Abstract
The progesterone (P4) metabolite and neurosteroid, 5α-pregnan-3α-ol-20-one (3α,5α-THP) acts in the midbrain ventral tegmental area (VTA) to modulate lordosis of female rats. 3α,5α-THP also mediates exploratory, affective, and social behaviors; whether actions of 3α,5α-THP in the VTA mediate these behaviors is of interest. To elucidate the role of the VTA in mediating exploratory, affective, and social behaviors, the present study examined effects of inhibiting 3α,5α-THP formation in the VTA. Rats received intra-VTA infusions of either PK11195 (400 ng/μl, which inhibits de novo 3α,5α-THP production), indomethacin (10 μg/μl, which blocks metabolism of P4 to 3α,5α-THP), PK11195 and indomethacin together, or β-cyclodextrin vehicle and tested on a battery of anxiety (open field and elevated plus maze), social (partner preference and social interaction), and sexual (paced mating) tasks. Compared to rats infused with vehicle to the VTA, rats infused with inhibitor(s) demonstrated significant reductions in central entries in the open field, time on open arms of an elevated plus maze, time spent interacting with a conspecific, initiation and intensity of lordosis, sexual solicitations, and midbrain 3α,5α-THP levels. These findings suggest that actions of 3α,5α-THP in the VTA are important for mediating aspects of exploration, anxiety, and social behavior related to mating.
Keywords: Neurosteroid, Biosynthesis, Non-genomic , GABAA receptors, Homeostasis
1. Introduction
The ovarian hormones 17β-estradiol (E2) and progesterone (P4) have diverse functional effects via classic actions at cognate steroid receptors and/or other non-traditional mechanisms to modulate female sexual behavior. E2 and P4 initiate sexual behavior of female rodents through actions at intracellular progestin receptors in the ventromedial hypothalamus (VMH). In the midbrain ventral tegmental area (VTA), progestins have actions to mediate the intensity and duration of lordosis (the stereotypic behavior female rodents engage in when sexually receptive) that occur independent of the few cognate intracellular progestin receptors in the VTA [37]. The primary actions of P4 in the VTA to mediate the intensity and duration of sexual responsiveness of rodents involve formation of 5α-pregnan-3α-ol-20-one (3α,5α-THP), a P4 metabolite and neurosteroid, and its subsequent actions at GABAA, NMDA, and/or dopamine-like type 1 receptors, and their downstream signal transduction processes [32,33,39,41,76]. These data support a diverse role of E2 and P4 in the VMH and VTA to modulate sexual behavior via actions at intracellular and membrane receptors, respectively. However, there are other factors that influence sexual behavior.
Female sexual behavior requires receiving and integrating a number of sensory stimuli. In order for successful mating to occur, aggressive and non-social behaviors normally exhibited by female rodents must be dampened. 3α,5α-THP has been shown to mediate these behaviors. Rats in behavioral estrus, which have elevated 3α,5α-THP concentrations, demonstrate more exploration, anti-anxiety, and pro-social behaviors compared to rats in diestrous, with lower 3α,5α-THP levels [34,52,82]. Removal of the ovaries, the primary source of E2 and progestins, decreases exploration, anti-anxiety, and pro-social behaviors of female rats and administration of 3α,5α-THP re-instates these behaviors akin to that of rats in behavioral estrus [36]. Thus, 3α,5α-THP plays a role in modulating responses to a suite of incoming sensory cues important for mating but the mechanisms by which these actions occur are still being elucidated.
Evidence suggests that activation of the mesolimbic dopamine system appears to play a particularly important role in mating. First, the midbrain VTA is characterized by the presence of dopamine cell bodies that project to regions important for reward and 3α,5α-THP infusion to the VTA, but not surrounding regions, facilitates consummatory (lordosis) and appetitive (exploratory, anti-anxiety, and social) aspects of sexual behavior among E2-primed ovariectomized rats [36,37,45,77]. Second, lesions caused by ablation, electrical stimulation, or 6-hydroxydopamine (which selectively kills dopaminergic and noradrenergic neurons) to the midbrain VTA of rats or hamsters, disrupts mating and/or maternal behavior [5,12,13,50,58,71,72,80]. Third, physiological dosing of P4 with E2 to ovariectomized rats enhances central dopamine levels [8,54]. Fourth, engaging in sexual behavior or exposure to mating-relevant cues produces increases in dopaminergic activity in the nucleus accumbens and the ventral striatum of male and female rodents [3,56]. Fifth, increasing dopaminergic activity with D-amphetamine or amfonelic acid enhances mating behavior of male rats [2]. Lastly, blocking actions at D1/D2 receptors with flupenthixol reduces sexual behavior of male rats and rabbits [1,2]. Together, these data suggest that the mesolimbic dopamine system is sensitive to, and influences, sexual behavior.
We have previously observed that inhibiting 3α,5α-THP’s actions at GABAA or dopamine-like type 1 receptors attenuates lordosis [37,39], but the role of 3α,5α-THP in the midbrain VTA for other reproductively relevant behaviors is not well-understood. The present experiments were designed to elucidate the role of 3α,5α-THP in the VTA in mediating behavioral responses to sensory stimuli associated with reproductively relevant processes. We hypothesized that if actions of 3α,5α-THP in the VTA are necessary for appropriate behavioral responses associated with mating (exploratory, anti-anxiety, and pro-social behaviors), then blocking formation of 3α,5α-THP in the VTA should attenuate these behaviors in a manner similar to that seen with sexual behaviors.
2. Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY.
2.1. Animals and housing
Adult, intact female Long–Evans rats (N = 41) were obtained from the breeding colony of the Life Sciences Research Laboratory Animal Care Facility at The University at Albany-SUNY (original stock Charles River, Raleigh, NC). Rats were group-housed in a temperature- and humidity-controlled room on a reverse light cycle (lights off at 8:00 a.m.) with ad libitium access to water and rat chow in their cages.
2.2. Surgery
Rats were stereotaxically implanted with bilateral guide cannulae aimed at the medial aspect of the VTA (from bregma: AP = −5.3, ML = ± 0.4, DV = −7.0) [61] under xylazine (12 mg/kg) and ketamine (60 mg/kg) anesthesia. Guide cannulae consisted of modified 23-gauge, thin-wall stainless steel needles with 30-gauge, removable inserts. Following surgery, rats were monitored for loss of weight, righting response, flank stimulation response, and/or muscle tone [53]. Eight rats that failed these assessments were killed immediately and were excluded from analyses.
