Abstract
Steroid hormones have pervasive functional effects. Although steroids are generally known to have actions via binding to their cognate steroid receptors, it is becoming more clear that steroids can have non-traditional actions that do not require activation of cognate steroid receptors. We have found that progestogen-facilitated lordosis of rodents is enhanced by activation of dopamine type 1 (D1) or GABAA receptors and their downstream effectors, such as second messengers, in the ventral tegmental area (VTA). The role of phospholipase C in these effects is not clear. If progestins’ actions through D1 and GABAA receptors in the VTA are mediated through PLC, then inhibiting PLC formation in the VTA, via infusions of U73122 (400 nM/side), should reduce progestin (5α-pregnan-3α -ol-20-one; 3α,5α -THP; 100 or 200 ng/side)-facilitated lordosis and its enhancement by D1 (SKF38393; 100 ng/side) or GABAA (muscimol; 100 ng/side) receptor agonists in ovariectomized, estradiol-primed rats. We found that 3α, 5α -THP-, SKF38393-, and muscimol-facilitated lordosis was attenuated by infusions of the PLC inhibitor, U73122, but not vehicle, to the VTA. Thus, progestogens’ non-traditional actions in the VTA to enhance lordosis through D1 and/or GABAA include activity of PLC.
Keywords: neurosteroids, GABAA, dopamine type 1 receptors, cAMP, protein kinase C, 5α-pregnan-3α-ol-20-one
1. Introduction
Steroid hormones can have profound, potent, and diverse actions to modulate physiology and behavior. Among the more dramatic effects of steroid hormones are their facilitatory effects on reproductive behaviors of male and female rodents. Actions of steroid hormones at cognate intracellular steroid receptors can account in part for this regulation of mating behavior. However, evidence is emerging that steroids may have actions at non-traditional targets, such as membrane steroid receptors and/or neurotransmitter targets ([Bryant et al., 2006], [Rønnekleiv and Kelly, 2005] and [Zhu et al., 2003a,b]). By examining steroid-mediated mating behavior, the neural substrates and mechanisms underlying some functionally-relevant actions of steroids can be elucidated. For instance, sexual behavior of male rodents is regulated by androgens and their actions at cognate androgen receptors, glutamatergic and dopaminergic targets (Hull and Dominquez, 2006). In female rodents, progesterone (P) initiates sexual receptivity (i.e. lordosis) of estrogen (E2)-primed rodents in part through actions at cognate intracellular progestin receptors (PRs) in the ventromedial hypothalamus (VMH) (Frye, 2001). However, in the midbrain ventral tegmental area (VTA), P modulates the magnitude and duration of lordosis indirectly through “non-genomic” rapid, membrane-mediated actions ([Frye, 2001] and [Frye et al., 1992]). There is data from clinical and basic research studies to support that hormone-replacement therapies can have beneficial effects on brain function, such as reducing potential for dementia or Alzheimer’s Disease and improving mood (Frye, 2008). However, as was demonstrated in the publication of some of the Women’s Health Initiative Studies, estrogen and progesterone replacement therapies may not be beneficial for all women, and may even pose a risk for some women’s health (Sherwin, 2007). More information about the mechanisms of steroid hormones and their metabolic products for their functional effects is needed to begin to address some of the inconsistencies in the effects of hormone-based treatments on brain health of people. The well-characterized neural circuitry of, and hormonal requirements for, lordosis, have enabled us to use lordosis as a bioassay to investigate progestogens’ nontraditional mechanisms in the VTA (Frye and Walf, 2008). We have then been able to start applying this approach using other behavioral endpoints to investigate the mechanisms of progestogens or other steroids, such as estradiol or 3α-androstanediol, that have non-traditional actions in other brain regions, such as the hippocampus or cortex, for learning and memory, affective behavior, and/or neuroprotection ([Frye, 2007], [Frye et al., 2007], and [Walf and Frye, 2008]).
