Abstract
Although androgen secretion is reduced with aging, and may underlie decrements in cognitive and affective performance, the effects and mechanisms of androgens to mediate these behaviors are not well understood. Testosterone (T), the primary male androgen, is aromatized to estrogen (E2), and reduced to dihydrotestosterone (DHT), which is converted to 5α-androstane, 3α, 17β-diol (3α-diol). To ascertain whether actions of the neuroactive metabolite of T, 3α-diol, mediates cognitive and affective behaviors, intact, aged male C57/B6 mice (24 month old) as well as young, intact and gonadectomized (GDX; 12 week old) mice were administered s.c. T, 3α-diol, E2, or sesame oil vehicle (1 mg/kg; n = 4–5/group) at weekly intervals and 1 h later mice were tested in the activity box, roto-rod, open field, elevated plus maze, zero maze, mirror maze, dark-light transition, forced swim, or Vogel tasks. Mice were trained in the inhibitory avoidance or conditioned contextual fear and were administered hormones following training and then were tested. After the last test occasion, tissues were collected for evaluation of hormone levels and effects on γ-aminobutyric acid (GABA)-stimulated chloride flux. T, 3α-diol, or E2 increased anti-anxiety and antidepressant behavior of aged, intact mice in the open field, light-dark transition, mirror maze, and forced swim tasks. T or 3α-diol, but not E2, enhanced anti-anxiety behavior in the elevated plus maze, zero maze, and the Vogel task, and increased motor behavior in the activity monitor, latency to fall in the Roto-rod task, and cognitive performance in the hippocampally-mediated, but not the amygdala-mediated, portion of the conditioned fear task and in the inhibitory avoidance task. Anti-anxiety and enhanced cognitive performance was associated with regimen that increased plasma and hippocampal 3α-diol levels and GABA-stimulated chloride flux. Similar patterns were seen among young, adult GDX but not in intact mice. Thus, 3α-diol can enhance affective and cognitive behavior of male mice.
Keywords: testosterone, aging, senescent anxiety cognition, 3α-diol
INTRODUCTION
Cognitive and affective performance can decline with aging. For example, aged men and women show decreased performance in measures of attention and working memory, learning and memory retrieval, language, visuospatial function, sensori-motor, and executive function compared to their middle-aged counterparts (Clark et al, 2006). In addition, aging is associated with more self-reported feelings of depression (Butler, 2006) and increased incidence of anxiety disorders (Delhez et al, 2003). Some of these aged-related changes may be due to a decline in endogenous steroid levels, which also occurs with aging (Beer et al, 2006; Janowsky 2006; Markou et al, 2005). Menopause is characterized by decline in estrogen (E2) and progesterone secretion from the ovaries, and is associated with decline in cognitive function and increased incidence of affective and depressive symptoms (Markou et al, 2005). Although there has been interest and investigation in how decline in steroid hormone levels influences cognitive and affective behavior of women, the effects of testosterone (T) decline among men has received less attention (Beer et al, 2006; Janowsky, 2006). One of the reasons for this may be that T decline in men is more gradual than is ovarian cessation among women. Endogenous androgen levels slowly decline decade-by-decade among healthy men until, at the age of 70, T levels are approximately 40% lower than that of men in their twenties (Janowsky, 2006).
Evidence that androgens influence cognitive and/or affective measures of men is as follows. First, men with high levels of androgens demonstrate enhanced mood. For example, anabolic steroid users report enhanced mood and positive well-being while using these illicit drugs, irrespective of their effect on appearance (Cafri et al, 2006). Second, young or aged men with lower androgen levels show poorer cognitive and/or affective function. For example, young hypogonadal men, with low endogenous T levels, exhibit decreased performance in cognitive tasks, and are more likely to be diagnosed with an anxiety or depressive disorder (Howell and Shalet, 2001; Kaminetsky, 2005). Aged men with lower T levels exhibit decreased performance in visuospatial tasks, such as route-learning via a map, surface development, paper folding, and hidden patterns (Janowsky, 2006; Janowsky et al, 1994; Li et al, 2002). Aged men with lower T levels also have increased incidence of affective disorders (Davis, 2001; Lund et al, 1999; Orengo et al, 2004; Seidman, 2003; Sternbach, 1998). Third, T replacement to young or aged men with low T levels improves cognitive and affective behavior. T replacement to young hypogonadal men enhances performance in cognitive tasks, as well as self-reported indices of mood (Howell and Shalet, 2001; Kaminetsky, 2005). T replacement to aged men also improves cognitive (Alexander et al, 1998; Janowsky, 2006) and affective (Delhez et al, 2003) performance. However, some reports have suggested that these results are inconsistent and/or not robust (Delhez et al, 2003; Haren et al, 2002, 2005; Wolf, 2003). This variability may be due to androgen milieu investigated, which could influence androgen metabolism. Given these data, and that the population is aging, it is particularly important to characterize the effects and mechanisms of androgens, as such information may contribute to the development of better hormonal therapies to improve age-related decline.
Results from animal studies also support a role for steroid hormones to enhance cognitive and affective performance. E2 may influence cognitive and affective performance. Aged female rats and mice have age-related decrements in cognitive and affective performance that may be associated with a decline in endogenous E2 levels (Frye et al, 2005; Wise, 2006). These decrements in cognitive and affective performance can be abrogated through systemic E2 replacement (Frye et al, 2005; Savonenko and Markowska, 2003). Although there are few reports regarding effects of androgens on aging, androgens’ enhancement of cognitive and affective performance has been observed in adult intact and gonadectomized (GDX) rodents. Systemic T administration to intact rats enhances performance in cognitive tasks such as the object recognition (Ceccareli et al, 2001), inhibitory avoidance (Edinger and Frye, 2004), and conditioned contextual fear (Edinger et al, 2004) tasks. Systemic administration of T (Bitran et al, 1993; Bing et al, 1998) or anabolic steroids (Rojas-Ortiz et al, 2006) to intact rats also decreases anxiety-like behavior in the elevated plus maze and Vogel paradigm. GDX results in decreased cognitive performance (Ceccareli et al, 2001; Edinger and Frye, 2004; Edinger et al, 2004; Frye and Seliga, 2001) and increased anxiety-like behavior (Adler et al, 1999; Bitran et al, 1993; Fernandez-Guasti and Martinez-Mota, 2003; Frye and Seliga, 2001) across these same tasks. These GDX-associated decrements in cognitive and affective performance are abrogated by systemic administration of T (Fernandez-Guasti and Martinez-Mota, 2003; Frye and Seliga, 2001). However, as in studies with people, some studies have found disparate behavioral results in studies of androgens and cognitive and affective performance (Naghdi et al, 2003).
Variability in behavior among studies in people and in animals may be due to T′s different routes of metabolism. For example, T can be aromatized to E2, which decreases anxiety (Nathorst-Boos et al, 1993; Pearlstein et al, 1997) and enhances cognitive performance (Drake et al, 2000; Sherwin, 2002) in some women. E2 to female rats also reduces anxiety (Palermo-Neto and Dorce, 1990; Walf and Frye, 2005, 2006) and enhances learning and memory (Frick et al, 2002; Frye and Rhodes, 2002; Gresack and Frick, 2006). T can also be metabolized to dihydrotestosterone (DHT), which is subsequently converted by 3α-hydroxy-steroid dehydrogenase (3αHSD) to the nonaromatizable metabolite, 5α-androstane, 17β-diol (3α-diol). 3α-Diol administration increases anti-anxiety behavior and enhances cognitive performance in the inhibitory avoidance and place-learning tasks (Edinger and Frye, 2004, 2005, 2007a; Edinger et al, 2004; Frye and Lacey 2001; Frye et al, 2001). Blocking metabolism to 3α-diol with indomethacin decreases cognitive performance in intact and/or DHT-replaced rats (Frye and Edinger, 2004; Frye et al, 2004a). Together, these findings suggest that some of T′s beneficial effects may be due, in part, to T′s reduction to 3α-diol.
In order to assess the effect of different androgen treatment on affective and cognitive behavior, three different groups of male mice have been tested. In experiment 1, aged (24 months of age) male mice, were administered E2, T, 3α-diol, or sesame oil vehicle, s.c. 1 h before testing in motor (activity box and roto-rod) or anxiety (open field, elevated plus maze, dark-light transition, mirror maze, zero maze, and forced swim) tasks. Mice were administered E2, T, 3α-diol, or sesame oil vehicle immediately following training in the cognitive (conditioned contextual fear and inhibitory avoidance) tasks. Young, intact (experiment 2) and GDX (experiment 3) mice, 12 week old, were similarly tested. We hypothesized that if 3α-diol is important for androgens’ anxiety-reducing and cognitive-enhancing effects, then administration of the nonaromatizable metabolite, 3α-diol, should enhance cognitive and affective performance of aged male mice equally as well as T, and should result in increased 3α-diol levels in the hippocampus and plasma. We expected a similar but less robust pattern in GDX young adult mice, that would be expected to have fewer deficits than their aged counterparts, and minimal effects in androgen-replete young intact mice.