2.3. Determination of sexual receptivity
Daily (between 10:00 and 11:00 a.m.), females were vaginally masked and paired briefly with a stimulus male (that was conditioned to show consistent, high levels of sexual contact). Sexual receptivity was determined by the response of experimental females to stimulus male investigation. Rats that demonstrated receptive (lordosis) and proceptive behaviors (hopping, darting, and ear wiggling) were considered to be in behavioral estrus, while those that exhibited aggressive behaviors (vocalizing, defensive posturing, boxing, and avoidance) were considered not in behavioral estrus. Vaginal cytology was not used to determine estrous cycle phase because vaginal–cervical stimulation that occurs during sample collection could have altered subsequent behavioral responses.
2.4. Central manipulations
Immediately following determination of sexual-receptivity rats received randomly assigned central infusions. Formation of 3α,5α-THP was inhibited with infusions of either PK11195 (400 ng/μl, Alexis Biomedicals Inc., San Diego, CA), a peripheral-type benzodiazepine receptor partial agonist, which attenuates 3α,5α-THP biosynthesis, or indomethacin (10 μg/μl, Sigma Chemical Co., St. Louis, MO), a 3α-hydroxysteroid dehydrogenase inhibitor, which blocks P4’s metabolism to 3α,5α-THP. Each of these inhibitor regimen have been shown previously to attenuate lordosis and/or anti-anxiety behavior, and decrease midbrain 3α,5α-THP levels [7,40,70].
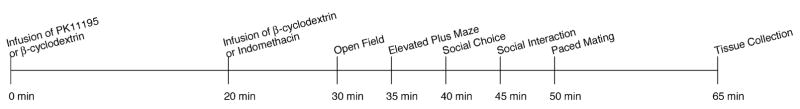
Rats were randomly assigned to one of four conditions and tested throughout the behavioral battery described below. One group of rats (n = 9) was infused with PK11195 followed 20 min later by vehicle infusions (β-cyclodextrin), and then tested 10 min following the last infusion. A second group of rats (n = 8) was infused with vehicle, followed 20 min later by indomethacin infusions, and tested 10 min following these infusions. A third group of rats (n = 8) was infused with PK11195, followed 20 min later by infusions of indomethacin, and tested 10 min later. A fourth group of rats (n = 8) was infused with vehicle, followed 20 min later by a second vehicle infusion, and tested 10 min later (see Fig. 1).
Fig. 1.
Depicts timeline and course of testing for each rat from first infusion to tissue collection.
2.5. Behavioral testing
Following infusions, rats in behavioral estrus were tested in the battery of tasks described below. We have utilized these behavioral measures in the past as individual tasks [31], small batteries of anxiety, social, or sexual measures only [31], or as a single battery of testing [35]. We find that behavioral and neuroendocrine status is not significantly affected by exposure to any task other than mating [31]. Because the present study was designed to examine effects of progestins on exposure to novel stimuli, rats were not habituated to behavioral apparatus prior to testing. All testing apparatus were brightly lit from above.
All behavioral data were collected with the ANY-Maze data collection program (Stoelting Co., Wheat Dale, IL) by one of two observers. There was at least a 95% concordance rating between data that was collected by ANY-Maze and that collected by observers.
2.5.1. Open field
Behavior in the open field is an index of exploration, anxiety, and motor behavior [9,34]. The open field (76 cm × 57 cm × 35 cm) has a 48-square grid floor (6 × 8 squares, 9.5 cm per side): there is an overhead light illuminating the central squares (all but the 24 perimeter squares were considered central). Per previous methods, rats were placed in the open field and the path of their exploration was recorded for 5 min. The number of central, peripheral, and total entries was then calculated from these data as indices of anxiolysis and motor behavior, respectively.
2.5.2. Elevated plus maze
Behavior in the elevated plus maze is also utilized to assess exploration, anxiety, and motor behavior [21,34]. The plus maze was elevated 50 cm off the ground and consisted of four arms (49 cm long and 10 cm wide). Two arms were enclosed by walls 30 cm high and the other two arms were exposed. As per previous methods, rats were placed at the juncture of the open and closed arms and the number of entries into, and the amount of time spent on, the open and closed arms were recorded during a 5-min test. Time spent on the open arms is an index of anxiety and the total number of arm entries is measure of motor activity.
2.5.3. Partner preference
A modified version of the previously established partner preference task was utilized to assess preference for an intact male or a conspecific [4,34]. Experimental rats were placed in the center of an open field (76 cm × 57 cm × 35 cm) that contained an ovariectomized stimulus female and an intact stimulus male in opposite corners. While, physical contact was prohibited by containing the stimulus rats in Plexiglass compartments, sensory contact was made possible via small, center-facing holes (1 cm diameter) drilled in the bottom portion of the enclosures that allowed the experimental rat to make visual and olfactory contact with the stimulus rats. The amount of time that experimental rats spent within a body’s length of stimulus animals was recorded in a 5-min test. Increased time spent in close proximity to one stimulus rat versus another is an indication of a preference for that animal.
2.5.4. Social interaction
The social interaction task assessed exploratory and anxiety behavior associated with interacting with a novel conspecific [22,34]. Each member of a pair of rats (one experimental, one stimulus) was placed in opposite corners of an open field (76 cm × 57 cm × 35 cm). The total duration of time that experimental rats engaged an ovariectomized stimulus rat in crawling over and under, sniffing, following with contact, genital investigation, tumbling, boxing, and grooming was recorded during a 5-min test [34]. An ovariectomized rat was utilized as the stimulus animal in order to avoid the possibility of vaginocervical stimulation of experimental rats, which might occur if a male had been used as the stimulus animal. Duration of time spent interacting with a conspecific is an index of anxiety behavior.
2.5.5. Paced mating
Paced mating was utilized over standard mating because of its greater ethological relevance and procedures were carried out as previously reported [16,28,42,55]. Paced mating tests were conducted in a chamber (37.5 cm × 75 cm × 30 cm), which was equally divided by a partition that had a small (5 cm in diameter) hole in the bottom center, to allow a female free access to both sides of the chamber, but which prevented the larger stimulus male from moving between sides. Females were placed in the side of the chamber opposite the stimulus male. Rats were behaviorally tested for an entire ejaculatory series. Behaviors recorded were the frequency of mounts and intromissions that preceded an ejaculation. As well, the frequency (lordosis quotient = incidence of lordosis/number of mounts) and intensity (lordosis rating) of lordosis, quantified by rating of dorsiflexion on a scale of 0–3 [47], was recorded. The percentage of proceptive (i.e. hopping, darting, ear wiggling; proceptivity quotient) and aggressive (i.e. vocalizations, defensive postures; aggression quotient) behaviors prior to contacts was also recorded. Pacing measures included the percentage of times the female left the compartment containing the male after receiving a particular copulatory stimuli (% exits after mounts, intromissions, and ejaculations) and latencies in seconds to return to the male compartment after these stimuli. The normal pattern of pacing behaviors for percent exits and return latencies to be longer after more intensive stimulation (ejaculations > intromissions > mounts) was observed in the present study.