Using lordosis as an endpoint, we have examined progestogens’ non-traditional actions in the VTA. 5α -pregnan-3α -ol-20-one (3α,5α -THP) in the VTA is formed from metabolism of P or de novo synthesis and has subsequent actions via γ-aminobutryic acid type A (GABAA) and/or dopamine type 1-like (D1) receptors, but not PRs, to mediate lordosis of hamsters and rats (Frye et al., 2006). Given that 3α,5α -THP has rapid and membrane receptor mediated actions in the VTA, we have begun to investigate whether second messenger, signal transduction pathways are involved. This is a novel question because actions of protein hormones, rather than steroid hormones, are typically attributed to membrane targets and downstream second messenger events (Kow et al., 1994). However, progestogens can have actions through D1 receptors, which are well known G-protein coupled receptors (Girault and Greengard, 2004). As well, accumulating evidence suggest that some of progestogens’ agonist-like actions at GABAA receptors may also be G-protein mediated. In hypothalamic or hippocampal slice preparations, inhibiting G-proteins reduces 3α,5α -THP-mediated increases in GABAA receptor-induced inhibitory post-synaptic potentials ([Fancsik et al., 2000] and [Lui et al., 2002]). Blocking actions of G-proteins, adenosine 3’,5’ monophosphase (cAMP), and protein kinase A inhibit lordosis of E2- and progestogen-primed rats ([Beyer et al., 1981], [Frye and Walf, 2007] and [Uphouse et al., 2000]). Moreover, infusions to the VTA of the D1 antagonist, SCH23390, attenuate lordosis, and these effects are reversed by subsequent infusions of the cAMP analogue, 8-bromo-cAMP (Petralia and Frye, 2006). Although these data support the notion that progestogens may have novel actions for lordosis in the VTA through the adenylyl cyclase second-messenger system, given their ubiquitous nature, the role of other signaling pathways is of interest. The phosphoinositide pathway may also be a target for actions of progestogens in the VTA, but this has received far less attention than adenylyl cyclase. Hormones and neurotransmitters can activate phospholipase C (PLC) and, subsequently initiate the hydrolysis of phosphotidylinositol into inositol triphosphate and diacylglycerol, mobilize intracellular calcium, and PLC-dependent protein kinase C (PKC). Indeed, G-protein-dependent PLC can confer sensitivity to cAMP (Majerus et al, 1990) and, as described above, and elsewhere (Liu and Simon, 1996), progestogens’ actions can involve cAMP. Lordosis of E2- and P-primed hamsters, which was enhanced by co-administration of D1 or GABAA receptor agonists, can be terminated with infusions of a PLC or PKC inhibitor to the VTA, but not missed sites (Frye and Walf, 2007). Whether these effects are dependent upon actions of 3α,5α -THP in the VTA are of interest and have not been determined. Unlike hamsters that require actions of progestogens in both the VTA and VMH, rats exhibit sexual receptivity following E2-priming alone, and these responses are enhanced with subsequent administration of progestogens (P or 3α,5α -THP) to the VTA (Frye et al., 2006). As such, we tested the hypothesis that: if progestogens’ actions in the VTA require PLC, then a PLC inhibitor to the VTA will attenuate 3α,5α -THP- or 3α,5α -THP plus D1- or GABAA- (but not E2) -mediated increases in lordosis of ovariectomized, E2-primed rats.
2. Results
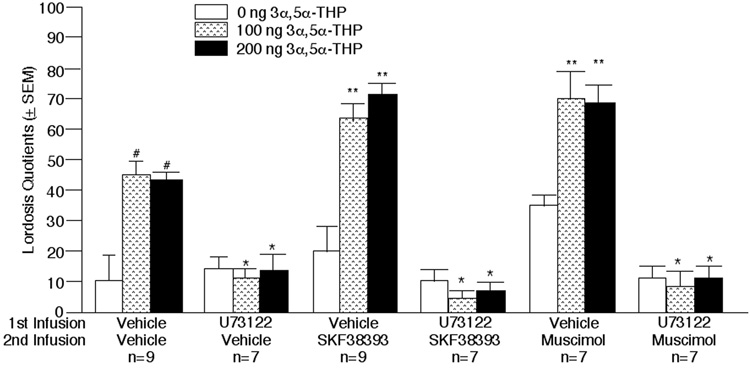
Infusions of U73122 to the VTA significantly decreased lordosis quotients (F1,80 = 48.04, P<0.01) compared to vehicle. Infusions of 3α,5α -THP (100 or 200 ng; F2,80 = 443.39, P<0.01), or the agonists SKF38393 or muscimol (F2,80 = 5.10, P≤0.01), to the VTA significantly increased lordosis quotients compared to vehicle. There was a significant interaction between these variables (F4,80 = 3.84, P<0.01). As has previously been demonstrated, SKF38393 or muscimol infusions enhanced the subsequent effect of intra-VTA administration of 3α,5α -THP to facilitate lordosis. However, U73122 blocked the subsequent effects of 3α,5α -THP, SKF38393 and/or muscimol to facilitate lordosis (Figure 1). There were no differences between groups in baseline motor activity during the pre-test, when rats were E2-primed only (data not shown). No effects of drug infusions were observed in the motor behavior of rats when they were tested in the activity monitor 60 minutes following 3α,5α -THP or vehicle infusions (Table 1).