METHODS
All procedures were approved by the Animal Care and Use Committee at the University at Albany-SUNY.
Animals and Housing
Aged intact, male mice (N = 16, mean age 24 months, range 20–28 months), were bred in the Social Sciences Laboratory Animal Care Facility at SUNY-Albany, and young intact and GDX male mice (N =40, 12 week old), were bred in the Life Science Laboratory Animal Care Facility at SUNY-Albany on a congenic C57/B6 background. Mice were group-housed, on a 12/12 h light/dark cycle (lights on 0800) with free access to Purina Rat Chow and water in their home cages.
Hormonal Milieu
Mice were randomly assigned to receive a 1 mg/kg subcutaneous injection of T, 3α-diol, E2, or sesame oil vehicle (n = 4/group for aged group and n = 5/group for young, intact and GDX mice) 1 h before testing in the anxiety tasks, and immediately following training in the cognitive tasks. These androgen regimens, when administered to young GDX rats, produce endogenous levels of androgens in the plasma and hippocampus that are akin to that of their gonadally intact counterparts (Edinger and Frye, 2004, 2005). Further, this E2 regimen has been demonstrated to reinstate E2 levels in the hippocampus among aged mice (Frye et al, 2005).
Surgery
Young adult mice were gonadectomized (GDX; n = 20) or received sham surgery (n = 20) under sodium pentobarbital anesthesia (70 mg/kg) around 55 days of age. At least 4 weeks following GDX, or 1 week after sham surgery, mice were injected with the assigned androgens or vehicle, and behaviorally tested.
Procedure
Mice were tested approximately once weekly until all tasks were completed. In experiment 1, intact aged mice (N = 16) were tested. In experiment 2, we tested young, intact mice (N =20), and young GDX mice (N =20) were tested in the experiment 3. To investigate the effects of androgens on anxiety and related behaviors of aged and young mice, methods similar to those used by Frye et al (2006) to examine effects of progesterone on these behaviors on aged and mid-aged mice were employed. Animals were randomly assigned each hormone condition and administered androgens once a week for 8 weeks for anxiety test or cognitive test. At week 1, animals were tested in the activity box and open field. At week 2, testing examined effects in the elevated plus maze. At week 3, behavior in the elevated zero maze and mirror chamber were investigated. In the fourth week, mice were tested in the roto-rod and dark/light transition tasks. Because the Vogel conflict task, inhibitory avoidance, and fear conditioning task involve shock, and forced swim testing can be stressful, behavior in these tasks were examined after the completion of all other behavioral testing. At week 5, animals were tested in the inhibitory avoidance task. At week 6, testing examined effects in the Vogel conflict task. At week 7, mice were tested in the contextual conditioned fear task. During week 8, mice were tested in the forced swim task. At week 9, tissues of animals were collected, 1 h after hormone or vehicle administration.
Behavioral Testing
Motor behavior
Activity monitor
A Digiscan Optical Animal Activity Monitor (39×39×30 cm; Accuscan Instruments) recorded the number of horizontal beam breaks in 5 min (Frye et al, 2006).
Roto-rod
The latency to fall (3 min maximum, a 48cm fall height) from The Accurotor Roto-Rod Apparatus (Accuscan Instruments Inc.), with a 70mm drum, set to rotate at accelerating speeds (0–60 r.p.m./min), was recorded (Frye et al, 2006).
Affective behavior
Open field
Affective behavior was assessed in the open field. Mice were placed in the open field arena (39 × 39 × 30cm) a 16-square grid floor, and an overhead light illuminating the central squares (Frye et al, 2004b). Entries into central and peripheral squares were recorded for 5 min (Frye et al, 2006).
Elevated plus maze
Affective behavior was assessed in the elevated plus maze (Frye et al, 2004b, 2006). Mice were placed in the center of the maze and the duration of open and closed arm entries (maximum latency = 300s) was measured. The time spent in the two open (5 × 40 cm) or closed (5 × 40 × 20 cm) arms was recorded for 5 min.
Dark/light transition task
Affective behavior of mice was assessed in the dark-light transition task. Mice are placed in the dark side of the chamber (24.5 × 23.5 × 35 cm) and allowed to move between the two chambers. Time spent in the light side of the box was recorded for 5 min (Frye et al, 2006).
Zero maze
Affective behavior of mice was assessed in the elevated zero maze (Frye et al, 2006; Rizk et al, 2004). Mice were placed in an open section of the maze, and the duration of open and closed section entries (maximum latency = 300 s) was measured for a period of 5 min.
Mirror chamber
Affective behavior of mice was assessed in the mirror chamber task. This behavioral task is based upon the image-induced acute changes in behavior that occur when animals observe themselves in a mirror (Houri, 1986; Lamberty, 1998). The mirrored chamber consists of a cubed chamber (30 × 30 × 30 cm), with mirrors on each of the four walls and adjoined to an alleyway (30 × 5 × 30 cm) without mirrors. Mice were placed in the center of the mirrored-chamber and the time spent in the mirrored chamber was recorded for 5 min. (Frye et al, 2006; Henderson et al, 2004).
Vogel conflict task
The number of licks made by water-deprived (24 h) mice of an electrified water bottle that delivered a shock (0.25 mA) every 20 licks was recorded for 15 min (Frye et al, 2006).
Porsolt forced swim task
The duration of immobility when mice were placed in a glass cylinder (20.5 cm diameter, 21.5 cm depth), which contained 18 cm of 25°C water, was recorded for 5 min (Frye et al, 2004b).
Cognitive measures
Inhibitory avoidance
Cognitive performance was assessed in the inhibitory avoidance task (Fugger et al, 2000). The inhibitory avoidance apparatus consists of a two-compartment (14 × 8.5 × 6.5 cm each) stainless steel box as described in Fugger et al (2000). One chamber is white and brightly lit from above. The other chamber is black and dark. A door, which corresponds on each side to the color of the chamber that it faces, separates the two chambers. The flooring consists of stainless steel bars (0.2 cm in diameter) spaced 1 cm apart. On training day, all mice were habituated for 2 min to the light side. Following habituation, mice were placed in the light side for 1 min, at which point the door dividing the two chambers was lifted. Mice were permitted to cross over the dark side, where they received a mild shock (0.2 mA, 0.2 Hz, 1 s). Twenty-four hours later, mice were tested by placing them in the light side of the chamber for 1 min and allowing them to crossover to the dark, shock-associated side (maximum latency = 300s).
Conditioned fear
On training day, the mice were placed in the apparatus for a habituation period of 4 min. Following habituation, a tone was sounded, followed by the administration of an electric shock (2s duration, 0.5 mA). After 1 min, the tone and shock pairing was re-administered until three training trials were received. Five days later, mice were tested in either the contextual and cued conditions, with a minimum of 4h between testing occasions. Contextual and cued conditions were counter balanced to minimized order effects. In the contextual condition, which is mediated by the hippocampus (Kim et al, 1993; Sanders et al, 2003), animals were placed in the original chamber without the tone and were habituated for 4 min. The freezing behavior, indicative of an association between the environment and the aversive stimuli, were observed for eight 1 min intervals. In the cued learning condition, which is mediated by the amygdala (Kim et al, 1993), a black insert was placed in the chamber, along with almond extract. After a 4 min habituation period, the tone was sounded, and freezing behavior was observed for eight 1 min trials following the tone.
Radioimmunoassay
Following testing, animals were again administered, E2, T, 3α-diol, or vehicle, and killed 1 h later, a time analogous to testing. Mice were killed via cervical dislocation, trunk blood was collected, and whole brains were immediately put on dry ice. Tissue was stored at −80°C until radioimmunoassay.
Androgens were extracted from plasma with diethyl ether and trace amounts of 3H ligand. Ether was evaporated, and the pellets were reconstituted in phosphate assay buffer (pH = 7.4). For brain tissue, tissues were homogenized with a glass/teflon homogenizer in distilled water. Androgens were extracted from the homogenate with diethyl ether and dried down in a savant. Androgens were extracted from plasma with diethyl ether and trace amounts of 3H ligand. The ether was evaporated, and the pellets were reconstituted in phosphate assay buffer (pH = 7.4). Androgens were extracted from the homogenate with diethyl ether and dried down in a savant.
Plasma and hippocampal concentrations of T, 3α-diol, and E2 were measured according to previously published methods (Frye and Bayon, 1999; Frye et al, 1996a,b). The T antibody (T3-125; Endocrine Sciences, Calabasas Hills, CA) is moderately specific to T, with modest cross reactivity with DHT and negligible binding to other androgens. The 1:20000 dilution of this antibody binds between 60 and 65% of [3H]T (NET-387: specific activity = 51.0 Ci/mmol). The 3α-diol antibody (X-144; Dr PN Rao, Southwest Foundation for Biomedical Research, San Antonio, TX) is highly specific to 3 α-diol (Rao et al, 1977) and binds approximately 96% of [3H]3α-diol (NET-806: specific activity = 41.0 Ci/mmol). The E2 antibody (Dr Niswender, #244, Colorado State University, Fort Collins, CO) is highly specific to E2 (Hotchkiss et al, 1971) and binds approximately 90% of [3H]E2 (NET-317, 51.3 Ci/mmol).
