Abstract
17β-Estradiol (E2) and progesterone (P4) influence the onset and duration of sexual behavior and are also associated with changes in behaviors that may contribute to mating, such as exploration, anxiety, and social behaviors (socio-sexual behaviors). In the midbrain ventral tegmental area (VTA), the P4 metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), modulates lordosis of E2-primed rodents; 3α,5α-THP can also influence anxiety and social behaviors. To examine if 3α,5α-THP in the VTA mediates socio-sexual behaviors, we infused 3α,5α-THP to the VTA of diestrous and proestrous rats. As expected, proestrous, compared to diestrous, rats showed more exploratory (open field), anxiolytic (elevated plus maze), pro-social (partner preference, social interaction), and sexual (paced mating) behavior and had increased E2, P4, dihydroprogesterone (DHP), and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, and cortex. Infusions of 3α,5α-THP to the VTA, but not control sites, such as the substantia nigra (SN) or central grey (CG), of diestrous rats produced behavioral and endocrine effects akin to that of proestrous rats and increased DHP and 3α,5α-THP levels in midbrain, hippocampus, and diencephalon. Levels of DHP and 3α,5α-THP, but neither E2 nor P4 concentrations, in midbrain, hippocampus, diencephalon, and/or cortex were positively correlated with socio-sexual behaviors. Thus, 3α,5α-THP infusions to the VTA, but not SN or CG, can enhance socio-sexual behaviors and increase levels in midbrain, hippocampus, and diencephalon.
Keywords: Progesterone, Allopregnanolone, Lordosis, Affect, GABA
1. Introduction
Increases in the ovarian hormones, 17α-estradiol (E2) and progesterone (P4), which occur during behavioral estrus of rodents, modulate mating behavior, typically operationally defined as the occurrence and incidence of lordosis (the stereotypical posture that female rodents exhibit in response to male-typical stimuli in order for successful mating to occur). Systemic or intra-brain administration of E2 and/or P4 to the ventromedial hypothalamus (VMH) of ovariectomized rats reveals that actions of these hormones in this area are sufficient to initiate lordosis [1,2]. However, P4 also has effects in other brain regions, such as the ventral tegmental area (VTA) to influence the duration and intensity of lordosis [3,4]. In addition to differences in the effects of P4 in the VMH and VTA to alter sexual behavior, there are also differences in P4's mechanisms of action in these regions. In the VMH, E2 and P4 initiate lordosis through classical actions at intracellular progestin receptors (PRs). However, in the VTA, P4 modulates the duration and intensity of lordosis responses following metabolism to, and/or de novo synthesis of, 5α-pregnan-3α-ol-20-one (3α,5α-THP) and its subsequent actions at GABAA, dopamine-like type 1, and/or NMDA receptors and subsequent downstream signal transduction processes [5-12]. By using lordosis as a bioassay, we have elucidated that these are some of the mechanisms by which 3α,5α-THP has actions in the VTA to mediate mating. However, whether 3α,5α-THP may also influence other components of female reproductive behavior is also of interest.
Separate reports suggest that E2 and 3α,5α-THP can modulate behaviors other than lordosis. Exploration, anti-anxiety, and pro-social behaviors of female rodents are increased during behavioral estrus, when E2 and 3α,5α-THP levels are high, relative to other phases of the estrous cycle [13-17]. Increasing levels of 3α,5α-THP in brain enhances exploratory, anti-anxiety, and social behaviors. Systemic administration of the atypical anti-psychotic, olanzapine, increases whole brain levels of 3α,5α-THP and latency to freeze in response to shock, time spent on the open arms of the elevated plus maze and time spent in social interaction with a conspecific [18]. Increasing levels of 3α,5α-THP in the hippocampus by activating mitochondrial benzodiazepine receptors, which enhances neurosteroidogenesis, reduces fear and anxiety behaviors in the shock-probe burying test and the elevated plus maze [19]. Systemic or intra-hippocampal administration of E2 alone also increases central entries in the open field and open arm time in the elevated plus maze [17,20]. Together, these data suggest that E2 and/or 3α,5α-THP may serve a broader role beyond facilitating lordosis by modulating behavioral processes that may precede mating, such as exploration, anxiety, and social behaviors. Notably, E2 increases 3α,5α-THP in the hippocampus [8,9,21], which is an important area for affective processes [17]. Further, stressful or challenging environmental experiences increase biosynthesis of E2 and 3α,5α-THP [22-25]. As such, we were interested in the effects of E2 and/or 3α,5α-THP on behaviors that may promote sexual interactions (exploration, anxiety, social behaviors) and whether actions of E2 and/or 3α,5α-THP in the VTA are sufficient to modulates these behaviors.
In order for female rodents to mate successfully, other behavioral processes, including aggression and anxiety, have to be dampened [26]. E2 and P4 have modulatory effects on reproductive behaviors, but have also been shown, in separate studies, to influence aggression, anxiety, and social behavior processes. As such, the extent to which actions of E2 and/or progestins in the VTA may be important for mediating functional effects that may contribute ultimately to the expression of lordosis is not known. Hence, in these studies, we manipulated and/or controlled E2 and progestin levels and examined effects on exploration, anxiety, social, and reproductive behaviors (socio-sexual behaviors) and investigated E2 and progestin levels after these behaviors occurred. Behavior of diestrous (low E2 and progestins) and proestrous (moderate E2 and progestins) rats with or without infusions of 3α,5α-THP to the VTA, or control sites [substantia nigra (SN) and central grey (CG)] were compared and tissues were collected for measurement of E2 and progestins. We hypothesized that if progestins (rather than E2) in the VTA are critical for mediating socio-sexual behaviors, then infusions of 3α,5α-THP to the VTA, but not other CNS sites, should enhance these behaviors (irrespective of E2 elevations) and there should be a greater association between progestin (rather than E2) levels and behavior.
2. Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY.
2.1. Animals and housing
Adult, intact, Long-Evans female rats were bred in the Social Sciences or Life Sciences Laboratory Animal Care Facilities at The University at Albany. Rats were group-housed in polycarbonate cages (45 cm × 24 cm × 21 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility. Rats were maintained on a 12-h/12-h reversed light cycle (lights off 8:00 a.m.) with continuous access to Purina Rat Chow and tap water in their home cages.
2.2. Surgery
All rats were stereotaxically implanted with bilateral guide cannulae aimed at the medial aspect of the VTA (from bregma: AP=−5.3, ML=±0.4, DV=−7.0) under xylazine (12 mg/kg) and ketamine (70 mg/kg) anesthesia. Guide cannulae consisted of modified 23-gauge thin-wall stainless steel needles with 30-gauge removable inserts. Following surgery, animals were monitored for loss of weight, righting response, flank stimulation response, and/or muscle tone [27]. No rats failed these assessments.
Findings from 3α,5α-THP infusions that missed the central VTA suggested there were site-specific effects of VTA infusions to modulate the behaviors of interest. As such, we investigated further effects of 3α,5α-THP infusions to other sites. In Experiments 2 and 3, bilateral cannulae were aimed at the SN (Experiment 2, from bregma: AP=−5.0, ML=±2.0, DV=−8.0) or CG (Experiment 3, from bregma: AP=−6.5, ML=±0.5, DV=−5.5) to further examine the behavioral consequences of 3α,5α-THP infusions to these regions. The SN and CG were used as control sites based upon prior reports that the SN and CG are progestin sensitive. Actions of progestins in the SN have different effects on seizure susceptibility [28], than do progestins anti-seizure effects in other areas, such as the hippocampus [29]. Infusions of 3α,5α-THP to the CG increase lordosis responses, albeit at higher concentrations than that used in the present studies, but do not alter anxiety behavior in the open field [30]. Moreover, the proximity of the CG to the cerebral aqueduct makes it a good control for possible effects of infusions due to diffusions through the ventricles. Hence, the SN and CG are sensitive to effects of progestins and are ideal controls for the present experiments.
