Abstract
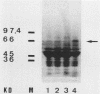
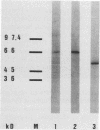
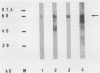
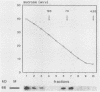
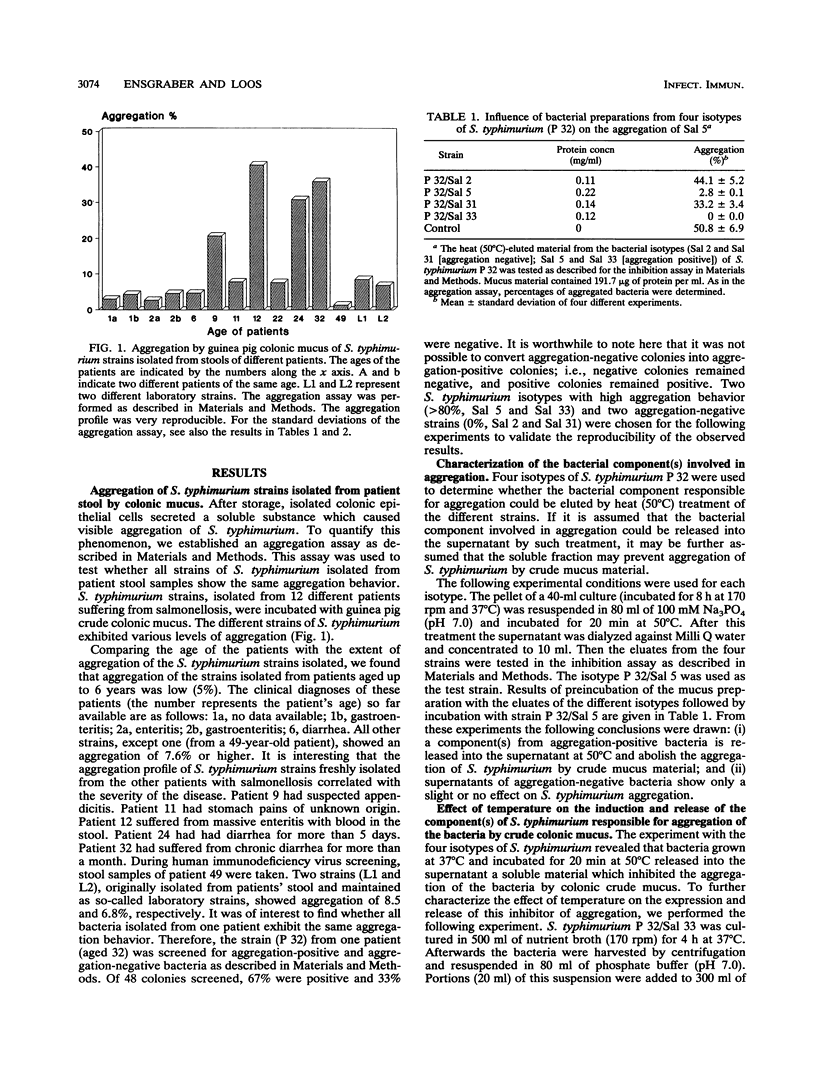
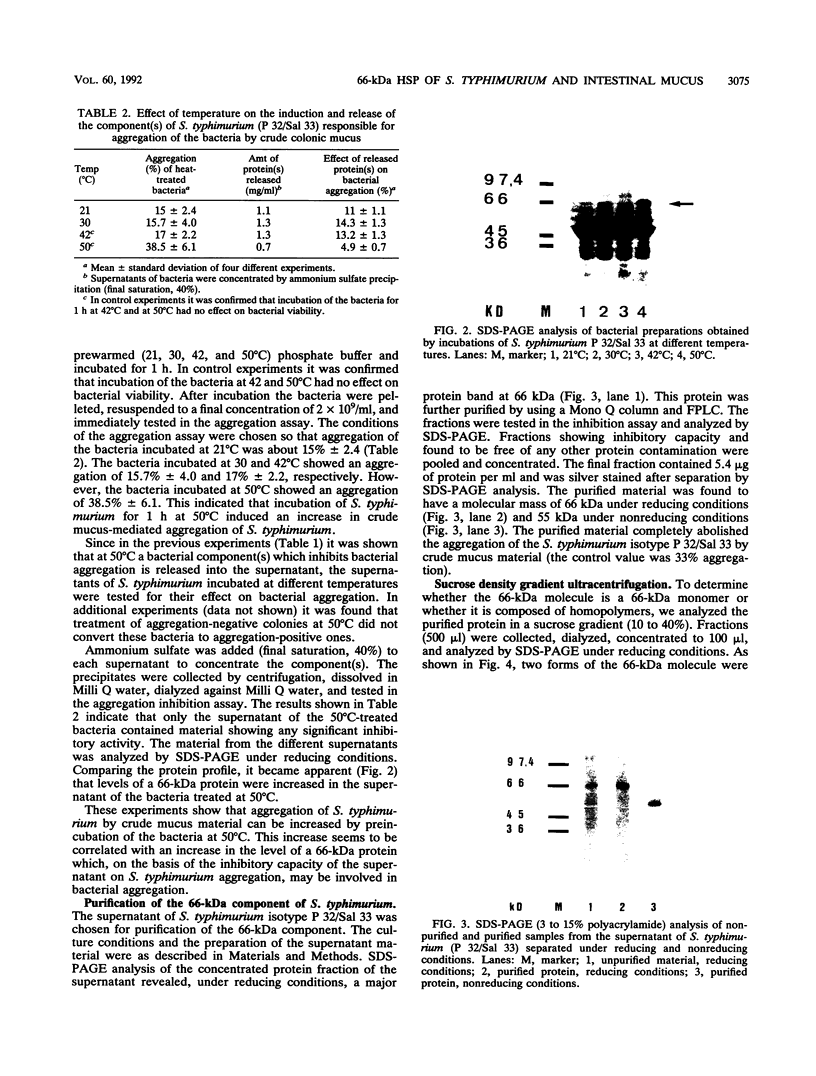
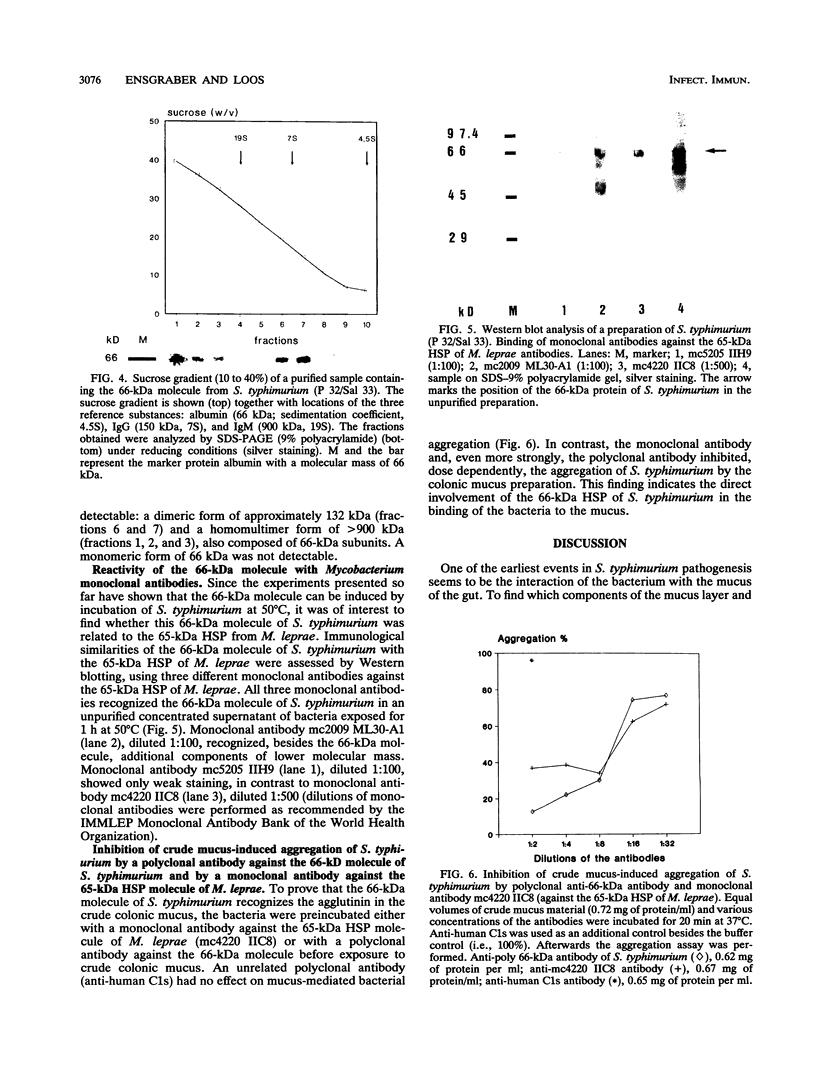
Salmonella typhimurium infections have increased during the last few years. However, the interplay of virulence factors in S. typhimurium pathogenesis is still poorly understood, particularly with regard to the mechanisms and components of the bacterium which are involved in its interaction with the intestinal mucus. We have observed that S. typhimurium is aggregated by incubation with colonic mucus (guinea pig model). To quantify this phenomenon, an aggregation assay was established. By using this assay, it was found that the aggregation profile of S. typhimurium strains freshly isolated from patients (age 9 and older) with salmonellosis correlated with the severity of the disease. An isolate with high aggregation behavior was chosen for characterization of the bacterial component involved in binding to colonic mucus material. The component of