Standard curves for all steroids were prepared in duplicate (range: 50–2000 pg). The standards were added to assay buffer, followed by addition of the appropriate antibody and [3H] steroid. T assay was incubated overnight at 4°C and the 3α-diol assay was incubated overnight at room temperature. The E2 assay was incubated at room temperature for 50 min.
Separation of bound and free was completed using rapid addition of dextran-coated charcoal. Following charcoal incubation, samples were centrifuged at 1200g. The supernatant was pipetted into a glass scintillation vial with scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt (1974), interpolation of the standards, and correction for recovery.
GABA-Stimulated Chloride Flux
Cortical synaptoneurosomes were prepared as described in Frye et al (1996a) from animals in vehicle-, T-, and 3 α-diol-treated groups. Freshly dissected cortices were homogenized in 7 vol of 20 μM Hepes-Tris buffer (118mM NaCl, 4.7 mM KCl, 1.189 mM MgSO4, 2.5 mM CaCl, pH 7.4) using six strokes of a glass-glass homogenizer. The homogenate was diluted to 30 vol with buffer and centrifuged at 1000g for 15 min. The pellet was re-suspended in buffer to yield a final protein concentration of 15–20 mg/ml and centrifuged again at 1000g for 15 min. Synaptoneurosomes and reaction tubes containing increasing concentrations of γ-aminobutyric acid (GABA, 0–1000 μM) and 0.5 μCi of 36Cl (specific activity 13.25–14.75 μCi/g: Dupont New England Nuclear, Boston, MA) were separately equilibrated at 30°C for 10 min. GABA-stimulated chloride influx was initiated by adding 100μl of synaptoneurosomes to test tubes containing GABA (0–1000 μM) and 0.5 μCi of 36Cl−. This reaction was terminated 10s later by addition of ice-cold picrotoxin (100μM) in Hepes-Tris buffer and vacuum filtration over GF/C filters. Filters were dried overnight in scintillation vials. The following day, scintillation cocktail (3ml) was added. The flux of 36Cl− through synaptoneurosomes was determined by standard liquid scintillation spectrometry, expressed as nanomoles 36Cl−/mg protein in the synaptoneurosomal preparation, which was determined using Bradford’s method (Bradford, 1976).
Data Analyses
One-way analyses of variance (ANOVAs), with Fisher’s post hoc tests, as appropriate, were used to evaluate effects of hormone condition (T, 3α-diol, E2, or vehicle) on behavioral and endocrine indices, as well as the Ec50 for GABA-stimulated chloride flux. The α level for statistical significance was P<0.05, and a trend was considered P<0.10.
RESULTS
Motor Behavior
Activity monitor
Aged intact mice administered T or 3α-diol tended to make more beam breaks in the activity monitor than did vehicle-administered mice (F(3,12) =2. 87, P = 0.08. However, hormone administration did not significantly influence the number of beam breaks made by young, intact (F(3,16) = 0.13, P = 0.94.) or young, GDX mice (F(3,16) = 0.17, P = 0.99; Table 1).
Table 1.
Behavior in the Activity Monitor, Roto-Rod, Open Field, Dark-Light Transition and Vogel Tasks of Male Mice Administered Vehicle or 1 rng/kg s.c. E2, T, or 3α-diol, 1 h before Testing
| Condition | Beam breaks | Roto-rod (fall time) | Total entries open field | Total entries dark– light transition | Number of licks vogel |
|---|---|---|---|---|---|
| Aged, intact mice | |||||
| Vehicle (n = 4) | 439 ± 53 | 22 ± 8 | 125 ± 29 | 11 ± 2 | 74 ± 13 |
| E2(n = 4) | 533 ± 39 | 16 ± 0.4 | I29±7* | 10 ± 0.3 | 104±7 |
| T(n = 4) | 713 ± 114# | 5 ± 2* | 245 ± 24* | 12 ± 4 | 143 ± 18* |
| 3α-diol(n = 4) | 677 ± 71* | 5 ± 1* | I84 ± 18* | 10 ± 2 | 219 ± 21* |
| Young, intact mice | |||||
| Vehicle (n = 5) | 1075 ± 45 | 61 ± 19 | 180±18 | 14 ± 1 | 50 ± 16 |
| E2(n = 5) | 1069 ± 127 | 83 ± 25 | 178 ± 37 | 14 ± 4 | 52 ± 16 |
| T(n = 5) | 1063 ± 116 | 65 ± 15 | 187 ± 14 | 13 ± 1 | 42 ± 15 |
| 3α-diol(n = 5) | 1148 ± 133 | 69 ± 13 | 222 ± 37 | 17 ± 2 | 41 ± 20 |
| Young, GDX mice. | |||||
| Vehicle(n = 5) | 855 ± 191 | 37 ± 16 | 194 ± 44 | 14 ± 2 | 39 ± 14 |
| E2(n = 5) | 883 ± 75 | 56 ±18 | 189 ± 17 | 15 ± 3 | 43 ± 7 |
| T(n = 5) | 886 ± 54 | 35 ± 8 | 197 ± 11 | 16 ± 1 | 46 ± 15 |
| 3α-diol (n = 5) | 871 ± 55 | 64 ± 19 | 193 ± 9 | 17 ± 1 | 89 ± 31 |
Denotes significant difference from vehicle (P <0.05)
denotes tendency to be different from control (P<0.10).
Roto-rod
Aged intact mice administered T or 3α-diol had a longer latency to fall in the roto-rod task than did vehicle-administered mice (F(3,12) = 3.51, P = 0.05). There was no effect on latency to fall from the roto-rod bar of hormone administration to young, intact (F(3,16) = 0.27, P = 0.85) and young, GDX mice (F(3,16) = 0.81, P = 0.51; Table 1).
Affective Measures
Open field
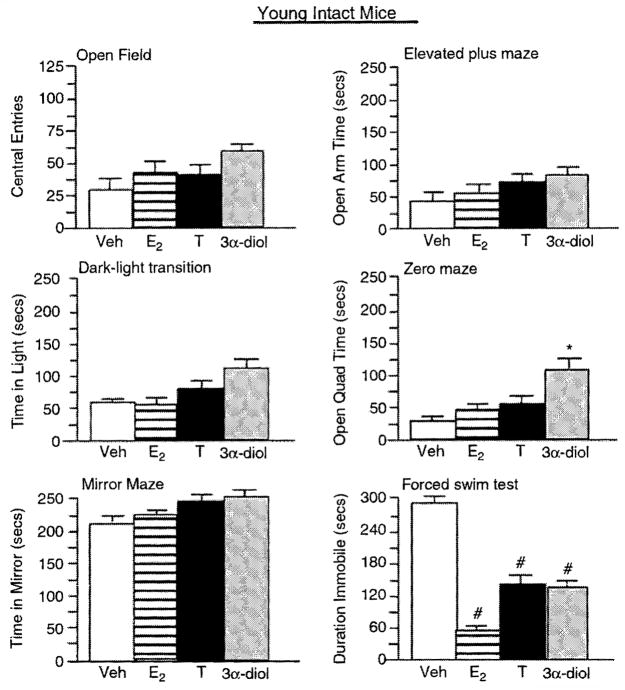
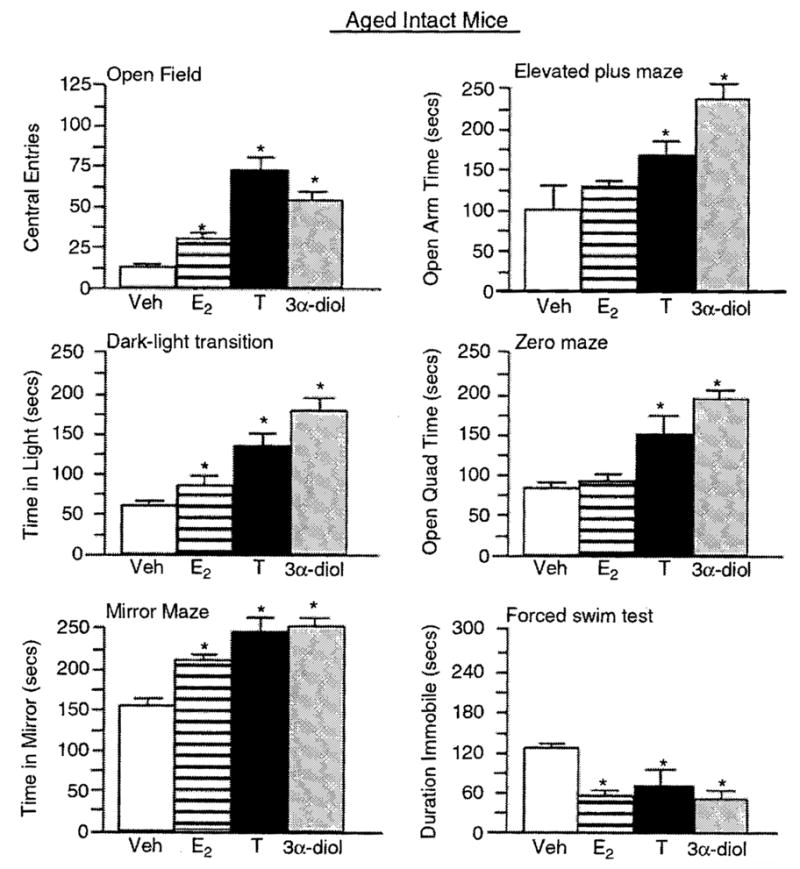
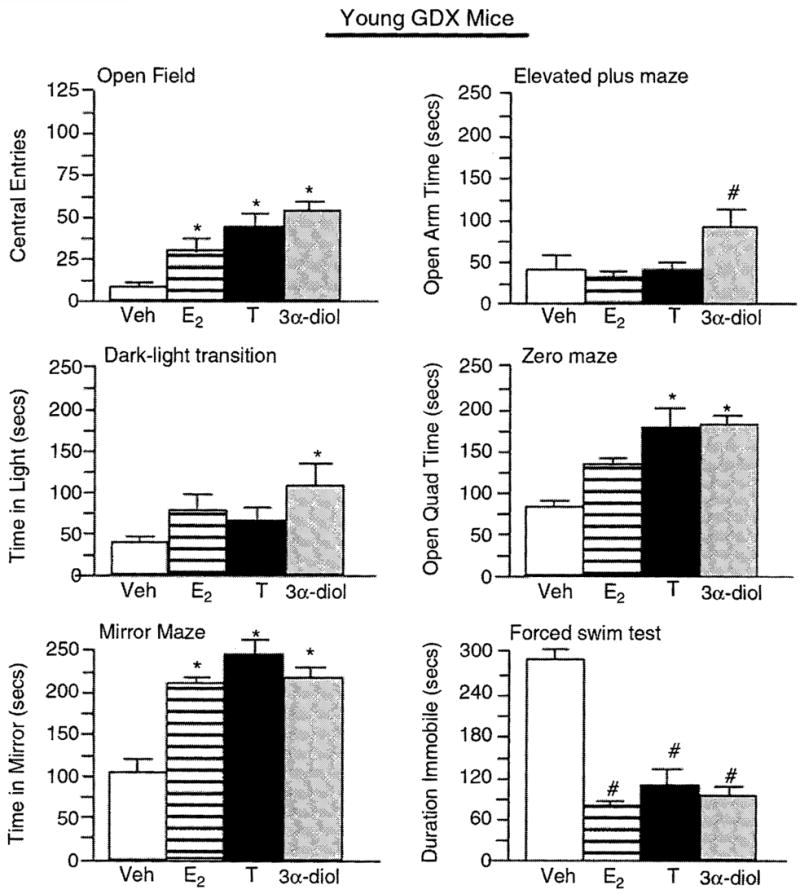
Aged intact male mice administered T, 3α-diol, or E2 made significantly more central (F(3,12) = 26.5, P = 0.001; Figure 1) and total (F(3,12) = 6.95, P = 0.006; Table 1) entries in the open field than did vehicle-administration. There was no significant effect of hormone administration to young, intact mice on central (F(3,16) = 1.18, P = 0.347; Figure 2) or total (F(3,16) = 0.515, P =0.67; Table 1) entries in the open field. However, T, 3α-diol, or E2 significantly increased central (F(3,16) = 7.526, P = 0.002; Figure 3) and total (F(3,16) = 0.018, P = 0.99; Table 1) entries in the open field when administered to young, GDX mice.