Figure 1.
In the VTA, SKF38393- or muscimol-mediated increases in 3α,5α -THP-facilitated lordosis quotients (LQs) of estradiol-primed rats are blocked by pre-treatment with the PLC inhibitor, U73122. # indicates a significant (P < 0.05) effect of 3α,5α -THP vs. vehicle infusions to enhance lordosis. * indicates a significant (P < 0.05) decrement produced by U73122 vs. vehicle infusions at that same 3α,5α -THP dosage (p<0.05). ** indicates a significant (P < 0.05) increase in 3α,5α -THP-facilitated lordosis following SKF38393 or muscimol compared to vehicle infusions (p<0.05).
Table 1.
Total number of beam breaks made in the activity monitor (± SEM) of rats that received drug infusions to the VTA. Motor behavior data was collected in rats after they were tested for lordosis 60 minutes following 3α,5α-THP infusions.
| First Infusion | Second Infusion | Total number of Beam Breaks | |||
|---|---|---|---|---|---|
| n | Infusions of 3α,5α-THP (ng) | ||||
| 0 | 100 | 200 | |||
| Vehicle | Vehicle | 9 | 702 ±81 | 673 ±75 | 868 ±157 |
| SKF38393 | 9 | 703 ±89 | 601 ±111 | 975 ±226 | |
| Muscimol | 7 | 663±140 | 678 ± 43 | 706 ±182 | |
| U73122 | Vehicle | 7 | 842 ±107 | 645 ±131 | 953 ±90 |
| SKF38393 | 7 | 910 ±162 | 730 ±108 | 830 ±76 | |
| Muscimol | 7 | 825 ±134 | 788 ±106 | 855 ±83 | |
3. Discussion
These findings support our hypothesis that, in the VTA, D1- and/or GABAA-receptor mediated increases in 3α,5α -THP-facilitated lordosis involve PLC. Intra-VTA infusions of the PLC inhibitor, U73122, prevented the lordosis-facilitating effects of subsequent infusions of SKF38393, muscimol, and/or 3α,5α -THP in E2-primed rats, but had no effect on motor behavior, suggesting that the effects of U73122 for lordosis were not secondary to general effects on arousal/motor behavior. Thus, inhibiting PLC in the VTA prevents 3α,5α -THP’s actions for lordosis and attenuates D1- or GBR-mediated increases in progestogen-facilitated lordosis.
The present findings confirm and extend the notion that progestogens’ actions in the VTA for lordosis are mediated, in part, by activation of D1 and/or GABAA receptors and subsequent initiation of second messenger signaling. Infusions of SKF38393 or muscimol to the VTA (but not substantia nigra) enhanced progestogen-facilitated lordosis of rodents in this and previous studies ([Frye et al., 2006] and [Petralia and Frye, 2006]). Interfering with factors downstream of D1 and/or GABAA receptors by blocking actions of G-proteins, adenylyl cyclase, or PKA attenuates sexual behavior facilitated by progestogens, D1 and/or GABAA receptors. In the present study, we see that inhibiting PLC in the VTA attenuates progestogen-, SKF38393-, and muscimol- facilitated lordosis ([Frye and Walf, 2007] and [Petralia and Frye, 2006]). In addition to blocking the facilitative effects of SKF38393 or muscimol on progestogen-mediated lordosis of rats, U73122 to the VTA attenuated 3α,5α -THP-mediated lordosis of rats. Notably, there was no effect of U73122 on lordosis of rats primed with E2 alone, which suggests that the effects observed are progestogen-dependent. However, the lack of effect of U73122 to inhibit E2- facilitated lordosis could have been due in part to a basement effect, which could be addressed systematically in future investigations. Together, these findings confirm that P’s actions in the VTA to facilitate lordosis occur following its conversion to 3α,5α -THP. Moreover, these findings imply that actions of progestogens outside of the VTA may not compensate for the loss of PLC activity within the VTA. Thus, blocking PLC appears to affect the actions of progestogens, which are necessary for D1 and/or GABAA agonists to have their augmenting behavioral effects.