2.3. Hormonal milieu
2.3.1. Endogenous
Vaginal epithelium was examined daily (between 7:00 and 8:00 h) to determine phase of the estrous cycle, per previous methods [14,31]. Rats were cycled through two normal estrous cycles (4–5 day cycle) prior to testing. Rats were tested on either early diestrus or on the evening of proestrus. Early diestrus is characterized by low E2 and progestin levels. On the evening of proestrus, E2 levels are declining, but progestin levels are high [32,33].
2.3.2. Exogenous
In Experiment 1, diestrous or proestrous rats received bilateral infusions of 3α,5α-THP (100 ng/1 μl, n = 10 diestrus, n = 10 proestrus) or vehicle (β-cyclodextrin, n = 10 diestrous, n = 9 proestrus) to the VTA. Diestrous and proestrous rats in Experiment 2 received bilateral infusions of 3α,5α-THP (n = 7 diestrus, n = 12 proestrus) or β-cyclodextrin (n = 13 diestrus, n = 7 proestrus) to the SN. In Experiment 3, diestrous or proestrous rats received bilateral infusions of 3α,5α-THP (n = 8 diestrus, n = 8 proestrus) or β-cyclodextrin (n = 8 diestrus, n = 8 proestrus) to the CG. Infusions were administered at a rate of 1 μl/min through a 30-gauge needle attached to PE-20 tubing and a 5-μl Hamilton syringe. The infusion needle was left in place for 60 s following infusions to reduce possible displacement of infusate. Ten minutes following infusions, rats were tested in the behavioral battery described below. This 3α,5α-THP infusion regimen to the VTA has been demonstrated previously to facilitate lordosis of ovariectomized E2-primed rats, albeit effects on other behaviors have not yet been investigated [10]. Previous reports, and pilot data for this project, have demonstrated that infusions to the anterior medial aspect of the VTA are most effective at enhancing lordosis [10]. Because of the importance of site-specificity in these investigations and the inability to perform comprehensive histological site analyses concomitant with endocrine measures, we ran a pilot study to examine the spread of 3α,5α-THP infusions to the VTA, SN, and CG. These data revealed that infusions of 3α,5α-THP to the VTA spread ∼1 mm and did not extend beyond the midbrain. Further, infusions of 3α,5α-THP to the SN and CG, diffused a similar extent and did not impringe upon these other sites (Fig. 1), which is consistent with prior findings [10,34].
Fig. 1.
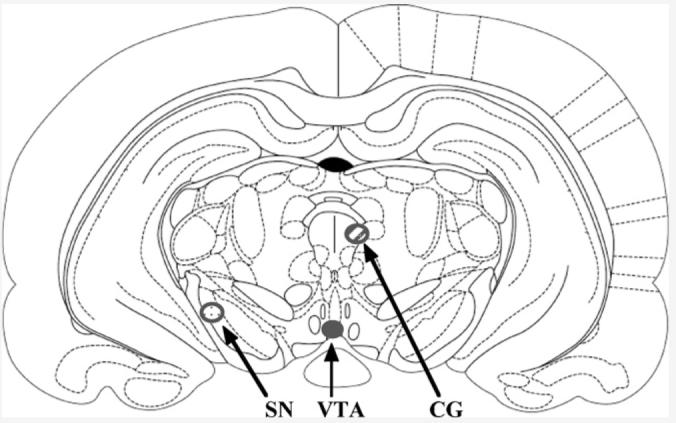
Depicts spread of 3α,5α-THP infusions to the VTA (solid), SN (dotted), and CG (striped).
2.4. Behavioral testing
Every rat was individually tested through the following battery in the order described below. Because the present study was designed to examine effects of 3α,5α-THP on exposure to novel stimuli and effects of novel stimuli on 3α,5α-THP secretion, rats were not habituated to the behavioral apparatus prior to testing. The testing apparatus were brightly lit from above with three fluorescent bulbs (32 W each). Testing occurred in a single room, in a sequential manner, with no breaks between individual tasks (other than time needed to clean apparatus and move rats from one task to the next). Although, it is possible that exposure to prior tests may influence performance in subsequent tasks, previous reports comparing males tested in a battery of anxiety tasks versus individual anxiety tasks did not reveal differences on behavioral or endocrine (5α-reduced androgens) measures [35]. It took approximately 45–50 min for each rat to be tested through the battery described below.
Behavioral data were collected with the ANY-Maze data collection program (Stoelting Co., Wheat Dale, IL) and by an observer blind to the condition of experimental rats and the hypothesized outcome of the study. There was a 97% concordance rate between data that was collected by ANY-Maze and that collected by the uninformed observer.
2.4.1. Open field
Behavior in the open field is used as a measure of exploration, anxiety, and locomotor behavior [14,36]. The open field (76 cm × 57 cm × 35 cm) has a 48-square grid floor (6 × 8 squares, 9.5 cm/side): there is an overhead light illuminating the central squares (all but the 24 perimeter squares were considered central). Per previous methods, rats were placed in the open field and the path of their exploration was recorded for 5 min. The number of central, peripheral, and total entries was then calculated from these data as indices of anti-anxiety, thigmotaxis, and motor behavior, respectively.
2.4.2. Elevated plus maze
Behavior in the elevated plus maze is also utilized to assess exploration, anxiety, and motor behavior [14,37]. The elevated plus maze consists of four arms, 49 cm long and 10 cm wide, elevated 50 cm off the ground. Two arms were enclosed by walls 30 cm high and the other two arms were exposed. As per previous methods, rats were placed at the juncture of the open and closed arms and the number of entries into, and the amount of time spent on, the open and closed arms were recorded during a 5-min test. Time spent on the open arms is an index of anxiety and the total number of arm entries is measure of motor activity.
2.4.3. Partner preference
A modified version of the previously established partner preference task was utilized to assess preference for an intact male or a conspecific [14,38]. Experimental rats were placed in the center of an open field (76 cm × 57 cm × 35 cm) that contained an ovariectomized stimulus female and an intact stimulus male in opposite corners. Stimulus rats were enclosed in Plexiglass compartments with small holes (1 cm diameter) drilled in the bottom portion of the enclosure exposed to the center of the open field, so that experimental rats could receive visual and olfactory stimulation from stimulus rats in the absence of physical contact. The amount of time that experimental rats spent within a body's length of stimulus animals was recorded in a 5-min test. Increased time spent in close proximity to one stimulus rat versus another is an indication of a preference for that animal.
2.4.4. Social interaction
The social interaction task was used to assess exploratory and anxiety behavior associated with interacting with a novel conspecific [14,39]. Each member of a pair of rats (one experimental, one stimulus) was placed in opposite corners of an open field (76 cm × 57 cm × 35 cm). The total duration of time that experimental rats engaged an ovariectomized stimulus rat in crawling over and under partner, sniffing of partner, following with contact, genital investigation of partner, tumbling, boxing and grooming was recorded during a 5-min test [14]. An ovariectomized rat was utilized as the stimulus animal in order to avoid exposure of experimental rats to vaginocervical stimulation, which might occur if a male had been used as the stimulus animal. Duration of time spent interacting with a conspecific is an index of anxiety behavior.
2.4.5. Paced mating
Paced mating was utilized over standard mating because of its greater ethological relevance and procedures were carried out as previously reported [40-43]. Paced mating tests were conducted in a chamber (37.5 cm × 75 cm × 30 cm), which was equally divided by a partition that had a small (5 cm in diameter) hole in the bottom center, to allow a female free access to both sides of the chamber, but which prevented the larger stimulus male from moving between sides. Females were placed in the side of the chamber opposite the stimulus male. Rats were behaviorally tested for an entire ejaculatory series. Behaviors recorded were the frequency of mounts and intromissions that preceded an ejaculation. As well, the frequency (lordosis quotient = incidence of lordosis/number of mounts) and intensity (lordosis rating) of lordosis, quantified by rating of dorsiflexion on a scale of 0–3 [44] was recorded. The percentage of proceptive (i.e. hopping, darting, ear wiggling, proceptivity quotient) and aggressive (i.e. vocalizations, defensive postures, aggression quotient) behaviors prior to contacts was also recorded. Pacing measures included the percentage of times the female left the compartment containing the male after receiving a particular copulatory stimuli (%exits after mounts, intromissions, and ejaculations) and latencies in seconds to return to the male compartment after these stimuli. The normal pattern of pacing behaviors for percent exits and returns latencies to be longer after more intensive stimulation (ejaculations > intromissions > mounts) was observed in the present study.