2.6. Tissue collection
Immediately following testing, rats were rapidly decapitated, trunk blood was collected by inverting bodies over a chilled funnel and culture tube, and whole brains were removed and stored for later measurement of corticosterone, E2, P4, DHP, and 3α,5α-THP. Trunk blood was centrifuged at 3000 × g for 10 min and serum was stored at −80 °C. Brains were rapidly frozen on dry ice and stored at −80 °C for approximately 3 months prior to radioimmunoassay.
2.7. Tissue preparation
Serum was thawed on ice and steroids were extracted as described below. Brains were thawed on ice and midbrain, hippocampus, diencephalon, cortex, and interbrain were dissected as previously described [35,36]. Because endocrine measurements precluded histological analyses, all brains were visually inspected during dissection to ascertain the site of infusion. All animals that were behaviorally tested had infusions to the VTA. We have previously shown that the effects of 3α,5α-THP are very specific to the VTA, and do not occur with manipulations to the substantia nigra or central grey [35,36]. Following dissection, steroids were extracted from brain tissue as described below.
2.8. Radioimmunoassay for steroid hormones
Corticosterone, E2, P4, DHP, and 3α,5α-THP concentrations were measured as described below, using previously reported methods [11,25,38].
2.8.1. Radioactive probes
[3H] corticosterone (NET-182: specific activity = 48.2 Ci/mmol), E2 (NET-317: specific activity = 51.3 Ci/mmol), P4 (NET-208: specific activity = 47.5 Ci/mmol), and 3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol), were purchased from PerkinElmer (Boston, MA).
2.8.2. Extraction of steroids from serum
Corticosterone was extracted from serum by heating at 60 °C for 30 min [11]. E2, P4, DHP, and 3α,5α-THP were extracted from serum with ether following incubation with water and 800 cpm of [3H] steroid [25]. After snap-freezing twice, test tubes containing steroid and ether were evaporated to dryness in a Savant speed drier. Dried down tubes were reconstituted with phosphate assay buffer to the original serum volume.
2.8.3. Extraction of steroids from brain tissues
E2, P4, DHP, and 3α,5α-THP were extracted from brain tissues following homogenization with a glass/glass homogenizer in 50% MeOH, 1% acetic acid. Tissues were centrifuged at 3000 × g and the supernatant was chromatographed on Sepak-cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH). Solvents were removed using a speed drier. Samples were reconstituted in 300 μl assay buffer.
2.8.4. Antibodies
The corticosterone antibody (#B3-163, Endocrine Sciences), which typically binds 40–60% of [3H] corticosterone was used in a 1:20,000 dilution and bound 45% in the present study. The E2 antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO), which generally binds between 40% and 60% of [3H] E2, was used in a 1:40,000 dilution and bound 54% in the present study. The P4 antibody (P#337 from Dr. G.D. Niswender, Colorado State University) used in a 1:30,000 dilution typically binds between 30% and 50% of [3H] P4, and bound 48% in the present study. The DHP (X-947) and 3α,5α-THP antibodies (#921412-5, purchased from Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, CA) used in a 1:5000 dilution binds between 40% and 60% of [3H] 3α,5α-THP and bound 47% in the present study.
2.8.5. Set-up and incubation of radioimmunoassays
The range of the standard curves was 0–4 ng for corticosterone, 0–1000 pg for E2, and 0–8000 pg for P4, DHP, and 3α,5α-THP. Standards were added to assay buffer followed by addition of the appropriate antibody (described above) and 3H steroid. Total assay volumes were 900 μl for corticosterone, 800 μl for E2 and P4, 950 μl for DHP, and 1250 μl for 3α,5α-THP. All assays were incubated overnight at 4 °C, except for corticosterone which incubated at room temperature for 60 min.
2.8.6. Termination of binding
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000 × g and the supernatant was pipetted into a glass scintillation vial with 5 ml scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt [69], interpolation of the standards, and correction for recovery with Assay Zap. The inter- and intra-assay reliability coefficients were as follows: corticosterone 0.05 and 0.06, E2 0.07 and 0.05, P4 0.11 and 0.10, DHP 0.11 and 0.09, and 3α,5α-THP 0.09 and 0.10.
2.9. Statistical analyses
One-way analyses of variance (ANOVAs) were used to examine effects of progestin synthesis and/or metabolism inhibitors (PK11195, indomethacin, PK11195/indomethacin, vehicle) on neuroendocrine and behavioral outcomes. ANOVAs were run on all conditions and Fisher’s protected least significant differences post hoc tests were performed to ascertain differences between inhibitor and vehicle infusions. p values for post hoc tests are reported in text with the corresponding inhibitor condition. Alpha level for statistical significance was p < 0.05. Tendencies towards significance were noted when p < 0.10. Power analyses were utilized to verify that all inferential statistics reported were valid with sufficient power.
3. Results
3.1. Endocrine measures
Infusions of PK11195 (p < 0.0001), indomethacin (p = 0.0005), or PK11195 and indomethacin (p = 0.0001) to the VTA significantly decreased midbrain 3α,5α-THP levels [F(3, 29) = 10.44, p = < 0.0001] compared to vehicle administration (Fig. 2, top), but did not alter 3α,5α-THP levels in plasma, hippocampus, diencephalon, or cortex (Table 1).
Fig. 2.
