hepatic expression of multidrug resistance-associated protein-like proteins maintained in eisai hyperbilirubinemic rats. Mol Pharmacol. 1998;53:1068–1075. [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3) J Biol Chem. 1999;274:15181–15185. doi: 10.1074/jbc.274.21.15181. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KL. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm Res. 2004;21:1294–1302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- Izbicka E, Lawrence R, Raymond E, Eckhardt G, Faircloth G, Jimeno J, Clark G, Von Hoff DD. In vitro antitumor activity of the novel marine agent, ecteinascidin-743 (ET-743, NSC-648766) against human tumors explanted from patients. Ann Oncol. 1998;9:981–987. doi: 10.1023/A:1008224322396. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KL. Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos. 2006;34:556–562. doi: 10.1124/dmd.105.005793. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chen ZS, Wada M, Uchiumi T, Ono M, Akiyama S, Kuwano M. Enhanced transport of anticancer agents and leukotriene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2) FEBS Lett. 1999;456:327–331. doi: 10.1016/s0014-5793(99)00979-5. [DOI] [PubMed] [Google Scholar]
- Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3) FEBS Lett. 1998;433:149–152. doi: 10.1016/s0014-5793(98)00899-0. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Laverdiere C, Kolb EA, Supko JG, Gorlick R, Meyers PA, Maki RG, Wexler L, Demetri GD, Healey JH, Huvos AG, Goorin AM, Bagatell R, Ruiz-Casado A, Guzman C, Jimeno J, Harmon D. Phase II study of ecteinascidin 743 in heavily pretreated patients with recurrent osteosarcoma. Cancer. 2003;98:832–840. doi: 10.1002/cncr.11563. [DOI] [PubMed] [Google Scholar]
- LeCluyse E, Bullock P, Madan A, Carroll K, Parkinson A. Influence of extracellular matrix overlay and medium formulation on the induction of cytochrome P-450 2B enzymes in primary cultures of rat hepatocytes. Drug Metab Dispos. 1999;27:909–915. [PubMed] [Google Scholar]
- LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. Pharm Biotechnol. 1996;8:121–159. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Watkins PB, Kim RB, Brouwer KL. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther. 2007;321:1170–1178. doi: 10.1124/jpet.106.119073. [DOI] [PubMed] [Google Scholar]
- Liu X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15:1533–1539. doi: 10.1023/a:1011994831139. [DOI] [PubMed] [Google Scholar]
- Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KL. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999;27:637–644. [PubMed] [Google Scholar]
- Lu SC, Cai J, Kuhlenkamp J, Sun WM, Takikawa H, Takenaka O, Horie T, Yi J, Kaplowitz N. Alterations in glutathione homeostasis in mutant Eisai hyperbilirubinemic rats. Hepatology. 1996;24:253–258. doi: 10.1002/hep.510240140. [DOI] [PubMed] [Google Scholar]
- Luttringer O, Theil FP, Lave T, Wernli-Kuratli K, Guentert TW, de Saizieu A. Influence of isolation procedure, extracellular matrix and dexamethasone on the regulation of membrane transporters gene expression in rat hepatocytes. Biochem Pharmacol. 2002;64:1637–1650. doi: 10.1016/s0006-2952(02)01382-5. [DOI] [PubMed] [Google Scholar]
- Newton DJ, Wang RW, Evans DC. Determination of phase I metabolic enzyme activities in liver microsomes of Mrp2 deficient TR- and EHBR rats. Life Sci. 2005;77:1106–1115. doi: 10.1016/j.lfs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Kuffel MJ, Ruben SL, Morales JJ, Rinehart KL, Squillace DP, Ames MM. Rat and human liver cytochrome P-450 isoform metabolism of ecteinascidin 743 does not predict gender-dependent toxicity in humans. Clin Cancer Res. 2002;8:2952–2962. [PubMed] [Google Scholar]
- Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38:374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- Ryan DP, Supko JG, Eder JP, Seiden MV, Demetri G, Lynch TJ, Fischman AJ, Davis J, Jimeno J, Clark JW. Phase I and pharmacokinetic study of ecteinascidin 743 administered as a 72-hour continuous intravenous infusion in patients with solid malignancies. Clin Cancer Res. 2001;7:231–242. [PubMed] [Google Scholar]
- Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2) J Biol Chem. 2002;277:6497–6503. doi: 10.1074/jbc.M109081200. [DOI] [PubMed] [Google Scholar]
- Silva VM, Thibodeau MS, Chen C, Manautou JE. Transport deficient (TR-) hyperbilirubinemic rats are resistant to acetaminophen hepatotoxicity. Biochem Pharmacol. 2005;70:1832–1839. doi: 10.1016/j.bcp.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Sinbandhit-Tricot S, Cillard J, Chevanne M, Morel I, Cillard P, Sergent O. Glutathione depletion increases nitric oxide-induced oxidative stress in primary rat hepatocyte cultures: involvement of low-molecular-weight iron. Free Radic Biol Med. 2003;34:1283–1294. doi: 10.1016/s0891-5849(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Taamma A, Misset JL, Riofrio M, Guzman C, Brain E, Lopez Lazaro L, Rosing H, Jimeno JM, Cvitkovic E. Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumors. J Clin Oncol. 2001;19:1256–1265. doi: 10.1200/JCO.2001.19.5.1256. [DOI] [PubMed] [Google Scholar]
- Tian Q, Zhang J, Chan SY, Tan TM, Duan W, Huang M, Zhu YZ, Chan E, Yu Q, Nie YQ, Ho PC, Li Q, Ng KY, Yang HY, Wei H, Bian JS, Zhou SF. Topotecan is a substrate for multidrug resistance associated protein 4. Curr Drug Metab. 2006;7:105–118. doi: 10.2174/138920006774832550. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Meier PJ, Brouwer KL. Effect of dexamethasone treatment on the expression and function of transport proteins in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2004;32:834–839. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- Westley IS, Brogan LR, Morris RG, Evans AM, Sallustio BC. Role of Mrp2 in the hepatic disposition of mycophenolic acid and its glucuronide metabolites: effect of cyclosporine. Drug Metab Dispos. 2006;34:261–266. doi: 10.1124/dmd.105.006122. [DOI] [PubMed] [Google Scholar]
- Xiong H, Turner KC, Ward ES, Jansen PL, Brouwer KL. Altered hepatobiliary disposition of acetaminophen glucuronide in isolated perfused livers from multidrug resistance-associated protein 2-deficient TR(-) rats. J Pharmacol Exp Ther. 2000;295:512–518. [PubMed] [Google Scholar]
- Yovine A, Riofrio M, Blay JY, Brain E, Alexandre J, Kahatt C, Taamma A, Jimeno J, Martin C, Salhi Y, Cvitkovic E, Misset JL. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KL. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther. 2006;319:459–467. doi: 10.1124/jpet.106.101840. [DOI] [PubMed] [Google Scholar]
- Zelek L, Yovine A, Brain E, Turpin F, Taamma A, Riofrio M, Spielmann M, Jimeno J, Misset JL. A phase II study of Yondelis (trabectedin, ET-743) as a 24-h continuous intravenous infusion in pretreated advanced breast cancer. Br J Cancer. 2006;94:1610–1614. doi: 10.1038/sj.bjc.6603142. [DOI] [PMC free article] [PubMed] [Google Scholar]