2.5. Tissue collection
Immediately following testing in the entire battery of behavioral tasks described above (which requires approximately 50 min to complete), whole brains and trunk blood were collected for later measurement of corticosterone, E2, P4, DHP, and 3α,5α-THP. Trunk blood was centrifuged at 3000 × g for 10 min, serum was stored in eppendorfs at −80 °C. Brains were rapidly frozen on dry ice and stored at −80 °C for ∼3 months prior to radioimmunoassay.
2.6. Tissue preparation
Serum was thawed on ice and steroids extracted as described below. Brains were thawed and midbrain, hippocampus, diencephalon, and cortex were dissected out for all experiments. For Experiments 2 and 3, remaining subcortical tissue (interbrain) was also measured as an additional control. As endocrine analyses precluded histological site analyses, brains were visually inspected during dissection to determine infusion site. Upon inspection, two diestrous rats from Experiment 1 received 3α,5α-THP infusions to the lateral aspect of the VTA, bordering on the medial lemniscus and substantia nigra. Notably, endocrine and behavioral data of these rats was different than that of other rats in this group and was omitted from the statistical analyses. Following dissection, steroids were extracted from midbrain, hippocampus, diencephalon, cortex, and interbrain as described below. Steroid levels in these regions were investigated because they are involved in the mediation of exploration, anxiety, social, and sex behaviors (midbrain, hippocampus, diencephalon, and cortex) or as control sites (interbrain [3,45]).
2.7. Radioimmunoassay for steroid hormones
E2, P4, DHP, and 3α,5α-THP concentrations were measured as described below, using previously reported methods [33,46].
2.7.1. Radioactive probes
[3H] E2 (NET-317: specific activity = 51.3 Ci/mmol), P4 (NET-208: specific activity = 47.5 Ci/mmol), and 3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol), were purchased from Perkin-Elmer (Boston, MA).
2.7.2. Extraction of steroids from serum
E2, P4,DHP, and 3α,5α-THP were extracted from serum with ether following incubation with water and 800 cpms of 3H steroid [33]. After snap-freezing twice, test tubes containing steroid and ether were evaporated to dryness in a Savant. Dried down tubes were reconstituted with phosphate assay buffer to the original serum volume.
2.7.3. Extraction of steroids from brain tissues
E2, P4, DHP, and 3α,5α-THP were extracted from brain tissues following homogenization with a glass/glass homogenizer in 50% MeOH, 1% acetic acid. Tissues were centrifuged at 3000 × g and the supernatant was chromatographed on Sepak-cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH). Solvents were removed using a speed drier. Samples were reconstituted in 300 μl assay buffer.
2.7.4. Set-up and Incubation of radioimmunoassays
The range of the standard curves was 0–1000 pg for E2, and 0–8000 pg for P4, DHP, and 3α,5α-THP. Standards were added to assay buffer followed by addition of the appropriate antibody (described below) and3H steroid. Total assay volumes were 800 μl for E2 and P4, 950 μl for DHP, and 1250 μl for 3α,5α-THP. All assays were incubated overnight at 4 °C, except for E2, which incubated at room temperature for 50 min.
2.7.5. Antibodies
The E2 antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO) was used in a 1:40,000 dilution, which generally binds between 40% and 60% of [3H] E2 [33], and bound 48% in the present study. This E2antibody has negligible (<1%) cross-reactivity with other steroid hormones including, esterone, 17α-estradiol, P4, 17-hydroxyprogesterone [14]. The P4 antibody (P#337 from Dr. G.D. Niswender, Colorado State University), used in a 1:30,000 dilution, typically binds between 30% and 50% of [3H] P4 [33], and bound 43% in the present study. The P4 antibody has very low levels (<4%) of cross-reactivity with DHP and 3α,5α-THP [46]. The DHP (X-947) and 3α,5α-THP antibodies (#921412-5, purchased from Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, CA), were used in a 1:5000 dilution, typically bind between 40% and 60% of [3H] 3α,5α-THP [33], and bound 51% in the present study. The DHP antibody cross-reacts with 3α,5α-THP (100%), 5α-pregnan-3,20-dione (50%), 4-pregnen-3α-ol-20-one (50%), and P4 (17%) [47]. The 3α,5α-THP antibody cross-reacts with 3α-hydroxypregn-4en-20-one (84%) and DHP (11%) and its β isomer (7%), P4 (6%), and pregnenolone (<2%) [47,48].
2.7.6. Termination of binding
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000 × g and the supernatant was pipetted into a glass scintillation vial with 5 ml scintillation cocktail. Sample tube concentrations were calculated using the logit–log method of Rodbard and Hutt [49], interpolation of the standards, and correction for recovery with Assay Zap. The inter- and intra-assay reliability coefficients were: E2 0.09 and 0.10, P4 0.12 and 0.13, DHP 0.12 and 0.14, and 3α,5α-THP 0.13 and 0.15.
2.8. Statistical analyses
Two-way analyses of variance (ANOVA) were utilized to examine effects of hormonal milieu (estrous cycle phase, fusion condition) on endocrine and behavioral endpoints. Correlational analyses were used to determine the contribution of steroid levels in brain areas examined on performance in individual tasks. Alpha level for statistical significance was P≤0.05. Where appropriate, ANOVAs were followed by Fisher's PLSD post hoc tests to ascertain group differences. Power analyses were utilized to verify that all inferential statistics reported were valid with sufficient power.
3. Results
Expected estrous cycle variations were observed in all experiments. There were also effects of infusions of 3α,5α-THP to the VTA, but not the SN (Table 1, middle) or CG (Table 1, right), on behavioral and endocrine measures. The two diestrous rats from Experiment 1 that received infusions outside the medial aspect of the VTA had behavior and endocrine measures that were atypical of rats that received infusions of 3α,5α-THP to the VTA and more akin to rats that received infusions of 3α,5α-THP to the SN. As such, their data were excluded and the findings presented below are results of 3α,5α-THP infusions to the VTA on endocrine and behavioral measures from Experiment 1.
Table 1.