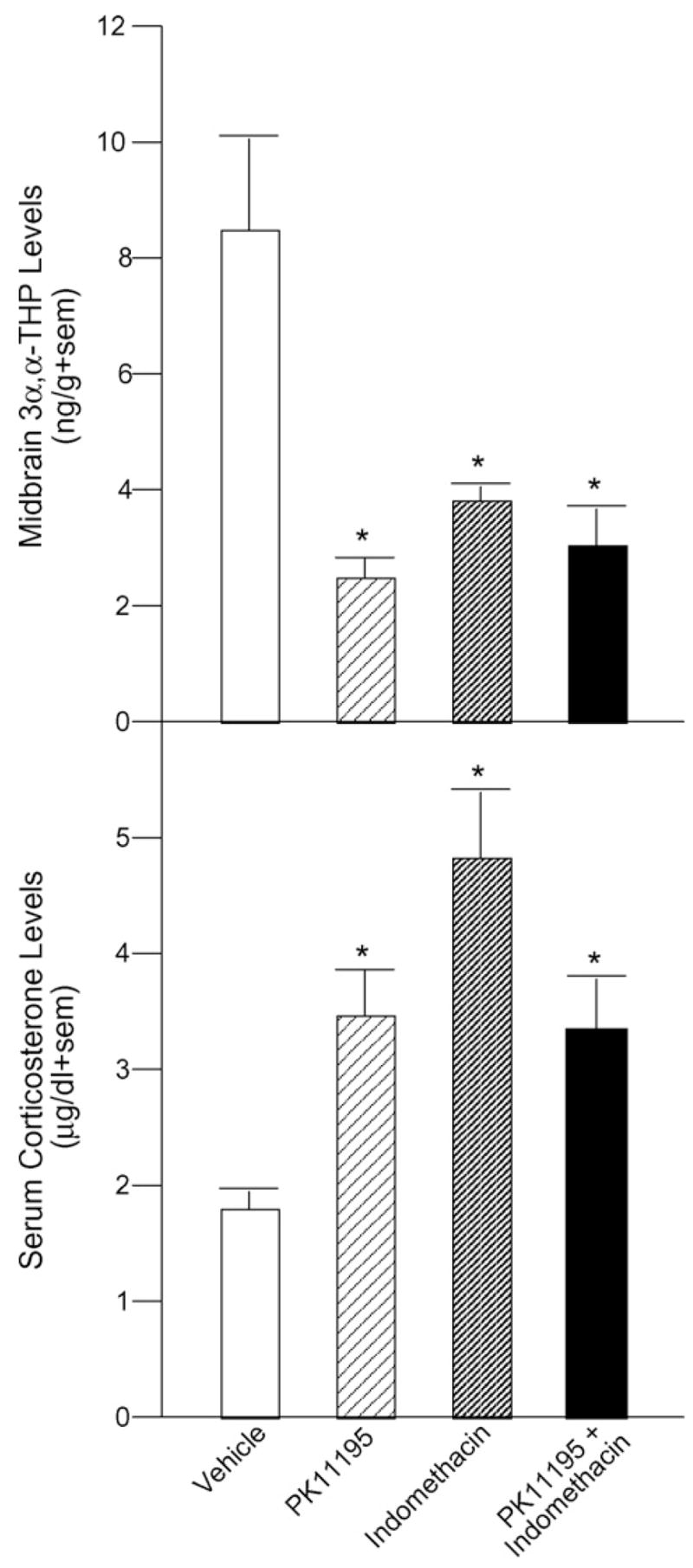
3α,5α-THP inhibition in VTA via infusion of PK11195 (n = 9), indomethacin (n = 8), or both (n = 8) significantly reduces midbrain 3α,5α-THP concentrations (top) and significantly increases serum corticosterone levels (bottom) compared to infusion of vehicle (n = 8). Significantly different from vehicle (*p < 0.05).
Table 1.
Endocrine data of proestrous rats that received infusions of PK11195, indomethacin, PK11195 and indomethacin, or vehicle to the VTA
| Vehicle | PK11195 | Indomethacin | PK11195 + indomethacin | |
|---|---|---|---|---|
| Midbrain E2 | 2.2 ± 0.3 | 2.2 ± 0.3 | 2.0 ± 0.3 | 2.1 ± 0.3 |
| Hippocampus E2 | 2.7 ± 0.5 | 2.3 ± 0.4 | 2.1 ± ± 0.5 | 2.3 ± 0.5 |
| Diencephalon E2 | 1.5 ± 0.2 | 1.9 ± 0.4 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| Cortex E2 | 1.8 ± 0.2 | 1.4 ± 0.3 | 1.7 ± 0.1 | 1.6 ± 0.2 |
| Interbrain E2 | 1.5 ± 0.2 | 1.9 ± 0.4 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| Midbrain P4 | 2.0 ± 0.2 | 1.9 ± 0.3 | 1.8 ± 0.2 | 2.0 ± 0.2 |
| Hippocampus P4 | 2.1 ± 0.4 | 2.8 ± 0.5 | 2.3 ± 0.2 | 2.0 ± 0.4 |
| Diencephalon P4 | 1.9 ± 0.4 | 2.2 ± 0.5 | 2.1 ± 0.7 | 2.5 ± 0.4 |
| Cortex P4 | 2.4 ± 0.3 | 2.8 ± 0.7 | 3.9 ± 1.0 | 3.1 ± 0.6 |
| Interbrain P4 | 5.1 ± 1.6 | 4.5 ± 0.8 | 4.9 ± 1.1 | 5.1 ± 1.2 |
| Midbrain DHP | 15.8 ± 0.8 | 16.6 ± 1.0 | 14.7 ± 1.1 | 15.0 ± 0.9 |
| Hippocampus DHP | 18.7 ± 1.6 | 18.0 ± 2.4 | 16.7 ± 1.1 | 17.4 ± 1.1 |
| diencephalon DHP | 15.4 ± 0.9 | 14.5 ± 0.9 | 15.3 ± 0.8 | 15.6 ± 1.4 |
| Cortex DHP | 34.0 ± 7.2 | 20.5 ± 6.0 | 43.1 ± 16.7 | 26.3 ± 7.2 |
| Interbrain DHP | 6.0 ± 3.4 | 2.9 ± 2.1 | 6.4 ± 3.7 | 6.6 ± 2.4 |
| Hippocampus 3α,5α-THP | 28.1 ± 3.9 | 25.3 ± 2.7 | 23.4 ± 3.8 | 31.3 ± 2.9 |
| Diencephalon 3α,5α-THP | 13.2 ± 1.6 | 10.7 ± 1.2 | 12.0 ± 2.0 | 10.1 ± 1.7 |
| Cortex 3α,5α-THP | 20.7 ± 2.4 | 15.7 ± 1.9 | 13.1 ± 1.3 | 17.7 ± 3.6 |
| Interbrain 3α,5α-THP | 13.2 ± 0.9 | 13.8 ± 1.2 | 12.3 ± 1.4 | 13.0 ± 1.1 |
Significantly different from vehicle (*p < 0.05).
Rats that received intra-VTA infusions of PK11195 (p = 0.007), indomethacin (p < 0.0001), or both inhibitors (p = 0.01) had significantly higher serum corticosterone levels than did vehicle-infused rats [F(3, 29) = 7.17, p = 0.001] (Fig. 2, bottom), but neither E2, P4, nor DHP levels in plasma, midbrain, hippocampus, diencephalon, cortex, or interbrain were different (Table 1).