Figure 1.
Represents mean affective behavior for aged, intact male mice administered vehicle control (white bar), E2 (horizontally-striped bar), T (black bar), or 3α-diol (gray bar) in the open field (upper left panel), elevated plus maze (upper right panel), dark—light transition (middle left panel), zero maze (middle right panel), mirror maze (lower left panel), and forced swim (lower nght panel) taste. *Significant difference (P<0.05).
Figure 2.
Represents mean affective behavior for young, intact male mice administered vehicle control (white bar), E2 (horizontally-striped bar), T (black bar), or 3α-diol (gray bar) in the open field (upper left panel), elevated plus maze (upper right panel), dark–light transrtion (middle left panel), zero maze (middle right panel), mirror maze (lower left panel), and forced swim (lower right panel) tasks, *Significant difference (P<0.05), #denotes significant trend (P<0.10).
Figure 3.
Represents mean affective behavior for young, gonadectomized (GDX) mice administered vehicle control (white bar), E2 (horizontally-striped bar), T (black bar), or 3α-diol (gray bar) in the open field (upper left panel), elevated plus maze (upper right panel), dark–light transition (middle left panel), zero maze (middle right panel), mirror maze (lower left panel), and forced swim (lower right panel) tasks. *Denotes significant difference (P<0.05), #denotes significant trend (P<0.10).
Elevated plus maze
T or 3α-diol administration significantly increased the amount of time spent on the open arms of the elevated plus maze (F(3,12) = 8.4, P = 0.003; Figure 1), as compared to that of vehicle-administered aged, intact mice. Hormone administration did not significantly influence the time spent on the open arms of the plus maze of young, intact mice (F(3,16) = 0.14, P = 0.94; Figure 2). However, when administered to young, GDX mice, 3α-diol tended to increase open arm time (F(3,16) = 2.585, P = 0.08; Figure 3) over that of their E2− or T-administered counterparts.
Dark-light transition
Aged intact mice administered E2, T, or 3α-diol administration had significantly increased the time spent in the light side (F(3,12) = 47.1, P = 0.001; Figure 1) compared to vehicle-administered mice, but hormone administration did not influence the total number of entries made in the dark-light task (F(3,12) = 0.13, P = 0.94; Table 1) of aged mice. Young, intact mice administered hormones neither differ in the time spent on the light side (F(3,16) = 0.67, P = 0.59; Figure 2) nor in the number of entries made (F(3,16) = 0.56, P = 0.65; Table 1) compared to vehicle administration. Among young, GDX mice, there were no overall significant differences in the time spent on the light side (F(3,16) = 1.72, P = 0.20; Figure 3) or the number of entries made (F(3,16) = 0.43, P = 0.73; Table 1), but compared to vehicle, 3α-diol administration increased the time spent on the light side (P = 0.04).
Zero maze
T or 3α-diol administration significantly increased the amount of time spent on the open quadrants of the elevated zero maze compared to that of vehicle-administered aged, intact (F(3,12) = 34.9, P = 0.001; Figure 1) or young, GDX (F(3,16) = 3.384, P = 0.04; Figure 3) mice. There were no overall significant effects of hormone condition to young, intact mice (F(3,16) = 2.23, P =0.12; Figure 2), but compared to vehicle, 3α-diol increased the open quadrant time (P =0.02).
Mirror maze
T, 3α-diol, or E2, compared to vehicle administration, significantly increased the amount of the time spent in the mirrored chamber of aged, intact (F(3, 12) = 15.75, P = 0.002; Figure 1) or young, GDX (F(3, 16) = 4.07, P = 0.02; Figure 3), but not young, intact (F(3, 16) = 0.66, P = 0.59; Figure 2) mice.
Vogel conflict task—punished drinking
T or 3α-diol administration significantly increased the number of licks made in the Vogel punished drinking task (F(3, 12) = 16.35, P = 0.002; Table 1) compared to that of vehicle-administered aged, intact mice. There were no overall significant effects of hormone condition among either young, intact (F(3, 16) = 1.01, P = 0.96; Table 1) or young, GDX (F(3, 16) = 1.57, P = 0.24; Table 1) mice but for GDX mice compared to vehicle, 3α-diol tended to increase punished drinking (P = 0.07).
Forced swim test
T, 3α-diol, or E2 administration, compared to vehicle, decreased the time spent immobile in the forced swirn test significantly among aged, intact mice (F(3, 12) = 10.33, P = 0.001; Figure 1) and tended to decease immobility among young, intact (F(3, 16) = 2.68, P = 0.08; Figure 2) and GDX mice (F(3, 16) = 2.63, P = 0.08; Figure 3).
Cognitive Behavior
Inhibitory avoidance
Among aged, intact mice, T or 3α-diol administration (F(3, 12) = 19.66, P = 0.001; Figure 4) significantly increased the crossover latency to shock-associated side in the inhibitory avoidance task compared to that of vehicle-administered controls. Among young, intact mice, 3α-diol administration (F(3, 16) = 3.99, P = 0.02; Figure 5) significantly increased the crossover latency to shock-associated side in the inhibitory avoidance task compared to that of vehicle-administered controls. There were no significant effects among young, GDX mice (F(3, 16) = 1.57, P = 0.23; Figure 6).
Figure 4.