Behavior in the open field (OF), elevated plus maze (EPM), social choice (SC), social interaction (SI), and lordosis quotients (LQ), lordosis rating (LR), proceptivity quotient (PQ), aggression quotient (AQ), and percentage of exits (%exits) in the paced mating task, and endocrine data of diestrous (Di) and proesterous (Pro) rats following infusions of 3α,5α-THP or vehicle to the SN or CG
| Di±vehicle | Pro±vehicle | Di±3α,5α-THP (SN) |
Pro±3α,5α-THP (SN) |
Di±3α,5α-THP (CG) |
Pro±3α,5α-THP (CG) |
|
|---|---|---|---|---|---|---|
| OF | ||||||
| Central entries | 27 ± 3 | 42 ± 7* | 26 ± 3 | 39 ± 3* | 34 ± 4 | 43 ± 5* |
| EPM | ||||||
| Open arm time | 18 ± 3 | 71 ± 16* | 18 ± 6 | 81 ± 8* | 24 ± 2 | 71 ± 13* |
| SC | ||||||
| Duration w/male | 100 ± 9 | 166 ± 23* | 119 ± 20 | 144 ± 12* | 91 ± 12 | 186 ± 11* |
| SI | ||||||
| Time engaging | 42 ± 7 | 78 ± 17* | 29 ± 7 | 91 ± 6* | 44 ± 9 | 66 ± 10* |
| Sex | ||||||
| LQ | 4 ± 3 | 82 ± 8* | 12 ± 12 | 100 ± 12* | 17 ± 11 | 96 ± 2* |
| LR | 0 ± 0 | 2.1 ± 0.3* | 0 ± 0 | 2.3 ± 0.3* | 0.1 ± 0.1 | 2.4 ± 0.2* |
| PQ | 0 ± 0 | 73 ± 14* | 9 ± 9 | 47 ± 17* | 0 ± 0 | 80 ± 11* |
| AQ | 68 ± 12 | 17 ± 9* | 62 ± 18 | 12 ± 10* | 56 ± 9 | 7 ± 2* |
| %Exits | 3 ± 0.3 | 25 ± 12* | 0 ± 0 | 25 ± 16* | 10 ± 10 | 33 ± 6* |
| E2 (pg/ml/mg) | ||||||
| Plasma | 5.5 ± 0.5 | 19.4 ± 1.2* | 4.6 ± 0.8 | 19.2 ± 2.9* | 5.9 ± 1.6 | 19.3 ± 2.2* |
| Midbrain | 1.8 ± 0.1 | 2.3 ± 0.2* | 1.7 ± 0.2 | 2.4 ± 0.2* | 1.6 ± 0.1 | 2.4 ± 0.2* |
| Hippocampus | 1.6 ± 0.3 | 2.3 ± 0.1* | 1.7 ± 0.2 | 2.4 ± 0.3* | 1.4 ± 0.1 | 2.5 ± 0.2* |
| Diencephalon | 1.3 ± 0.2 | 2.4 ± 0.3* | 1.7 ± 0.3 | 2.2 ± 0.2* | 1.2 ± 0.1 | 2.2 ± 0.2* |
| Cortex | 1.5 ± 0.2 | 2.5 ± 0.3* | 1.3 ± 0.2 | 2.1 ± 0.2* | 1.7 ± 0.1 | 2.9 ± 0.1* |
| Interbrain | 2.0 ± 0.3 | 5.5 ± 0.2* | 1.2 ± 0.1 | 1.8 ± 0.3* | 3.0 ± 0.2 | 3.1 ± 0.2* |
| P4 (ng/ml/mg) | ||||||
| Plasma | 2.4 ± 0.3 | 15.0 ± 2.0* | 2.6 ± 0.2 | 17.6 ± 0.7* | 1.9 ± 0.2 | 17.1 ± 1.3* |
| Midbrain | 1.6 ± 0.2 | 2.4 ± 0.1* | 1.5 ± 0.1 | 2.2 ± 0.2* | 1.5 ± 0.2 | 2.1 ± 0.1* |
| Hippocampus | 1.7 ± 0.3 | 2.5 ± 0.1* | 1.8 ± 0.1 | 2.6 ± 0.3* | 1.5 ± 0.2 | 3.3 ± 0.6* |
| Diencephalon | 1.7 ± 0.2 | 2.6 ± 0.2* | 1.4 ± 0.1 | 2.3 ± 0.4* | 2.0 ± 0.3 | 2.9 ± 0.2* |
| Cortex | 1.9 ± 0.2 | 3.0 ± 0.4* | 1.8 ± 0.2 | 2.4 ± 0.2* | 1.8 ± 0.4 | 2.4 ± 0.2* |
| Interbrain | 1.8 ± 0.2 | 2.7 ± 0.3* | 1.7 ± 0.2 | 2.6 ± 0.2* | 1.7 ± 0.2 | 3.6 ± 0.7* |
| DHP (ng/ml/mg) | ||||||
| Plasma | 3.1 ± 0.4 | 22.7 ± 1.7* | 4.4 ± 0.5 | 30.6 ± 2.7* | 3.9 ± 0.1 | 17.6 ± 1.1* |
| Midbrain | 3.1 ± 0.2 | 12.7 ± 2.4* | 4.5 ± 0.5 | 15.9 ± 2.5* | 2.5 ± 0.3 | 8.6 ± 1.0* |
| Hippocampus | 4.0 ± 0.5 | 22.3 ± 1.3* | 3.2 ± 0.2 | 22.5 ± 1.8* | 4.2 ± 0.5 | 21.8 ± 1.9* |
| Diencephalon | 5.7 ± 0.7 | 11.7 ± 0.9* | 5.6 ± 0.4 | 13.6 ± 0.5* | 6.1 ± 0.5 | 12.8 ± 1.1* |
| Cortex | 4.7 ± 0.3 | 14.1 ± 1.3* | 4.4 ± 0.1 | 15.4 ± 0.6* | 5.7 ± 1.0 | 13.1 ± 0.8* |
| Interbrain | 5.4 ± 0.7 | 10.5 ± 1.2* | 4.4 ± 0.3 | 11.1 ± 2.4* | 5.2 ± 0.3 | 10.5 ± 0.9* |
| 3α,5α-THP (ng/ml/mg) | ||||||
| Plasma | 2.8 ± 0.4 | 19.2 ± 1.7* | 2.5 ± 0.1 | 19.3 ± 1.8* | 2.9 ± 0.2 | 23.9 ± 2.0* |
| Midbrain | 1.8 ± 0.4 | 11.3 ± 1.7* | 1.8 ± 0.1 | 11.0 ± 1.1* | 2.6 ± 0.3 | 9.5 ± 1.2* |
| Hippocampus | 3.7 ± 0.7 | 17.4 ± 3.0* | 4.3 ± 0.7 | 15.4 ± 0.9* | 3.3 ± 0.5 | 22.8 ± 3.0* |
| Diencephalon | 1.7 ± 0.4 | 4.0 ± 0.5* | 1.4 ± 0.1 | 4.2 ± 0.3* | 2.0 ± 0.4 | 4.2 ± 0.4* |
| Cortex | 1.4 ± 0.2 | 2.7 ± 0.5* | 1.3 ± 0.1 | 2.4 ± 0.2* | 1.7 ± 0.2 | 2.5 ± 0.2* |
| Interbrain | 1.5 ± 0.2 | 2.1 ± 0.1* | 1.5 ± 0.1 | 2.1 ± 0.1* | 1.2 ± 0.2 | 2.3 ± 0.2* |
Significantly different from diestrous groups.
3.1. E2 and P4 levels
Estrous cycle, but not VTA infusion condition, influenced E2 (Table 2, top) and P4 (Table 2, bottom) levels. As previously reported, proestrous, compared to diestrous, rats had significantly higher E2 and P4 concentrations in all tissues examined. Neither E2 nor P4 levels in midbrain, hippocampus, diencephalon, or cortex correlated with behaviors examined.
Table 2.