3.2. Behavioral measures
Exploratory, anti-anxiety, social, and sexual behaviors were decreased among rats infused with PK11195, indomethacin, or PK11195 and indomethacin to the VTA.
3.3. Open field
Central square entries were significantly reduced in rats infused with PK11195 alone (18 + 5, p = 0.007) or in conjunction with indomethacin (13 ± 4, p = 0.001) and tended to be different after indomethacin alone (23 ± 4, p = 0.06) compared to rats infused with vehicle (35 ± 4) to the VTA [F(3, 29) = 4.86, p = 0.007] (Fig. 3, top).
Fig. 3.
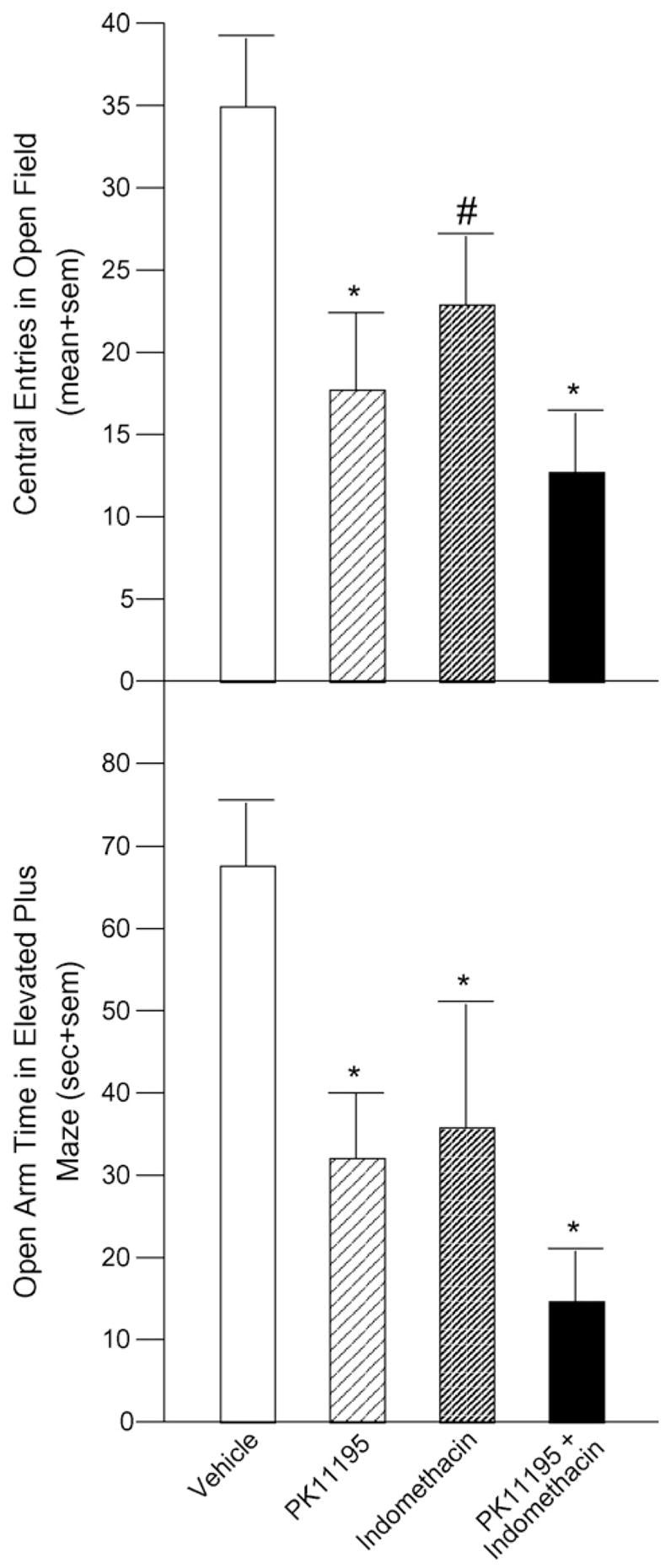
Inhibition of 3α,5α-THP in VTA via infusion of PK11195 (n = 9), alone or in conjunction with indomethacin (n = 8), significantly decreases central entries in an open field and indomethacin infusion alone (n = 8) tends to decrease central entries compared to vehicle infusion (n = 8, top). Time spent on the open arms of an elevated plus maze is significantly reduced with any inhibitor combination compared to vehicle infusion (bottom). Significantly different from vehicle (*p < 0.05). Tendency to be different from vehicle (#p < 0.10).
3.4. Elevated plus maze
Time on the open arms of the elevated plus maze was significantly decreased among rats infused with PK11195 (32 ± 8 s, p = 0.01), indomethacin (36 ± 15 s, p = 0.03), or PK11195 and indomethacin (15 ± 5 s, p = 0.0007) to the VTA compared to vehicle-infused rats (68 ± 7 s) [F(3, 29) = 5.03, p = 0.006] (Fig. 3, bottom).
3.5. Social choice
There was an apparent, albeit non-significant, effect for infusions of PK11195 and indomethacin to decrease time spent in close proximity to a stimulus male (58 ± 21 s) compared to vehicle-infused rats (105 ± 22 s). Infusions of PK11195 (88 + 14 s) or indomethacin (85 + 28 s) alone did not alter time in proximity to a stimulus male (Fig. 4, top).
Fig. 4.