PLC has many downstream effects and how each of these intracellular events alters progestogen-facilitated lordosis has not been fully elucidated ([Kow et al., 1994] and [Mobbs et al., 1991]), but there are some intriguing possibilities that have emerged from related model systems. First, PLC may have some of its effects by altering progestogen production. Steroidogenesis is modulated by the PLC pathway in a tissue-specific manner. Although it is possible that our manipulations of PLC altered neurosteroidogenesis in the midbrain and, thereby, behavior, this seems unlikely given that 3α,5α -THP administration did not reverse attenuation of lordosis by prior infusions of U73122. Second, it may be that some of the effects of progestogens via PLC involve downstream actions via protein kinases. In general, the present findings are consistent with the results presented by Gonzalez-Flores and colleagues which showed that 3α,5α -THP stimulated lordosis involved the protein kinase C system. Indeed, the elegant work of Gonzalez-Flores and colleagues (2006) demonstrated that P may have some of its actions to facilitate lordosis through protein kinase A, whereas 3α,5α -THP may have some of its effects through protein kinase C. As such, the PLC system may represent an important area of cross-talk for how P and 3α,5α -THP exert their respective genomic and non-genomic actions (Frye and Petralia, 2003). Third, Sinchak, Micevych and colleagues (2007) have shown that progestogens may have effects via mu-opioid receptor or opioid-like receptor circuits to regulate sexual receptivity of rats. P administration or biosynthesis can block E2-induced endogenous opiate release, which may relieve E2 inhibition, and, thereby, facilitate lordosis. Indeed, µ- opioid receptors are G-protein coupled receptors that are internalized after endogenous opiate binding. Many cells in the VTA are sensitive and/or responsive to opiates. As such, the involvement of peptidergic signaling in mediating progestogen-facilitated lordosis, is the subject of ongoing investigation.
In summary, the present findings include that prior treatment with U73122, a PLC inhibitor, to the VTA attenuated the subsequent facilitatory response to 3α,5α -THP, a D1 agonist, SKF38393, or a GABAA receptor agonist, muscimol. These findings suggest that progestogen-facilitation of lordosis involves PLC in the midbrain VTA. Understanding the signaling mechanisms through which progestogens act to regulate E2-primed behavioral neurocircuits is an important question in the field. Lordosis may be a particularly sensitive behavioral assay to address how steroids exert their effects via cross-talk between genomic and non-genomic actions to initiate transcriptional factors required for steroid regulation of gene expression and, thereby, function (Frye and Petralia, 2003). Like 3α,5α -THP (Frye, 2008), signal transduction pathways may be targets to consider in the etiology and/or treatment of neuropsychiatric conditions, such as anxiety, depression, and bipolar disorder ([Akin et al., 2005], [Dwivedi et al., 2002], [Marazziti et al., 2001], and Pandey et al., 2002]). For example, acute treatment with the selective serotonin reuptake inhibitor, fluoxetine, downregulates PKC and other kinases in whole rat brain with chronic, but not acute treatment (Rausch et al., 2002). In depressed people, the therapeutic effects of fluoxetine treatment are not typically observed for several weeks after their initiation. An intriguing question that needs to be investigated is whether changes in these signal transduction pathways may be related to therapeutic efficacy. As such, further investigation of how processes downstream of PLC may mitigate progestogen-facilitated lordosis and other behavioral processes in animal models, is warranted.
4. Experimental Protocol
4.1. Animal subjects
Experimental rats were bred and raised in The University at Albany Laboratory Animal Care Facility in the Social Sciences Building. Female Long-Evans rats (N=54), were group-housed (4–5 per cage), had unlimited access to rodent chow and water in their home cages. Animals were housed on reversed-light cycles (12:12 hrs, with lights off between 08:00 and 20:00 hrs) and were tested in the dark-phase of the cycle. All procedures utilized in the present study were pre-approved by The University at Albany Institutional Animal Care and Use Committee and comply with The NIH Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985).