E2 (top) and P4 (bottom) concentrations in plasma, midbrain, hippocampus, diencephalon, and PFC of diestrous and proestrous rats infused with vehicle or 3α,5α-THP to the VTA
| E2 concentrations |
||||||
|---|---|---|---|---|---|---|
| Plasma (pg/ml) | Midbrain (pg/mg) | Hippocampus (pg/mg) | Diencephalon (pg/mg) | PFC (pg/mg) | ||
| Diestrous + vehicle (n = 10) | 11.2 ± 2.6 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.4 ± 0.2 | |
| Diestrous + 3α,5α-THP (n = 8) | 11.3 ± 3.9 | 1.1 ± 0.1 | 1.9 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.3 | |
| Proestrous + vehicle (n = 9) | 33.3 ± 3.7* | 2.2 ± 0.1* | 2.5 ± 0.3* | 2.4 ± 0.3* | 2.2 ± 0.5* | |
| Proestrous + 3α,5α-THP (n = 10) | 30.1 ± 3.8* | 1.8 ± 0.2* | 2.7 ± 0.3* | 2.1 ± 0.3* | 1.9 ± 0.4* | |
| P4 concentrations |
||||||
| Plasma (ng/ml) | Midbrain (ng/mg) | Hippocampus (ng/mg) | Diencephalon (ng/mg) | PFC (ng/mg) | ||
| Diestrous + vehicle (n = 10) | 5.1 ± 0.7 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.3 ± 0.3 | 1.9 ± 0.6 | |
| Diestrous + 3α,5α-THP (n = 8) | 7.0 ± 0.7 | 1.4 ± 0.1 | 2.2 ± 0.2 | 1.5 ± 0.5 | 1.9 ± 0.2 | |
| Proestrous + vehicle (n = 9) | 16.1 ± 1.1* | 2.2 ± 0.2* | 2.6 ± 0.2* | 2.4 ± 0.3* | 3.2 ± 0.6* | |
| Proestrous + 3α,5α-THP (n = 10) | 17.0 ± 1.5* | 2.1 ± 0.2* | 3.3 ± 0.7* | 3.3 ± 0.2* | 2.4 ± 0.6* | |
| Plasma 3α,5α-THP |
||||||
| Diestrous±vehicle (n = 10) | Diestrous±3α,5α-THP (n = 8) | Proestrous±vehicle (n = 9) | Proestrous±3α,5α-THP (n = 10) | |||
| Plasma 3α,5α-THP (ng/ml) | 2.1 ± 0.2 | 1.8 ± 0.5 | 19.2 ± 0.6 | 20.1 ± 0.9 | ||
Significantly different from diestrous groups (P < 0.05).
3.2. DHP and 3α,5α-THP levels
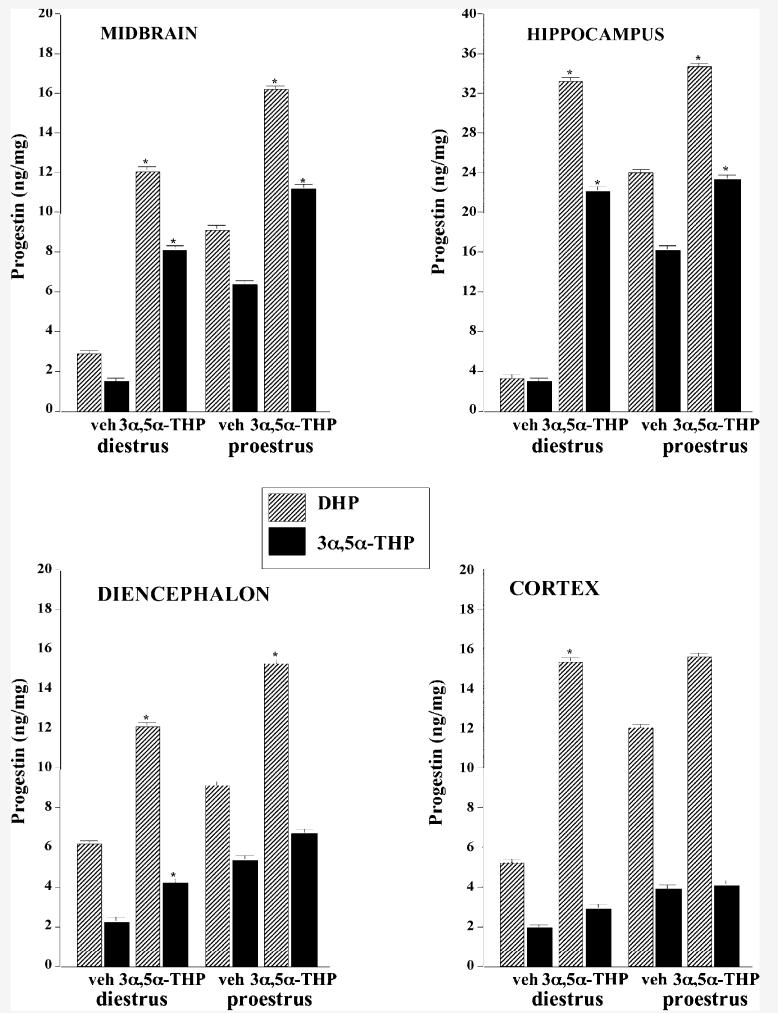
Proestrous, compared to diestrous, rats had significantly higher levels of DHP and 3α,5α-THP levels in serum, mid-brain, hippocampus, diencephalon, and cortex (Fig. 2 and Table 2). Although infusions of 3α,5α-THP to the VTA did not effect DHP levels in serum or 3α,5α-THP levels in serum or cortex, they did increase levels in midbrain [DHP: F(1,33) = 58.80, P < 0.001; 3α,5α-THP: F(1,33) = 49.41, P < 0.001], hippocampus [DHP: F(1,33) = 19.22, P < 0.001; 3α,5α-THP: F(1,33) = 29.20, P < 0.001], diencephalon [DHP: F(1,33) = 11.79, P < 0.001; 3α,5α-THP: F(1,33) = 5.88, P < 0.05], and cortex [DHP: F(1,33) = 8.67, P < 0.01]. There were significant correlations between DHP and 3α,5α-THP concentrations and behavior, which are described below for the individual tasks.
Fig. 2.
DHP (striped bars) and 3α,5α-THP (solid bars) concentrations in midbrain (top left), hippocampus (top right), diencephalon (bottom left), and cortex (bottom right) of proestrous (right side) and diestrous (left side) rats that received vehicle (first two bars, diestrus n = 10, proestrus n=9) or 3α,5α-THP (second two bars, diestrus n = 8, proestrus n = 10). Asterisk (*) indicates significantly different than respective vehicle control (P < 0.05).
3.3. Open field
Proestrous, compared to diestrous, rats entered significantly more central squares (F(1,33) = 8.78, P < 0.01) and 3α,5α-THP, but not vehicle, infusions (F(1,33) = 12.52, P < 0.01) increased central square entries (Fig. 3, top). The number of central square entries was significantly positively correlated with levels of DHP and 3α,5α-THP in midbrain (DHP: R = 0.7, P < 0.0001; 3α,5α-THP: R = 0.6, P < 0.0001), hippocampus (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.6, P < 0.001), and diencephalon (DHP: R = 0.3, P < 0.03; 3α,5α-THP: R = 0.5, P < 0.001). Neither estrous cycle phase, nor VTA infusions, influenced the total number of squares entered in the open field (Table 3).
Fig. 3.