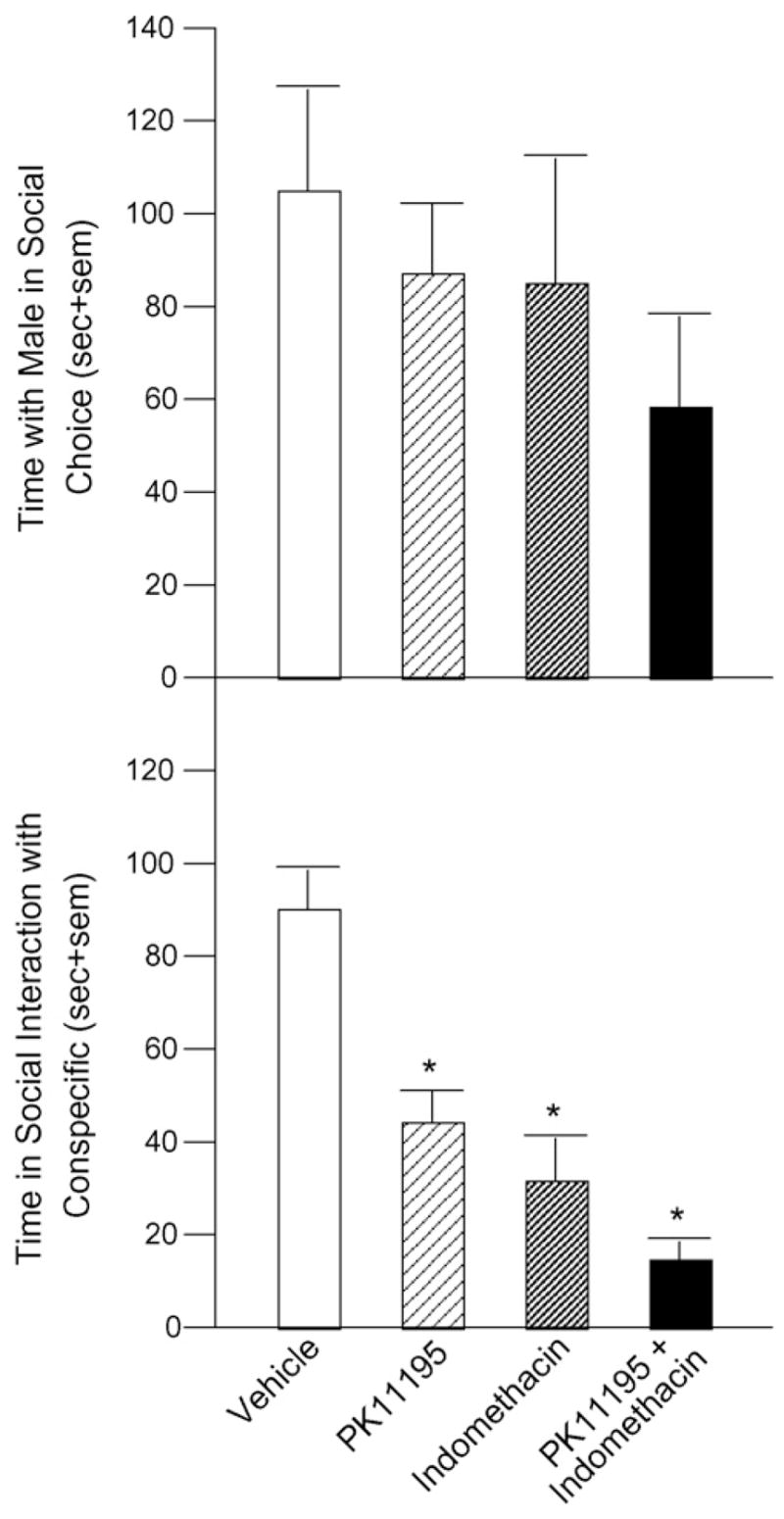
Infusion of PK11195 (n = 9), indomethacin (n = 8), or both (n = 8) to VTA appears to reduce time spent in proximity to a male in a social choice task (top) and significantly reduces time spent interacting with a female conspecific compared to infusion of vehicle (n = 8). Significantly different from vehicle (*p < 0.05).
3.6. Social interaction
Time spent in social interaction with an ovariectomized conspecific was significantly less in rats infused with PK11195 (43 ± 6 s, p = 0.0003), indomethacin (31 ± 10 s, p < 0.0001), or PK11195 and indomethacin (15 ± 4 s, p = 0.0003) to the VTA compared to vehicle-infused rats (105 ± 22 s) [F(3, 29) = 15.56, p < 0.0001] (Fig. 4, bottom).
3.7. Lordosis quotients
Lordosis quotients were significantly lower among rats infused with PK11195 (24 ± 14%, p = 0.0005), indomethacin (25 ± 16%, p = 0.0008), or PK11195 and indomethacin (24 ± 12%, p = 0.0006) to the VTA compared to vehicle-infused rats (93 ± 4%) [F(3, 29) = 7.32, p = 0.0008] (Fig. 5, top).
Fig. 5.
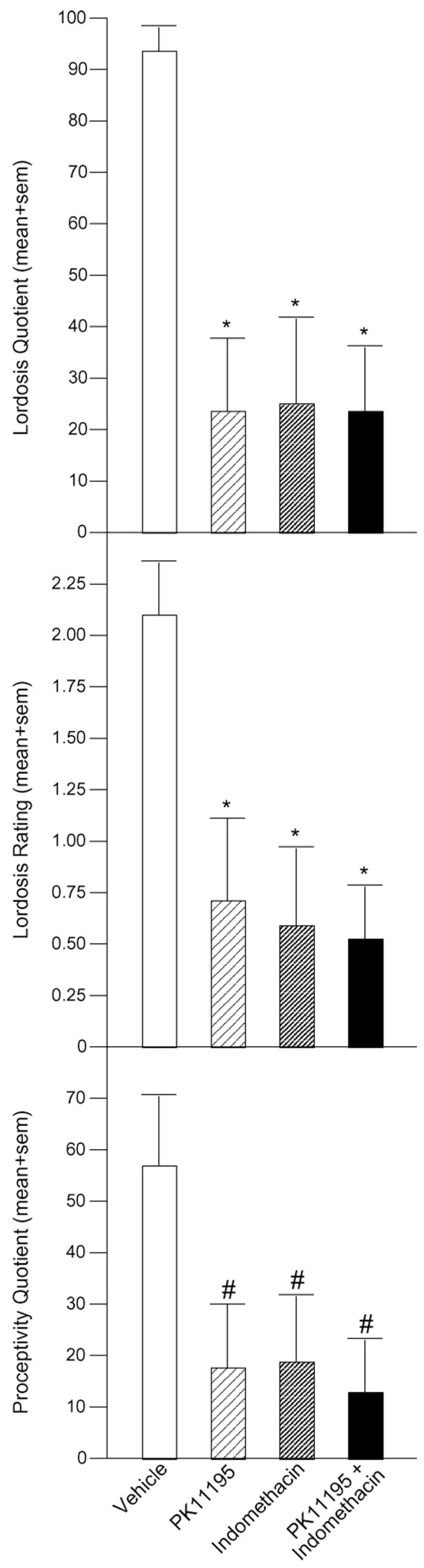
Compared to vehicle, inhibition of 3α,5α-THP in VTA via infusion of PK11195 (n = 9), indomethacin (n = 8), or both (n = 8) significantly decreases lordosis frequency (lordosis quotient, top) and intensity (lordosis rating; middle) in response to male mounting and tends to reduce frequency of proceptive (hopping, darting, ear wiggling) behavior (proceptivity quotient, bottom). Significantly different from vehicle (*p < 0.05). Tendency to be different from vehicle (#p < 0.10).