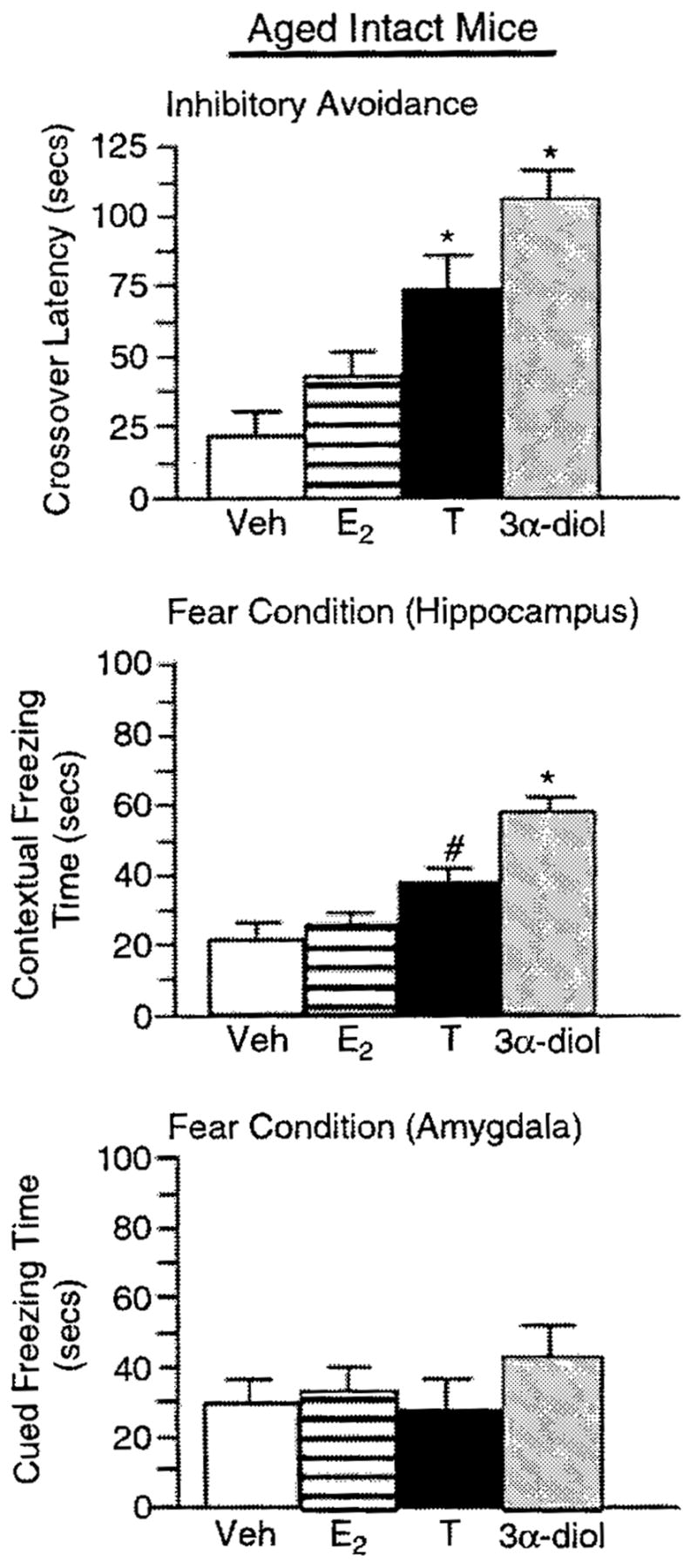
Represents mean cognitive behavior for aged, intact male mice administered vehicle control (white bar). E2 (horizontally-striped bar), T (black bar), or 3α-diol (gray bar) in the inhibitory avoidance task (top panel), the hippocampally-mediated portion of the conditioned contextual fear task (middle panel), and the amygdala-mediated portion of the conditioned contextual fear task (lower panel). *Denotes significant difference (P<0.05). #denotes significant trend (P<0.10).
Figure 5.
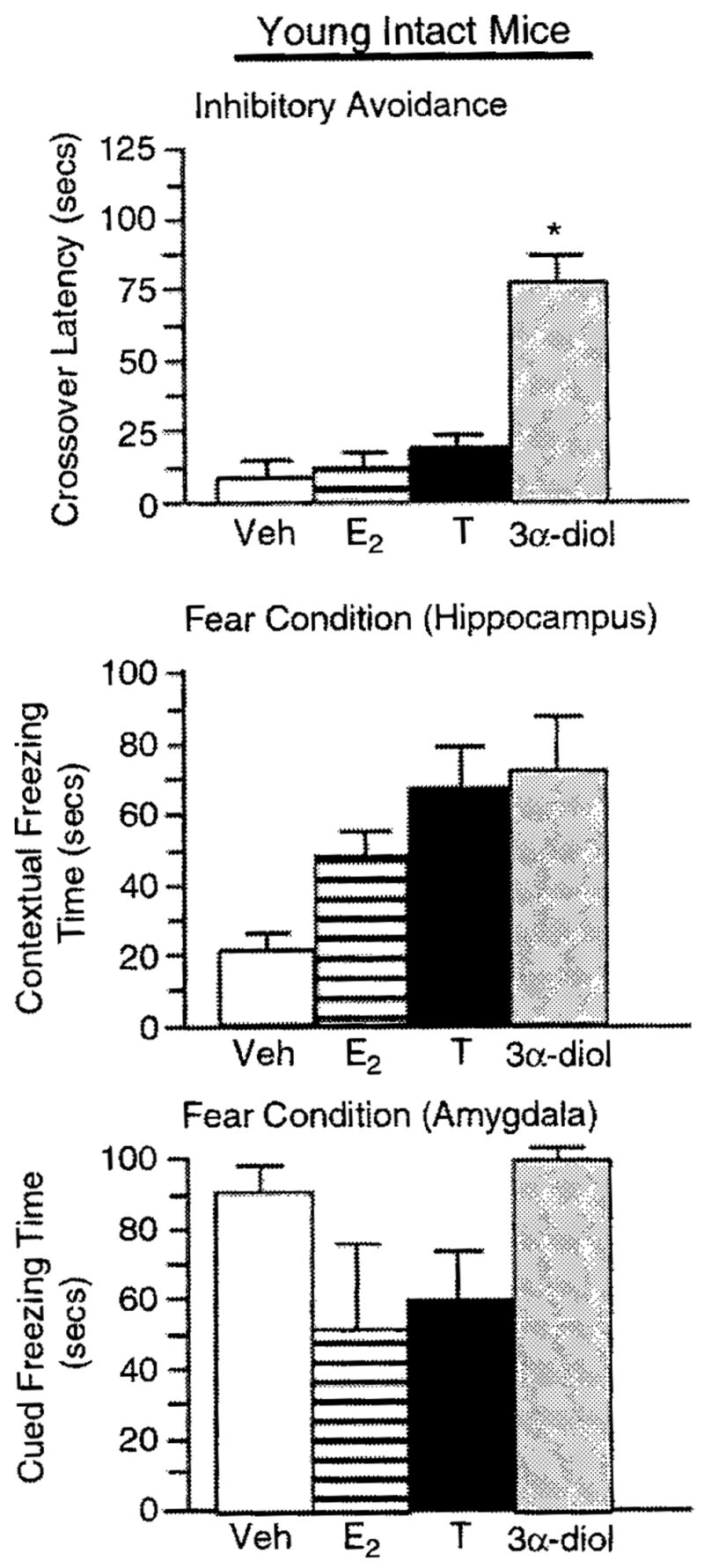
Represents mean cognitive behavior for young, intact male mice administered vehicle control (white bar), E2 (horizontally-striped bar). T (black bar), or 3α-diol (gray bar) in the inhibitory avoidance task (top panel), the hippocampally-mediated portion of the conditioned contextual fear task (middle panel), and the amygdala-mediated portion of the conditioned contextual fear task (lower panel). *Denotes significant difference (P<0.05).
Figure 6.