4.2. Surgery
Rats were ovariectomized and received stereotaxic implantation of guide cannulae (modified 23-gauge thin-walled stainless steel needles with 30-gauge removable inserts) aimed at the VTA (from bregma, AP=−5.3, ML=±0.4, DV=−7.0; Paxinos and Watson, 1986) under xylazine (12 mg/kg IP Bayer Corp., Shawnee Mission, KS) and ketamine hydrochloride (80 mg/kg, IP; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia. All rats in the present study demonstrated full recovery from surgery and testing began one week after surgery.
4.3. Hormone-priming
Rats were subcutaneously administered 17β-estradiol (10 µg/ 0.2 ml in corn oil vehicle; Steraloids) at hr 0. At hour 44, rats were pre-tested for motor behavior and lordosis before receiving drug infusions. After pre-testing, rats were bilaterally infused with U73122 (400 nM/µl; a PLC inhibitor (Frye and Walf, 2007) or saline vehicle and were re-tested for lordosis immediately afterward. After the post infusion 1 test, rats were infused with SKF38393 (100 ng/µl, a D1 agonist), muscimol (100 ng/µl, a GABAA receptor agonist), or saline vehicle (Petralia and Frye, 2006). Immediately after infusions, rats were re-tested for lordosis and then infused with 3α,5α -THP or β-cyclodextrin saline vehicle. Rats were re-tested for lordosis 10 and 60 mins later and motor behavior after the last test for lordosis. All rats were randomly assigned to one of six infusion conditions that were administered once a week for 3 weeks. Rats received an initial infusion of U73122 or vehicle, followed by a second infusion of SKF38393, muscimol, or vehicle. Rats were tested once a week for 3 weeks so that effects of the other two infusions could be examined at each 3α,5α -THP dosage (0, 100 or 200 ng/side). The order in which 3α,5α -THP infusions were administered was counterbalanced to prevent order effects. All pharmacological agents (obtained from Sigma Chemical Company, St. Louis, MO) were administered in 1 µl to each side. The multiple infusions over weeks did not appear to adversely affect the integrity of the infused tissue. In support, subjects were in the same inhibitor and agonist conditions throughout the experiment and there were no differences in lordosis of rats immediately following inhibitor or agonist drug infusions after the first or third week for these subjects. Whether subjects were infused with vehicle, or 100 or 200 ng 3α,5α -THP, was counterbalanced across the three weeks of behavioral testing. We could then determine if rats in the same inhibitor and agonist condition had similar behavior, irrespective of being infused with one dosage of 3α,5α -THP on the first, second, or third week, and no differences were observed based upon the order that rats received a particular dosage of 3α,5α -THP.
4.4. Behavioral testing
Rats were vaginally-masked to minimize mating-induced changes in sexual behavior, and 3α,5α -THP levels in the VTA, and placed in a 50 × 25 × 30 cm mating chamber with a sexually-vigorous male. For 10 minutes or ten mounts (whichever occurred first), the percentage of times female rats exhibited lordosis in response to mounting by a male (lordosis quotients; LQs) were monitored. Before the first lordosis test, and after the last lordosis task, rats were placed in a 39 × 39 × 30 cm Digiscan Optical Animal Activity Monitor (Accuscan Instruments Inc., Columbus, OH) for 5 mins to assess general motor behavior.
4.5. Euthanasia, tissue collection, and site analyses
At the end of the experiment, rats were deeply anesthetized with sodium pentobarbital (150 mg/kg or to effect; IP) and were ex-sanguinated with 0.9% saline. This was immediately followed by intra-cardiac perfusion with 10% formalin. Brains were fixed in 10% formalin, followed by 30% sucrose-saline, and then sliced on a cryostat at 40 µm. Infusion location was determined by a researcher who was blind to the experimental condition and behavioral data of each subject. This was accomplished by examination using light microscopy of cresyl violet-stained brain slices at the level of the VTA. Data from 8 rats that received infusions to the substantia nigra, rather than the VTA, were excluded from statistical analyses (data not shown).
4.6. Statistical Analyses
Lordosis quotients and number of beam breaks in the activity monitor at the final test time were analyzed with three-way ANOVAs. There were two between factors: one was U73122 or vehicle condition and the other was agonist infusion condition (SKF38393, muscimol or vehicle). The within-variable was the 3α,5α -THP concentration (0, 100, or 200 ng) variables. Group differences were determined with Fisher PLSD comparisons when significant main or interactive effects were found (p≤0.05).