3.8. Lordosis ratings
Rats infused with PK11195 (0.7 ± 0.4, p = 0.006), indomethacin (0.6 ± 0.4, p = 0.004), or PK11195 and indomethacin (0.5 ± 0.3, p = 0.003) to the VTA had significantly lower lordosis ratings than did vehicle-infused rats (2.1 ± 0.3) [F(3, 29) = 4.84, p = 0.008] (Fig. 5, middle).
3.9. Proceptivity and aggression quotients
Proceptivity quotients tended to be lower among rats infused with PK11195 (18 ± 12%, p = 0.03), indomethacin (19 ± 13%, p = 0.04), or PK11195 and indomethacin (13 ± 11%, p = 0.02) to the VTA compared to vehicle-infused rats (57 ± 14%) [F(3, 29) = 2.62, p = 0.07]. Aggression quotients did not differ significantly among groups (Fig. 5, bottom).
3.10. Percent exits
There was an apparent, albeit non-significant effect, of PK11195 (7 ± 5%), indomethacin (13 ± 7%), or PK11195 and indomethacin (4 ± 3%) to decrease the percentage of exits following mating contacts compared to vehicle-infusions (18 ± 6%).
4. Discussion
These data are consistent with prior reports demonstrating that 3α,5α-THP in the VTA is necessary for enhanced sexual responsiveness of female rats. Administration of 3α,5α-THP directly to the VTA produces higher levels of lordosis than does P4 [27,29,39]. Blocking P4’s metabolism to 3α,5α-THP in the VTA attenuates lordosis of naturally receptive or ovariectomized, hormone-primed rodents [6,24,26,30,40]. Conversely, enhancing 3α,5α-THP synthesis facilitates lordosis. In the present study, attenuating either biosynthesis of, or metabolism to, 3α,5α-THP significantly decreased lordosis responses concomitant with decreases in midbrain 3α,5α-THP concentrations. These findings confirm that formation of 3α,5α-THP in the VTA is critical for lordosis.
These results also extend these findings to suggest that 3α,5α-THP in the VTA can influence behavioral and neuroendocrine responses to stressors in normative, physiologically relevant situations. Previous reports have demonstrated that exposure to extreme stressors (i.e. cold-water swim, ether, footshock) increases biosynthesis of pregnane and androstane neurosteroids [14,17,68]. Such levels of stress can enhance dopamine secretion to agonistic levels which may underlie some reward processes such as those seen in acquisition of cocaine [43,44,48,49]. This response can also enhance 3α,5α-THP in response to elevated stress axis factors. Administration of P4 or 3α,5α-THP decreases behavioral responses to normally stress-inducing stimuli, including predator odor, forced swim, and footshock [78,79]. Further, administration of 3α,5α-THP reduces stress-induced elevations in adrenocorticotropin and corticosterone [60]. In the present study, rats with lower 3α,5α-THP concentrations in the VTA exhibited increased anxiety/stress responses in the behavioral tasks examined and had higher serum corticosterone levels than did vehicle-infused rats with higher 3α,5α-THP levels. Notably, infusions of either PK11195, which attenuates biosynthesis of 3α,5α-THP, or indomethacin, a metabolism inhibitor, decreased anti-anxiety behavior and 3α,5α-THP concentrations and increased corticosterone levels. As such, both biosynthesis of, and metabolism to, 3α,5α-THP may be involved in 3α,5α-THP’s modulation of stress responses. Together, these data suggest that 3α,5α-THP in the VTA plays a vital role in maintaining homeostasis in response to stress.
The mesolimbic dopamine system is a considered to be a critical component in mediation of motivated behaviors [10,15,20,46,51,57,59,62,81]. As such, these data contribute to lines of research aimed at assessing interactions between natural reward processes, such those associated with reproduction, and exogenous rewards, such as proclivity towards drugs of abuse. In the current study, inhibition of 3α,5α-THP increased corticosterone concomitant with reducing reproductive behavior. Rats with high stress reactivity are found to more readily self-administer drugs of abuse than rats with lower stress reactivity [63–65]. As well, glucocorticoid administration enhances proclivity of rats to self-administer psychostimulants [66,67]. Reports among people find that women who use cocaine report that it is more pleasurable when their cyclical 3α,5α-THP levels are low and they report less subjective pleasure from using when endogenous 3α,5α-THP levels are high [19,73,75]. Oral administration of P4 has also been reported to have similar effects on men and women [18,73,74]. As well, we have found that cocaine administration enhances corticosterone in rats, and pre-treatment with P4 can moderately attenuate this [23]. 3α,5α-THP may have effects to reduce reinforcing effects of drugs and this may be due, in part, to its ability to dampen stress reactivity.
Although these are exciting data that lend greater support to the idea that 3α,5α-THP is an important homeostatic modulator, there are a number of questions that remain to be addressed. First, the present study revealed that actions of 3α,5α-THP in the VTA are necessary for enhanced exploratory, anti-anxiety, social, and sexual behaviors of female rats. However, the present study did not investigate the extent to which 3α,5α-THP in other brain areas might also be involved in mediating these behaviors. Indeed, it is well-known that other areas, such as the hippocampus, amygdala, cortex, and striatum, are also involved in stress, anxiety, and/or fear responses. As such, ongoing studies in our laboratory are examining effects of manipulating 3α,5α-THP in these areas for effects on exploratory, anti-anxiety, social, and sexual behaviors. Second, there is always a concern that deficits in behavior may be due to non-specific effects of pharmacological inhibitors. Given that there were no differences in gross motor behavior among rats in the present study, it is unlikely that the effects that we saw on exploratory, anti-anxiety, social, and sexual behaviors were due to non-specific effects of PK11195 or indomethacin. Thus, although the present results strongly support a role of 3α,5α-THP in the VTA for modulating exploratory, anti-anxiety, social, and sexual behavior, further investigation is needed to fully elucidate the role of 3α,5α-THP in the VTA and other brain areas for mediation of these behaviors.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (MH06769801).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Contributor Information
Cheryl A. Frye, Email: cafrye@albany.edu.
Jason J. Paris, Email: jason.paris01@albany.edu.
Madeline E. Rhodes, Email: mrhodes@mcdaniel.edu.