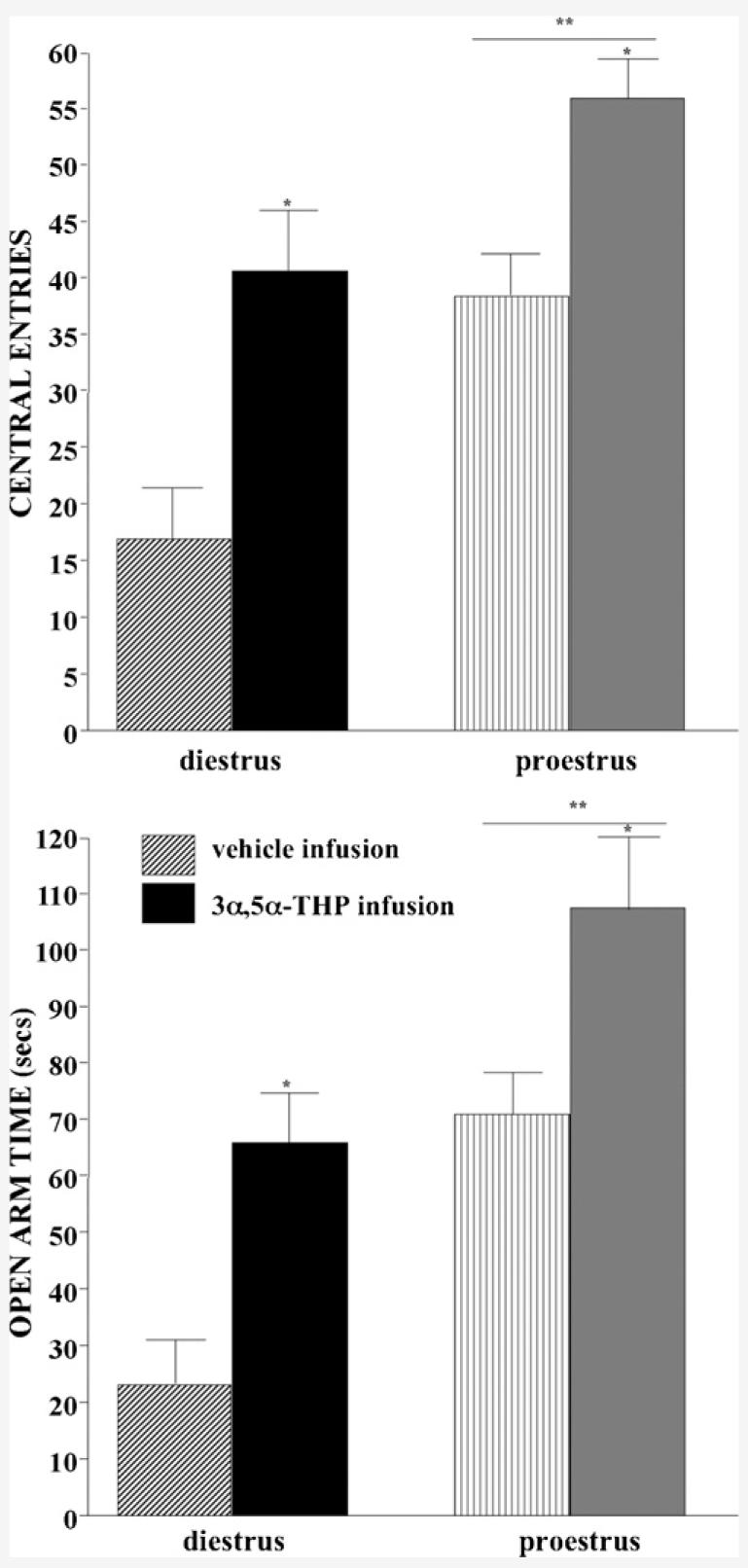
Central square entries (±S.E.M., top) and open arm time (±S.E.M., bottom) of diestrous (left) and proestrous (right) rats administered vehicle (striped bars, diestrus n = 10, proestrus n=9) or 3α,5α-THP (solid bars, diestrus n = 10, proestrus n = 9) infusions to the VTA.
Table 3.
Total square entries, total arm entries, time near female, proceptivity, and aggression quotients of diestrous and proestrous rats infused with vehicle or 3α,5α-THP to the VTA
| Total square entries | Total arm entries | Time near female (s) | Proceptivity quotient (%) | Aggression quotient (%) | |
|---|---|---|---|---|---|
| Diestrous + vehicle (n = 10) | 177 ± 17 | 11 ± 2 | 123 ± 19 | 0 ± 0 | 37 ± 7 |
| Diestrous + 3α,5α-THP (n = 8) | 188 ± 12 | 9 ± 2 | 69 ± 10 | 9 ± 8 | 7 ± 3 |
| Proestrous + vehicle (n = 9) | 188 ± 11 | 14 ± 1 | 58 ± 12 | 61 ± 7 | 2 ± 1 |
| Proestrous + 3α,5α-THP (n = 10) | 218 ± 16 | 12 ± 3 | 74 ± 13 | 86 ± 5 | 2 ± 1 |
3.4. Elevated plus maze
Proestrous, compared to diestrous, rats spent more time on the open arms (F(1,33) = 5.28, P < 0.05) and 3α,5α-THP, but not vehicle, infusions increased open arm time (F(1,33) = 4.47, P < 0.05; Fig. 3, bottom). Open arm time was significantly positively correlated with levels of DHP (R = 0.4, P < 0.05) and 3α,5α-THP (R = 0.3, P < 0.05) in hippocampus. Neither estrous cycle, nor VTA infusions, influenced total arm entries (Table 3).
3.5. Partner preference
Proesterous, compared to diestrous, rats spent more time in close proximity to a stimulus male (F(1,33) = 3.56, P < 0.01) and 3α,5α-THP, but not vehicle, infusions increased time spent in close proximity to a stimulus male (F(1,33) = 4.96, P < 0.05; Fig. 4, top). The interaction between estrous cycle and VTA infusion condition (F(1,33) = 4.19, P < 0.05) was due to 3α,5α-THP infusions to diestrous, but not proestrous, rats increasing time spent in close proximity to a stimulus male. Duration of time spent in close proximity to a stimulus male was significantly positively correlated with levels of DHP in hippocampus (R = 0.5, P < 0.01) and cortex (R = 0.3, P < 0.05) and 3α,5α-THP in hippocampus (R = 0.4, P < 0.01) and diencephalon (R = 0.5, P < 0.01). When given a choice, time spent in close proximity to a stimulus female was also influenced by estrous cycle and VTA infusion. As expected, diestrous rats that received vehicle infusions chose proximity to a female, rather than a male (Table 3).
Fig. 4.
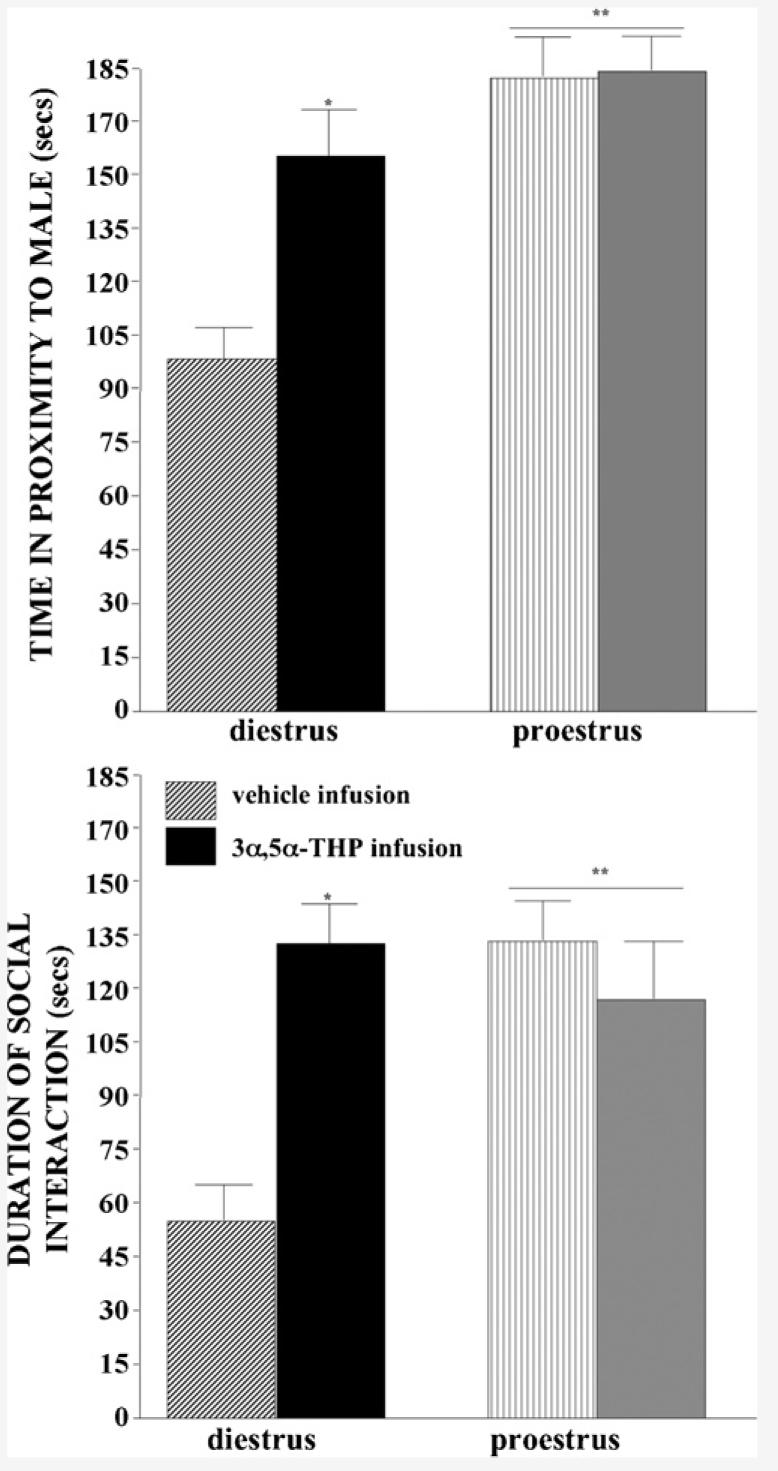
Time in proximity to a male (±S.E.M., top) and spent in social interaction (±S.E.M., bottom) of diestrous (left) and proestrous (right) rats administered vehicle (striped bars, diestrus n = 10, proestrus n=9) or 3α,5α-THP (solid bars, diestrus n = 10, proestrus n = 9) infusions to the VTA.