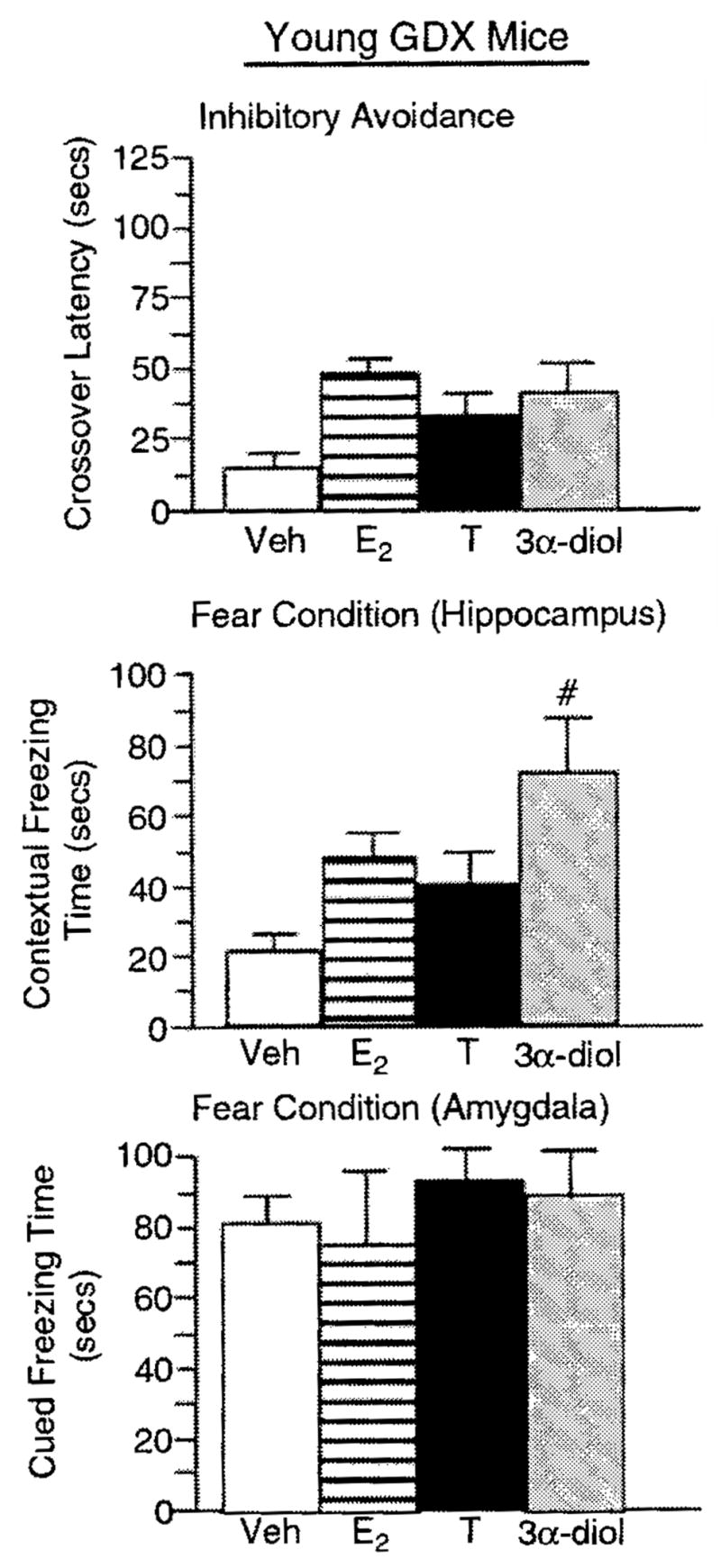
Represents mean cognitive behavior for young, gonadectomized (GDX) male mice administered vehicle control (white bar), E2 (horizontally-striped bar), T (black bar), or 3α-diol (gray bar) in the inhibitory avoidance task (top panel), the hippocampally-mediated portion of the conditioned contextual fear task (middle panel), and the amygdala-mediated portion of the conditioned contextual fear task (lower panel), #denotes significant trend (P<0.10).
Conditioned contextual fear
T administration tended to, and 3α-diol administration significantly, increased time spent freezing in the cued, hippocampally-mediated portion of the conditioned contextual fear task (F(3, 12) = 17.06, P = 0.001; Figure 4), but not in the contextual, amygdala-mediated portion of the task (F(3, 12) = 1.38, P = 0.29; Figure 4) compared to that of vehicle-administered aged, intact mice. There were no overall significant effects of hormone condition among young, intact (context-F(3, 16) = 0.508, P = 0.68; cued-F(3, 16) = 0.227, P = 0.87; Figure 5) or young, GDX (context-F(3, 16) = 1.472, P = 0.25; cued-F(3, 16) = 0.240, P = 0.99; Figure 6) mice but for GDX mice compared to vehicle, 3α-diol tended to increase freezing time in the contextual condition (P = 0.07).
Radioimmunoassay
Among aged, intact mice, systemic administration of E2 or T, but not 3α-diol, significantly increased plasma (F(3, 12) = 6.45, P = 0.007; Table 2) and hippocampal (F(3, 12) = 13.5 P = 0.004; Table 3) levels of E2 compared to that of vehicle-administered mice. Systemic administration of T, but neither 3α-diol nor E2, significantly increased plasma (F(3, 12) = 9.98, P = 0.001) and hippocampal (F(3, 12) = 3.37, P = 0.04) levels of T compared to that of vehicle-administered aged, intact mice. Administration of T or 3α-diol, but not E2, to aged male mice significantly increased plasma (F(3, 12) = 8.54, P = 0.002) and hippocampal (F(3, 12) = 25.6, P = 0.001) 3α-diol levels compared to that of vehicle-administered mice. Among young, intact mice, administration of E2 tended to increased levels of E2, in the hippocampus (F(3, 16) = 2.73, P = 0.078), but not plasma (F(3, 16) = 2.03, P = 0.149). Although administration of T or 3α-diol produced apparent increases in the levels of these hormones in plasma (T, F(3, 16) = 1.08, P = 0.385; 3α-diol, F(3, 16) = 1.50, P = 0.252) and the hippocampus (T, F(3, 16) = 1.17, P = 0.351; 3α-diol, F(3, 16)=1.50, P = 0.252), these effects were not statistically significant Among young, GDX mice, administration of E2 or T tended to increased plasma levels of E2 (F(3, 16) = 2.72, P = 0.078) and T (F(3, 16) = 2.84, P = 0.070). T or 3α-diol administration produced nonsignificant increases in circulating levels of 3α-diol (F(3, 16) = 1.537, P = 0.243). Effects in hippocampus were similar to plasma, but did not achieve statistical significance (E2, F(3, 16) = 2.38, P = 0.868; T, F(3, 16) = 0.490, P = 0.693; 3α-diol, F(3, 16) = 1.407, P = 0.277).
Table 2.
Mean Concentrations E2, T, or 3α-diol in the Plasma of aged, Intact; Young, Intact; and Young, GDX Male Mice 1 h Following Administration of Vehicle or 1 mg/kg s.c. E2, T, or 3α-diol, 1 h before Testing
| Condition | E2 (pg/mg) | T (ng/mg) | 3α-diol (ng/mg) |
|---|---|---|---|
| Aged, intact mice | |||
| Vehicle (n = 4) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| E2 (n = 4) | 2.0 ± 0.3* | 0.5 ± 0.2 | 0.8 ± 0.2 |
| T(n = 4) | 1.4 ± 0.3* | 2.9 ± 1.7* | 1.6 ± 0.2* |
| 3α-diol (n = 4) | 0.5 ± 0.2 | 0.4 ± 0.04 | 2.5 ± 0.6* |
| Young, intact mice | |||
| Vehicle (n = 5) | 7.5 ± 3.0 | 2.7 ± 1.5 | 6.4 ± 5.0 |
| E2 (n = 5) | 9.3 ± 1.2 | 1.8 ± 0.8 | 4.4 ± 2.3 |
| T(n = 5) | 2.6 ± 1.7 | 5.1 ±1.7 | 2.6 ± 1.5 |
| 3α-diol (n = 5) | 5.6 ± 1.7 | 4.2 ±1.5 | 9.5 ± 3.3 |
| Young. GDX mice | |||
| Vehicle (n = 5) | 0.8 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.1 |
| E2(n = 5) | 2.0 ± 0.7# | 0.4 ± 0.3 | 0.2 ± 0.1 |
| T(n = 5) | 2.8 ± 1.8# | 0.3 ± 0.2 | 0.6 ± 0.1 |
| 3α-diol (n = 5) | 0.6 ± 0.3 | 0.3 ± 0.2 | 0.6 ±0.2 |
Denotes significant difference from vehicle (P < 0.05).
denotes tendency to be different from control (P <0.10).
Table 3.
Mean Concentrations E2, T, or 3α-diol in the Hippocampus of Aged Intact, Young Intact, and Young GDX Male Mice 1 h Following Administration of Vehicle or 1 mg/kg s.c. E2, T, or 3α-diol, 1 h before Testing
| Condition | E2 (pg/mg) | T (ng/mg) | 3α-diol (ng/mg) |
|---|---|---|---|
| Aged, intact mice | |||
| Vehicle (n = 4) | 0.9 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 |
| E2 (n = 4) | 6.6 ± 1.3* | 0.6 ± 0.3 | 0.7 ± 0.1 |
| T(n = 4) | 6.7 ± 1.2* | 2.6 ± 0.4* | 2.0 ± 0.4* |
| 3α-diol (n = 4) | 1.3 ± 0.2 | 0.4 ± 0.1 | 3.2 ± 0.8* |
| Young intact mice | |||
| Vehicle (n = 5) | 0.8 ± 0.4 | 1.0 ± 0.3 | 0.8 ± 0.4 |
| E2 (n = 5) | 2.0 ± 0.2# | 0.6 ± 0.3 | 0.8 ± 0.4 |
| T(n = 5) | 0.6 ± 0.2 | 1.8 ± 0.4 | 0.6 ± 0.2 |
| 3α-diol (n = 5) | 0.8 ± 0.4 | 1.4 ± 0.4 | 2.0 ± 0.6 |
| Young, GDX mice | |||
| Vehicle (n = 5) | 0.4 ± 0.3 | 0.4 ± 0.1 | 0.8 ± 0.2 |
| E2(n = 5) | 0.6 ± 0.5 | 0.4 ± 0.2 | 0.3 ± 0.2 |
| T(n = 5) | 0.7 ± 0.5 | 0.7 ± 0.1 | 1.3 ± 0.4 |
| 3α-diol(n = 5) | 0.3 ± 0.2 | 0.4 ± 0.2 | 1.3 ± 0.2 |
Denotes significant difference from vehicle (P< 0.05),
denotes tendency to be different from control (P<0.10).