Acknowledgments
A grant from the National Science Foundation (03-16083) and National Institute of Mental Health (MH06769801) supported research conducted. The authors appreciate the technical assistance provided by Stephanie Youmans in these studies.
Abbreviations
- 3α,5α-THP
5α-pregnan-3α -ol-20-one
- D1
dopamine type 1-like
- E2
estradiol
- GABAA
γ-aminobutryic acid type A
- LQs
lordosis quotients
- PLC
phospholipase C
- P
progesterone
- PRs
progestin receptors
- PKC
protein kinase C
- VTA
ventral tegmental area
- VMH
ventral medial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beyer C, Canchola E, Larsson K. Facilitation of lordosis behavior in the ovariectomised estrogen-primed rat by dibutyryl cAMP. Physiol. Behav. 1981;26:249–251. doi: 10.1016/0031-9384(81)90019-6. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [(3) H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am. J. Psych. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J. Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res. Brain Res. Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5α- reduced metabolite 3α-androstanediol. Pharmacol. Biochem. Behav. 2007;86:354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. In: Neurosteroids-From Basic Research to Clinical Perspectives. Rubin Robert T, Pfaff Donald W., editors. 2008. [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM. Progestins have actions through GABAA receptors. In: Watson CS, editor. The Identities of Membrane Steroid Receptors: And Other Proteins Mediating Nongenomic Steroid Action. Boston, MA: Kluwer Academic Publishers; 2003. [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α- hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social sexual and affective behaviors. Neurosci. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. In the ventral tegmental area, progestins’ membrane-mediated actions for lordosis of hormone-primed hamsters involve phospholipase C and protein kinase C. J. Neuroendo. 2007;19:717–724. doi: 10.1111/j.1365-2826.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch. Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Ramirez-Orduna JM, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C. Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomised estrogen-primed rats. Horm Behav. 2006;49:398–404. doi: 10.1016/j.yhbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126(1):66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Kow LM, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci. Biobehav. Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Lui QY, Chang YH, Schaffner AE, Smith SV, Barker JL. Allopregnanolone activates GABAA receptor/Cl− channels in a multiphasic manner in embryonic rat hippocampal neurones. J. Neurophysiol. 2002;88:1147–1158. doi: 10.1152/jn.00942.2001. [DOI] [PubMed] [Google Scholar]
- Liu M, Simon MI. Regulation by cAMP-dependent protein kinase of a G-protein-mediated phospholipase C. Nature. 1996;382:83–87. doi: 10.1038/382083a0. [DOI] [PubMed] [Google Scholar]
- Majerus PW, Ross TS, Cunningham TW, Caldwell KK, Jefferson AB, Bansal VS. Recent insights in phosphatidylinositol signaling. Cell. 1990;63:459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Perez J, Cassano GB. Is obsessive-compulsive disorder caused by a second-messenger imbalance? CNS Spectrums. 2001;6:206–209. doi: 10.1017/s1092852900008579. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Kaplitt M, Kow LM, Pfaff DW. PLC-α: a common mediator of the action of estrogen and other hormones? Mol. Cell. Endocrinol. 1991;80:C187–C191. doi: 10.1016/0303-7207(91)90136-g. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Sridhararao J, Ren X, Janicak PG, Sharma R. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology. 2002;26:216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. New York: Academic Press; 1986. [Google Scholar]
- Petralia SM, Frye CA. In the ventral tegmental area, G-proteins mediate progesterone's actions at dopamine type 1 receptors for lordosis of rats and hamsters. Psychopharmacol. 2006;186:133–142. doi: 10.1007/s00213-006-0311-9. [DOI] [PubMed] [Google Scholar]
- Rausch JL, Gillespie CF, Fei Y, Hobby HM, Stoming T, Ganapathy V, Leibach FH. Antidepressant effects on kinase gene expression patterns in rat brain. Neurosci. Lett. 2002;334:91–94. doi: 10.1016/s0304-3940(02)01106-0. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J. Steroid Biochem. Mol. Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm. Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphouse L, Maswood S, Jackson A. Factors elevating cAMP attenuate the effects of 8- OH-DPAT on lordosis behavior. Pharmacol. Biochem. Behav. 2000;66:383–388. doi: 10.1016/s0091-3057(00)00179-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor β mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]