3.6. Social interaction
Proestrous, compared to diestrous, rats engaged in more social interaction (F(1,33) = 6.28, P < 0.05) and 3α,5α-THP, but not vehicle, infusions increased time spent in social interaction (F(1,33) = 9.18, P < 0.01). The interaction between estrous cycle and VTA infusion condition (F(1,33) = 29.44, P < 0.01) was attributable to 3α,5α-THP infusions to diestrous, but not proestrous, rats significantly increasing the amount of time spent in social interaction compared to vehicle infusions (Fig. 4, bottom). Time spent engaging in social interaction was significantly positively correlated with levels of DHP in midbrain (R = 0.4, P < 0.01), hippocampus (R = 0.4, P < 0.01), and cortex (R = 0.3, P < 0.05) and 3α,5α-THP in midbrain (R = 0.4, P < 0.05), hippocampus (R = 0.5, P < 0.001), and diencephalon (R = 0.4, P < 0.01).
3.7. Paced mating
3.7.1. Lordosis quotients
Proestrous, compared to diestrous, rats had greater lordosis quotients (F(1,33) = 363.71, P < 0.001) and 3α,5α-THP, but not vehicle, infusions increased lordosis quotients (F(1,33) = 72.48, P < 0.001). The interaction between estrous cycle and VTA infusions (F(1,33) = 85.21, P < 0.001) was due to 3α,5α-THP infusions to diestrous, but not proestrous, rats increasing lordosis quotients (Fig. 5, top). Lordosis quotients were significantly positively correlated with levels of DHP and 3α,5α-THP in midbrain (DHP: R = 0.6, P < 0.001; 3α,5α-THP: R = 0.06, P < 0.001), hippocampus (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.6, P < 0.001), diencephalon (DHP: R = 0.4, P < 0.01; 3α,5α-THP: R = 0.5, P < 0.01), and cortex (DHP: R = 0.4, P < 0.01; 3α,5α-THP: R = 0.7, P < 0.001).
Fig. 5.
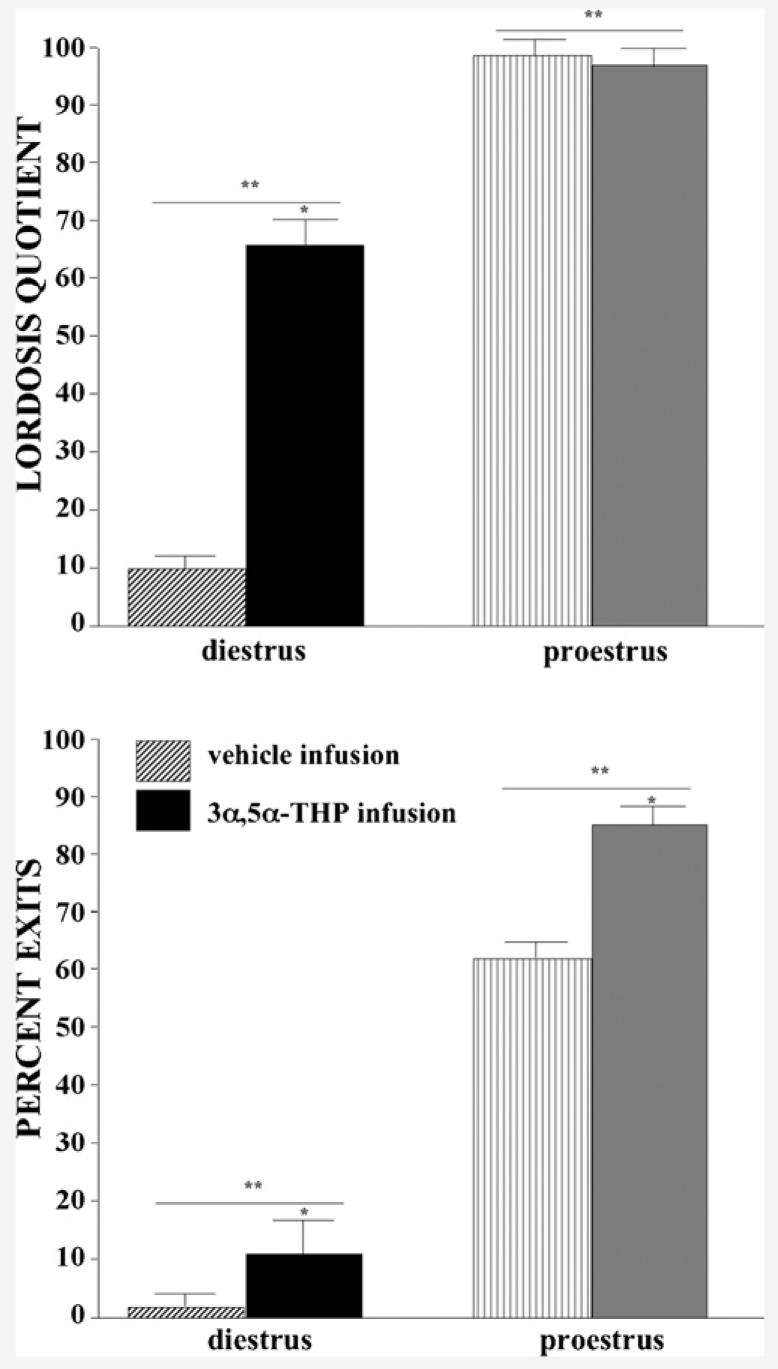
Lordosis quotients (±S.E.M., top) and percentage of exits (±S.E.M., bottom) of diestrous (left) and proestrous (right) rats administered vehicle (striped bars, diestrus n = 10, proestrus n=9) or 3α,5α-THP (solid bars, diestrus n = 10, proestrus n = 9) infusions to the VTA.
3.7.2. Percent exits
Proestrous, compared to diestrous, rats had a higher percentage of exits (F(1,33) = 62.55, P < 0.001) and 3α,5α-THP, but not vehicle, infusions increased the percentage of exits after contacts (F(1,33) = 6.13, P < 0.05; Fig. 5, bottom). Percentage of exits was significantly positively correlated with DHP and 3α,5α-THP concentrations in midbrain (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.5, P < 0.001), hippocampus (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.5, P < 0.01), and cortex (DHP: R = 0.4, P < 0.01; 3α,5α-THP: R = 0.4, P < 0.05) and levels of 3α,5α-THP in diencephalon (R = 0.7, P < 0.001).
3.7.3. Proceptivity quotients
Proestrous, compared to diestrous, rats exhibited a greater percentage of proceptive behaviors (F(1,33) = 133.35, P < 0.001) and 3α,5α-THP, but not vehicle, infusions increased the percentage of proceptive behaviors (F(1,33) = 7.99, P < 0.01; Table 3). Incidence of proceptive behaviors was significantly positively correlated with levels of DHP and 3α,5α-THP in mid-brain (DHP: R = 0.5, P < 0.01; 3α,5α-THP: R = 0.5, P < 0.001), hippocampus (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.4, P < 0.01), diencephalon (DHP: R = 0.4, P < 0.05; 3α,5α-THP: R = 0.4, P < 0.01), and cortex (DHP: R = 0.4, P < 0.01; 3α,5α-THP: R = 0.7, P < 0.001).