GABA-Stimulated Chloride Flux
Mice administered 3α-diol required less GABA to produce half-maximal increases in GABA-stimulated chloride influx. This was not statistically significant among aged, intact mice (F(2, 12) = 1.654, P =0.23: Table 4), tended to be significant among young, intact (F(2, 12) = 1.812, P = 0.09: Table 4) mice and achieved significance in young, GDX mice (F(2, 12) = 5.738, P = 0.01: Table 4).
Table 4.
Mean Concentrations of EC 50 in the GABA-Stimulated Chloride Flux of Aged, Intact; Young, Intact; and Young, GDX Male Mice 1 h Following Administration of Vehicle, 1 mg/kg s.c, T, or 3α-diol, 1 h before Testing
| Condition | Mean concentrations of Ec 50 (μM) |
|---|---|
| Aged, intact mice | |
| Vehicle | 60 ± 19 |
| T | 64 ± 22 |
| 3α-diol | 22 ± 11 |
| Young, intact mice | |
| Vehicle | 70 ± 12 |
| T | 44 ± 17* |
| 3α-diol | 26 ± 9.8* |
| Young, GDX mice | |
| Vehicle | 60 ± 10 |
| T | 18 ± 8.0* |
| 3α-diol | 26 ± 9.7* |
Denotes significant difference from vehicle (P <0.05),
denotes tendency to be different from control (P <0.10).
DISCUSSION
Our findings supported our hypothesis that 3α-diol administration would enhance cognitive and affective behavior of aged male mice with low endogenous androgen levels. In support, administration of T, 3α-diol, or E2 significantly increased anti-anxiety behavior of aged mice in the open field, dark-light transition, mirror maze, and forced swim tests. However, only T and 3α-diol significantly increased the anti-anxiety behavior of aged mice in the elevated plus maze and zero maze tasks. In the inhibitory avoidance task, only T and 3α-diol significantly enhanced the cognitive performance. In the conditioned contextual fear task, T tended to increase, and 3α-diol significantly increased, the cognitive performance in the hippocampally-mediated portion, but not the amygdala-mediated aspect, of the task. Administration of T or 3α-diol to aged mice enhanced the cognitive and/or affective performance in some or all of the tasks, and significantly increased the 3α-diol levels in plasma and in the hippocampus (without increasing E2 or T levels). In addition, 3α-diol administration was more effective than T and/or vehicle administration at enhancing GABA-stimulated chloride flux. Notably, these patterns of effects were similar to that of young, adult mice that were GDX, not intact. Together, these findings suggest that aging-induced decrements in affective and cognitive performance may be a result of decreased endogenous androgen levels, and that some of these effects may be reversed through administration of 3α-diol.
The present findings support previous findings that 5α-reduced metabolites are important for androgens’ beneficial influence on cognitive and affective behaviors. Previous findings have indicated that administration of T and the nonaromatizable metabolite, 3α-diol, to GDX rats significantly enhances behavior in affective and cognitive tasks (Edinger and Frye, 2004, 2005; Edinger et al, 2004; Frye and Seliga, 2001). In support, the present findings indicate that the administration of T or 3α-diol to aged male mice decreases anxiety-like behavior and increases cognitive performance. The present findings also support previous findings that some of androgens’ actions may take place in the hippocampus. Previous reports have found that intrahippocampal administration of T or 3α-diol to GDX male rats can increase anti-anxiety behavior and enhance cognitive performance to levels similar to GDX rats administered androgens systemically. In the present experiment, 3α-diol administration to aged male mice enhanced the performance in hippocampally-mediated portion of the conditioned contextual fear tasks, but not in the amygdala-mediated portion of this task (Kim et al, 1993; Sanders et al, 2003). In addition, systemic administration of T and 3α-diol increased 3α-diol levels in the hippocampus. Together, these findings suggest that androgens may have effects to decrease anxiety and enhance cognitive performance, which may reflect increases in central nervous system (CNS) arousal (Pfaff, 2006), in part through actions of the nonaromatizable metabolite, 3α-diol, in the hippocampus.
The present findings extend previous findings to suggest that androgen decline as a result of aging produces behavioral deficits in affective and cognitive tasks, and that these deficits can be reduced through systemic androgen replacement. Studies in female rodents have indicated that aging can result in a decline in cognitive and affective performance that can be reversed through systemic E2 administration (Frick et al, 2002; Gresack and Frick, 2006; Markham et al, 2002). Although a number of studies in men have suggested that deficits in affective and cognitive performance can be reduced through androgen administration (Gruenewald and Matsumoto, 2003; Harman, 2005), there have not been comprehensive studies to investigate the mechanism of this effect. The present study extends these findings to aged male mice to suggest that androgen administration can enhance affective and cognitive performance, and that this may be due, in part, to metabolism to 3α-diol. In support, the nonaromatizable metabolite, 3α-diol, was the only hormone administered that consistently enhanced the behavior across affective and cognitive measures. Together, these findings suggest that aging can result in deficits in affective and cognitive performance (perhaps due to effects on CNS arousal) that can be reversed through systemic androgen replacement.
Although the present findings indicated that androgens’ effects may be due, in part, to actions of 3α-diol, this does not preclude actions of T′s other metabolites. T can also be aromatized to E2, which, in the present experiment, increased anti-anxiety behavior in the open field, light-dark transition, mirror maze, and forced swim tasks, and enhanced cognitive performance in the inhibitory avoidance task. Declining E2 levels as a result of menopause have been shown to increase the incidence of anxiety and mood disorders, and to decrease performance in visuospatial tasks (Miller et al, 2002). In animals, E2 administration enhances cognitive performance and anti-anxiety behavior of female rodents (Frye et al, 2005; Palermo-Neto and Dorce, 1990; Rhodes and Frye, 2006; Walf and Frye, 2005). It is possible that T′s effects on cognitive and affective behavior are a result of its aromatization to E2. However, aromatase knockout mice, which lack the enzyme necessary to convert T to E2 and thus have very low endogenous E2 levels, exhibit normal anxiety levels and depressive symptomology (Dalla et al, 2005). In addition, in the present study, administration of the nonaromatizable metabolite, 3α-diol, consistently enhanced cognitive and affective behavior, and E2 administration did not improve cognitive performance, and was inconsistent at improving performance in affective tasks. It is also possible that 3α-diol was more effective than was E2 in this study because aged male mice were utilized, that may be more sensitive to androgens than E2.
As mentioned previously, T can be metabolized to DHT, which can be further metabolized to 3α-diol. While T and DHT have been shown to have actions at androgen receptors (ARs), 3α-diol does not typically bind to ARs, and has been shown to have actions at GABAA receptors (GBRs; Frye et al 1996a,b; Roselli et al, 1987) or at estrogen receptor (ER)-β in the hippocampus (Edinger and Frye 2007a; Kaminski et al, 2005; Pak et al, 2005). However, blocking ARs in the hippocampus with flutamide has been shown to increase anxiety-like behavior (Edinger and Frye, 2006) and to decrease cognitive performance (Edinger and Frye, 2007b) of intact and DHT-replaced male rats. Although blocking ARs can produce these negative cognitive and affective behaviors, blocking DHT’s metabolism to 3α-diol can also increase anxiety-like behavior and decrease cognitive performance of intact and DHT-replaced rats (Frye et al, 2004a; Frye and Edinger, 2004). In the present study, administration of the nonaromatizable metabolite, 3α-diol, consistently enhanced anti-anxiety behavior and cognitive performance, and that was the only hormone consistently elevated in treatment groups that produced beneficial behaviors. Thus, T′s effects to enhance affective and cognitive performance may be due, in part, to actions of 5α-reduced metabolites, such as 3α-diol, in the hippocampus.
In addition, androgens’ actions in the hippocampus do not preclude their actions in other brain regions. Androgen administration can increase c-Fos and Fos-related antigens in the central nucleus of the amygdala, the nucleus accumbens, and the frontal cortex (Johansson-Steensland et al, 2002). T administration, directly to the amygdala or nucleus accumbens, has been shown to enhance learning in the conditioned place preference (Frye et al, 2002; Rosellini et al, 2001) and water maze (Naghdi et al, 2003) tasks. In addition, female rats exposed to T have fewer cognitive deficits in response to frontal cortex lesions than do untreated controls (Forgie and Kolb, 1998). However, in this experiment, and others (Edinger et al, 2004), androgen administration enhanced the performance in hippocampally-mediated, but not in amygdala-mediated portion of the conditioned contextual fear task (Kim et al, 1993; Sanders et al, 2003). The 3α-diol regimen utilized here enhanced the GABA-stimulated chloride influx in cortical synaptoneurosomes, and presumably elsewhere in the brain. The extent to which effects on performance may be related to these actions of 3α-diol in cortical and/or other tissues is the subject of ongoing investigation.