References
- 1.Agmo A, Paredes RG, Ramos JI, Contreras JL. Dopamine and sexual behavior in the male rabbit. Pharmacol Biochem Behav. 1996;55:289–95. doi: 10.1016/s0091-3057(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 2.Agmo A, Picker Z. Catecholamines and the initiation of sexual behavior in male rats without sexual experience. Pharmacol Biochem Behav. 1990;35:327–34. doi: 10.1016/0091-3057(90)90164-d. [DOI] [PubMed] [Google Scholar]
- 3.Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–30. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- 4.Baum M. Paradoxical effect of alcohol on the resistance to extinction of an avoidance response in rats. J Comp Physiol Psychol. 1969;69:238–40. doi: 10.1037/h0028188. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer C, Gonzalez-Flores O, Ramirez-Orduna JM, Gonzalez-Mariscal G. Indomethacin inhibits lordosis induced by ring A-reduced progestins: possible role of 3α-oxoreduction in progestin-facilitated lordosis. Horm Behav. 1999;35:1–8. doi: 10.1006/hbeh.1998.1457. [DOI] [PubMed] [Google Scholar]
- 7.Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- 8.Bless EP, McGinnis KA, Mitchell AL, Hartwell A, Mitchell JB. The effects of gonadal steroids on brain stimulation reward in female rats. Behav Brain Res. 1997;82:235–44. doi: 10.1016/s0166-4328(96)00129-5. [DOI] [PubMed] [Google Scholar]
- 9.Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–8. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 10.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140:4081–8. doi: 10.1210/endo.140.9.6964. [DOI] [PubMed] [Google Scholar]
- 12.Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- 13.Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–91. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- 14.Drugan RC, Holmes PV, Scher DM, Luczak S, Oh H, Ferland RJ. Environmentally induced changes in peripheral benzodiazepine receptors are stressor and tissue specific. Pharmacol Biochem Behav. 1995;50:551–62. doi: 10.1016/0091-3057(94)00341-6. [DOI] [PubMed] [Google Scholar]
- 15.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–86. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–61. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- 17.Erskine MS, Kornberg E. Stress ACTH increase circulating concentrations of 3alpha-androstanediol in female rats. Life Sci. 1992;51:2065–71. doi: 10.1016/0024-3205(92)90157-k. [DOI] [PubMed] [Google Scholar]
- 18.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 19.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 20.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 21.File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- 22.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 23.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-diol. J Neuroendocrinol. 1999;11:839–47. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 26.Frye CA, Bayaon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 27.Frye CA, DeBold JF. 3α-OH-DHP and 5α-THDOC implants to the ventral tegmental area facilitate sexual receptivity in hamsters after progesterone priming to the ventral medial hypothalamus. Brain Res. 1993;612:130–7. doi: 10.1016/0006-8993(93)91653-a. [DOI] [PubMed] [Google Scholar]
- 28.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–85. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 29.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA, Leadbetter EA. 5α-Reduced progesterone metabolites are essential in hamster VTA for sexual receptivity. Life Sci. 1994;54:653–9. doi: 10.1016/0024-3205(94)00548-6. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frye CA, Petralia SM. Mitochondrial benzodiazepine receptors in the ventral tegmental area modulate sexual behaviour of cycling or hormone-primed hamsters. J Neuroendocrinol. 2003;15:677–86. doi: 10.1046/j.1365-2826.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 34.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 35.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 37.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-Hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3α-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and POA. Behav Neurosci. 1996;110:603–12. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- 39.Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA, Vongher JM. Ventral tegmental area infusions of inhibitors of the biosynthesis and metabolism of 3α,5α-THP attenuate lordosis of hormone-primed and behavioural oestrous rats and hamsters. J Neuroendocrinol. 2001;13:1076–86. doi: 10.1046/j.1365-2826.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Gans S, Erskine MS. Effects of neonatal testosterone treatment on pacing behaviors and development of a conditioned place preference. Horm Behav. 2003;44:354–64. doi: 10.1016/s0018-506x(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 43.Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–59. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 44.Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13:435–41. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett. 2007;427:165–70. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39:71–7. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- 47.Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–8. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- 48.Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–82. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–93. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Lisciotto CA, DeBold JF. Ventral tegmental lesions impair sexual receptivity in female hamsters. Brain Res Bull. 1991;26:877–83. doi: 10.1016/0361-9230(91)90252-f. [DOI] [PubMed] [Google Scholar]
- 51.Lonstein JS, Dominguez JM, Putnam SK, De Vries GJ, Hull EM. Intracellular preoptic and striatal monoamines in pregnant and lactating rats: possible role in maternal behavior. Brain Res. 2003;970:149–58. doi: 10.1016/s0006-8993(03)02315-1. [DOI] [PubMed] [Google Scholar]
- 52.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratficsh RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 53.Marshall JF, Teitelbaum P. Further analysis of sensory inattention following lateral hypothalamic damage in rats. J Comp Physiol Psychol. 1974;86:375–95. doi: 10.1037/h0035941. [DOI] [PubMed] [Google Scholar]
- 54.Matuszewich L, Lorrain DS, Hull EM. Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behav Neurosci. 2000;114:772–82. doi: 10.1037//0735-7044.114.4.772. [DOI] [PubMed] [Google Scholar]
- 55.McClintock MK, Adler NT. Induction of persistent estrus by airborne chemical communication among female rats. Horm Behav. 1978;11:414–8. doi: 10.1016/0018-506x(78)90041-7. [DOI] [PubMed] [Google Scholar]
- 56.Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55:151–7. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 57.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 58.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 59.Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005 March 7;158(1):53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–40. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- 61.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [Google Scholar]
- 62.Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–43. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- 63.Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 64.Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–72. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- 65.Piazza PV, Rougé-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93:8716–20. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piazza PV, Rougé-Pont F, Deminière JM, Kharouby M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–74. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- 68.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodbard D, Hutt DM International Atomic Energy Agency. Symposium on radioimmunoassay and related procedures in medicine. New York: Uniput; 1974. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting; pp. 209–33. [Google Scholar]
- 70.Romeo E, Auta J, Kozikowski AP, Ma D, Papadopoulos V, Puia G, et al. 2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR) J Pharmacol Exp Ther. 1992;262:971–8. [PubMed] [Google Scholar]
- 71.Rose JD. Brainstem influences on sexual behavior. In: Klemm WR, Vertes RP, editors. Brainstem mechanisms of behavior. New York: Wiley; 1990. pp. 407–63. [Google Scholar]
- 72.Sirinathsinghji DJ, Whittington PE, Audsley AR. Regulation of mating behaviour in the female rat by gonadotropin-releasing hormone in the ventral tegmental area: effects of selective destruction of the A10 dopamine neurones. Brain Res. 1986;374:167–73. doi: 10.1016/0006-8993(86)90406-3. [DOI] [PubMed] [Google Scholar]
- 73.Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- 74.Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 76.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 78.Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behav Brain Res. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 79.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamanouchi K, Arai Y. The role of mesencephalic tegmentum in regulating female rat sexual behaviors. Physiol Behav. 1985;35:255–9. doi: 10.1016/0031-9384(85)90346-4. [DOI] [PubMed] [Google Scholar]
- 81.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–11. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 82.Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84(2):279–86. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]