S. typhimurium responsible for aggregation was purified and characterized as a 66-kDa protein which was able to completely inhibit mucus-mediated bacterial aggregation. This protein was recognized by monoclonal antibodies against the 65-kDa heat shock protein (HSP) of Mycobacterium leprae. The 66-kDa protein of S. typhimurium was inducible by incubating the bacteria at 50 degrees C and was secreted into the supernatant, from which it could be isolated in both dimeric and polymeric forms. The monoclonal anti-HSP 65, as well as a polyclonal antibody against the 66-kDa protein of S. typhimurium, caused dose-dependent inhibition of the aggregation of S. typhimurium by crude mucus preparations. This is the first report showing that a bacterial HSP is involved in mucus-mediated interaction of pathogens with the host.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Mayet W. J., Lohse A. W., Grevenstein J., Meyer zum Büschenfelde K. H., Fleischer B. Proliferative response of synovial fluid and peripheral blood mononuclear cells to arthritogenic and non-arthritogenic microbial antigens and to the 65-kDa mycobacterial heat-shock protein. Med Microbiol Immunol. 1990;179(4):215–224. doi: 10.1007/BF00195252. [DOI] [PubMed] [Google Scholar]
- Hickey E. W., Hirshfield I. N. Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl Environ Microbiol. 1990 Apr;56(4):1038–1045. doi: 10.1128/aem.56.4.1038-1045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydyard P. M., van Eden W. Heat shock proteins: immunity and immunopathology. Immunol Today. 1990 Jul;11(7):228–229. doi: 10.1016/0167-5699(90)90091-m. [DOI] [PubMed] [Google Scholar]
- Mackey B. M., Derrick C. Heat shock protein synthesis and thermotolerance in Salmonella typhimurium. J Appl Bacteriol. 1990 Sep;69(3):373–383. doi: 10.1111/j.1365-2672.1990.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout W. R., Formal S. B., Dammin G. J., Giannella R. A. Pathophysiology of Salmonella diarrhea in the Rhesus monkey: Intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974 Jul;67(1):59–70. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Yano S., Slomiany B. L., Glass G. B. Lipid composition of the gastric mucous barrier in the rat. J Biol Chem. 1978 Jun 10;253(11):3785–3791. [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]