In summary, these findings suggest that aging can result in androgen decline that can produce decrements in affective and cognitive performance. Some of these deficits can be reduced through systemic administration of T, T′s nonaromatizable metabolite, 3α-diol, or T′s aromatized metabolite, E2. However, the only androgen that consistently and significantly enhanced affective and cognitive behavior across tasks, and was consistently elevated in plasma and in the hippocampus, was 3α-diol. Together, these findings suggest that aging-induced decrements in affective and cognitive performance can be attenuated through systemic administration of 3α-diol. Given the aging population, it will be particularly important to investigate the mechanism of this effect in order to produce more effective androgen replacement therapies.
Acknowledgments
Support for this research was provided by extramural funding to CAF from The National Institute of Mental Health (MH 06769801) and The National Science Foundation (IBN 98-96263, IBN 03-16083) and an intramural faculty research award grant to CAP.
Footnotes
DISCLOSURE/CONFLICT OF INTERESTS
The authors neither have any conflict of interest relating to the subject of this report nor do they have financial holdings (stocks, bonds or donations of supplies or equipment) that a reasonable person would construe as possibly influencing the objectivity of the report. Professor Frye has received compensation as a consultant for The Biocontinuum Group. She has received grant support from NSF, NIMH, NIDA, NIAA, The Department of Defense and The Epilepsy Foundation of America.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male mice. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, et al. Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M, et al. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wikland L, Eriksson E. High doses of testosterone increase anti-conflict behavior in rat. Eur Neuropsychopharmacol. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellog CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butler SS. Evaluating the Senior Companion Program A Mixed-Method Approach. J Gerontol Soc Work. 2006;47:45–70. doi: 10.1300/J083v47n01_05. [DOI] [PubMed] [Google Scholar]
- Cafri G, van den Berg P, Thompson JK. Pursuit of muscularity in adolescent boys: relations among biopsychosocial variables and clinical outcomes. J Clin Child Adolesc Psychol. 2006;35:283–291. doi: 10.1207/s15374424jccp3502_12. [DOI] [PubMed] [Google Scholar]
- Ceccareli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persist pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123:65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Clark CR, Paul RH, Williams LM, Arns M, Fallahpour K, Handmer C, et al. Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Arch Clin Neuropsychol. 2006;21:449–467. doi: 10.1016/j.acn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Male aromatase-knockout mice exhibit normal levels of activity, anxiety and ‘depressive-like’ symptomatology. Behav Brain Res. 2005;163:186–193. doi: 10.1016/j.bbr.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Davis S. Testosterone deficiency in women. J Reprod Med. 2001;46:291–296. [PubMed] [Google Scholar]
- Delhez M, Hansenne M, Legros JJ. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology. 2003;28:863–874. doi: 10.1016/s0306-4530(02)00102-6. [DOI] [PubMed] [Google Scholar]
- Drake EB, Henderson VW, Stanczyk FZ, McCleary CA, Brown WS, Smith CA, et al. Associations between circulating sex steroid hormones and cognition in normal elderly women. Neurology. 2000;54:599–603. doi: 10.1212/wnl.54.3.599. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav. 2006;50:216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens effects to enhance learning and memory may be mediated in part by actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007a;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol Learn Mem. 2007b;87:201–208. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned Fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Orchidectomy sensitizes male rats to the action of diazepam on burying behavior latency: role of testosterone. Pharmacol Biochem Behav. 2003;75:473–479. doi: 10.1016/s0091-3057(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Kolb B. Sex differences in the effects of frontal cortex injury: role of differential hormonal experience in early development. Behav Neurosci. 1998;112:141–153. doi: 10.1037//0735-7044.112.1.141. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synatophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3alpha, 5alpha-THP and 3alpha-Diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004a;29:1019–1027. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci. 2001;1:172–182. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-and rostanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5alpha-reduced metabolites. Pharmacol Biochem Behav. 2002;74:119–127. doi: 10.1016/s0091-3057(02)00968-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, et al. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996a;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3 alpha-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and PDA. Behav Neurosci. 1996b;110:603–612. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- Frye CA, Waif AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res. 2004b;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- Haren MT, Wittert GA, Chapman IM, Coates P, Morley JE. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas. 2005;50:124–133. doi: 10.1016/j.maturitas.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Haren MT, Morley JE, Chapman IM, O’Loughlin PD, Wittert GA. Defining ‘relative’ androgen deficiency in aging men: how should testosterone be measured and what are the relationships between androgen levels and physical, sexual, and emotional, health. Climacteric. 2002;5:15–25. [PubMed] [Google Scholar]
- Harman SM. Testosterone in older men after the Institute of Medicine Report: where do we go from here? Climacteric. 2005;8:124–135. doi: 10.1080/13697130500118001. [DOI] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J, Atkinson LE, Knobil E. Time course of serum estrogen and luteinizing hormone (LH) concentrations during the menstrual cycle of the rhesus monkey. Endocrinology. 1971;89:177–183. doi: 10.1210/endo-89-1-177. [DOI] [PubMed] [Google Scholar]
- Houri D. Effects of central acting drugs on the mirror staircase test. Nippon Yakurigaki Zasshi. 1986;87:135–142. doi: 10.1254/fpj.87.135. [DOI] [PubMed] [Google Scholar]
- Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res. 2001;56(Suppl 1):86–92. doi: 10.1159/000048142. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Johansson-Steensland P, Nyberg F, Chahl L. The anabolic androgenic steroid, nandrolone decanoate, increases the density of Fos-like immunoreactive neurons in limbic regions of guinea-pig brain. Eur J Neurosci. 2002;15:539–544. doi: 10.1046/j.0953-816x.2001.01877.x. [DOI] [PubMed] [Google Scholar]
- Kaminetsky JC. Benefits of a new testosterone gel formulation for hypogonadal men. Clin Cornerstone. 2005;7(Suppl 4):S8–S12. doi: 10.1016/s1098-3597(05)80091-2. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Lamberty Y. The mirror chamber test for testing anxiolytics: is there a mirror-induced stimulation? Physiol Behav. 1998;64:703–705. doi: 10.1016/s0031-9384(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Li JY, Zhu JC, Dou JT, Bai WJ, Deng SM, Li M, et al. Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male. 2002;5:47–51. [PubMed] [Google Scholar]
- Lund BC, Bever-Stille KA, Perry PJ. Testosterone and andropause: the feasibility of testosterone replacement therapy in elderly men. Pharmacotherapy. 1999;19:951–956. doi: 10.1592/phco.19.11.951.31574. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markou A, Duka T, Prelevic GM. Estrogens and brain function. Hormones. 2005;4:9–17. doi: 10.14310/horm.2002.11138. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Conney JC, Rasgon NL, Fairbanks LA, Small GW. Mood symptoms and cognitive performance in women estrogen users and nonusers and men. J Am Geriatr Soc. 2002;50:1826–1830. doi: 10.1046/j.1532-5415.2002.50511.x. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone ethanate and flutamide into the basolateral nucleus of the amygdala in the Morris Water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283–293. doi: 10.3109/01674829309084451. [DOI] [PubMed] [Google Scholar]
- Orengo CA, Fullerton G, Tan R. Male depression: a review of gender concerns and testosterone therapy. Geriatrics. 2004;59:24–30. [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Dorce VA. Influences of estrogen and/or progesterone on some dopamine-related behavior in rats. Gen Pharmacol. 1990;21:83–87. doi: 10.1016/0306-3623(90)90600-q. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279–294. doi: 10.1016/s0889-8529(05)70247-4. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Harvard University Press; Cambridge, MA: 2006. [Google Scholar]
- Rao PN, Khan AH, Moore PH., Jr Synthesis of new steroid haptens for radioimmunoassay. Part III. I5beta-Carboxyethyl-mercaptosteroid-bovine serum albumin conjugates. Specific antisera for radioimmunoassay of 5alpha-dihydrotestosterone, 5alpha-androstane-3beta, 17beta-diol and 5alpha-androstane-3alpha, 17beta-diol. Steroids. 1977;29:171–184. doi: 10.1016/0039-128x(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor−/− mice. Eur J Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hurt DM. Radioimmunoassays and Related Procedures in Medicine. International Atomic Energy Agency; Vienna, Austria: 1974. Statistical analysis of radioimmunoassay and immunoradiometric (labeled antibody) assays: a generalized weighted, iterative, least-squares method for logistic curve fitting; pp. 165–192. [Google Scholar]
- Rojas-Ortiz YA, Rundle-Gonzalez V, Rivera-Ramos I, Jorge JC. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Horm Behav. 2006;49:123–128. doi: 10.1016/j.yhbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Brain Res Rev. 2001;37:162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Seidman SN. Testosterone deficiency and mood in aging men: pathogenic and therapeutic interactions. World J Biol Psychiatry. 2003;4:14–20. doi: 10.3109/15622970309167905. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Trends Pharmacol Sci. 2002;23:527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- Sternbach H. Age-associated testosterone decline in men: clinical issues in psychiatry. Am J Psychiatry. 1998;155:1310–1318. doi: 10.1176/ajp.155.10.1310. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychophannacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Cognitive functions and sex steroids. Ann Endocrinol. 2003;64:158–161. [PubMed] [Google Scholar]