3.7.4. Aggression quotients
Proestrous, compared to diestrous, rats exhibited fewer aggressive behaviors (F(1,33) = 17.95, P < 0.001) and infusions of 3α,5α-THP, but not vehicle, decreased aggressive behavior (F(1,33) = 11.23, P < 0.01; Table 3). The significant interaction between estrous cycle and VTA infusions (F(1,33) = 11.11, P < 0.01) was due to infusions of 3α,5α-THP to diestrous, but not proestrous, rats decreasing aggressive behavior. Incidence of aggressive behaviors was significantly negatively correlated with DHP and 3α,5α-THP concentrations in midbrain (DHP: R = 0.5, P < 0.001; 3α,5α-THP: R = 0.6, P < 0.001), hippocampus (DHP: R = 0.4, P < 0.05; 3α,5α-THP: R = 0.4, P < 0.01), and cortex (DHP: R = 0.4, P < 0.05; 3α,5α-THP: R = 0.3, P < 0.05) and levels of 3α,5α-THP in diencephalon (R = 0.5, P < 0.001).
4. Discussion
Results of the present study supported our hypothesis that 3α,5α-THP in the VTA is sufficient to modulate exploratory, anxiety, social, and reproductive behaviors, independent of E2, and revealed that manipulating 3α,5α-THP in the VTA can increase DHP and/or 3α,5α-THP levels in midbrain, hippocampus, diencephalon, and cortex. First, infusions of 3α,5α-THP to the VTA, but not SN or CG, enhanced exploratory, anxiety, social, and reproductive behaviors of diestrous rats and increased DHP and 3α,5α-THP levels in midbrain, hippocampus, diencephalon, and cortex akin to that of proestrous rats. Second, rats that had higher levels of DHP and 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex engaged in more exploratory, anxiolytic, social, and sexual behaviors. Third, behavior in each of the tasks examined was positively correlated with levels of DHP and/or 3α,5α-THP, but not E2 nor P4, in midbrain, hippocampus, diencephalon, and/or cortex. Fourth, there were no estrous cycle/3α,5α-THP infusion interactions on behavioral or endocrine measures, which together with the correlations between behavior and progestins, rather than E2, suggests that the estrous cycle effects observed were due mainly to differences in progestin, rather than E2, concentrations. Together these data suggest that infusions of 3α,5α-THP facilitated behavior and concomitantly increased DHP and 3α,5α-THP levels in midbrain, hippocampus, and diencephalons.
The estrous cycle-dependent differences in endocrine and behavioral measures we observed were congruent with previous reports. Proestrous, compared to diestrous, rats had higher levels of E2 and progestins in serum, midbrain, hippocampus, diencephalon, and cortex, which were commensurate with previous reports that examined endocrine parameters across the estrous cycle [33,50]. In the present study, proestrous rats engaged in more exploratory, anxiolytic, social, and sexual behavior than did diestrous rats, which is consistent with prior reports that separately investigated changes in these behavioral processes across the estrous cycle [14,16,51,52]. The present experiment uniquely demonstrates contemporaneous changes in exploratory, anxiolytic, and social behaviors with reproductive behaviors. These data imply that similar endocrine effects may underlie these variations in behaviors and that exploration, anxiolysis, and social behaviors may be functionally linked to reproductive processes.
Results of the present study also extend previous findings on estrous cyclicity. For example, lordosis can be elicited on every day of the estrous cycle, even when E2 and progestin levels are low [53,54]. Congruent with this, there were low levels of lordosis among diestrous rats infused with vehicle. Notably, infusions of 3α,5α-THP to the VTA of diestrous rats was sufficient to enhance lordosis and increase exploratory, anti-anxiety, and social behaviors to levels commensurate with that of proestrous rats. These data suggest that low levels of E2 are a sufficient background milieu for progestins' facilitatory effects on lordosis and other socio-sexual behaviors. Further, manipulations of 3α,5α-THP in the VTA may serve as a trigger that is sufficient to initiate these effects even in rats with very low levels of E2.
The present findings confirm and extend previous effects of 3α,5α-THP infusions to the VTA to facilitate sex behavior to show that lordosis and behaviors related to lordosis are enhanced. Prior reports have demonstrated that ovariectomized, E2-primed rats that receive infusions of 3α,5α-THP to the VTA, but not the SN, show greater lordosis compared to rats that receive vehicle infusions [10,55]. Moreover, studies utilizing manipulations of 3α,5α-THP in the VTA have demonstrated that the anterior medial aspect of the VTA is the most important site for facilitating lordosis [10]. Similar site-specific effects of 3α,5α-THP infusions for lordosis and exploratory, anxiolytic, and social behaviors, were observed in the present study. Infusions of 3α,5α-THP to the anterior medial aspect of the VTA enhanced socio-sexual behaviors compared to vehicle infusions, while two diestrous rats that received 3α,5α-THP infusions outside the anterior medial aspect of the VTA had patterns of behavior and endocrine responses that were different from those of rats with infusions to this site and akin to that of rats in Experiments 2 and 3, which received infusions to the SN and CG, respectively. Further, rats that received infusions of 3α,5α-THP to control sites, the SN and CG, neither exhibited enhanced socio-sexual nor had subsequent increases in DHP and/or 3α,5α-THP levels in the midbrain, hippocampus, cortex, and/or diencephalon. Furthermore, the lack of effects observed among rats that had infusions to the CG imply that the increases in 3α,5α-THP were unlikely due to diffusion from the cerebral aqueduct. Thus, these data suggest that there are site-specific effects of 3α,5α-THP infusions to the VTA to enhance reproductively relevant behaviors that may be associated with lordosis.
The midbrain VTA is uniquely responsive to pharmacological and behavioral manipulations of 3α,5α-THP. In support, the midbrain VTA is very dynamic in terms of formation of 3α,5α-THP. The enzymes necessary for P4's conversion to 3α,5α-THP, 5α-reductase, and 3α-hydroxysteroid dehydrogenase (3-HSD), have been localized to the midbrain VTA [3] and activity of 5α-reductase, the limiting enzyme in the conversion of P4 to 3α,5α-THP, is greater in the midbrain tegmentum than other regions of the mouse or rat brain investigated (hypothalamus, hippocampus, and/or cortex; [56,57]). Pharmacologically blocking P4's conversion to 3α,5α-THP by means of application of 5α-reductase or 3-HSD inhibitors to the VTA, but not the surrounding regions, inhibits sexual receptivity of rodents [3,58-60]. Although, adequate 3α,5α-THP levels in the midbrain VTA are necessary for mating to occur, 3α,5α-THP concentrations in the midbrain are further increased with exposure to reproductively relevant stimuli [33]. Notably, mating-induced increases in 3α,5α-THP levels in the midbrain occur in gonadally intact, ovariectomized and/or adrenalectomized, E2-primed rodents [3,8,9,33,61,62], which implies that the source of 3α,5α-THP is independent of peripheral glands. The present findings that infusions of 3α,5α-THP to the midbrain VTA can alter levels of 3α,5α-THP in the hippocampus, diencephalon, and cortex suggest that the neurosteroidogenic capacity of the midbrain VTA may extent to its connections with these other regions. However, this point and the extent to which these behavioral effects of 3α,5α-THP infusions to the VTA are related to increases in 3α,5α-THP concentrations in other areas important for mediating these functional processes (i.e. hippocampus, cortex, diencephalon) are an intriguing possibility that will require further consideration once additional supporting evidence has been gathered.
The dynamic role, effects, and mechanisms of 3α,5α-THP in the VTA, associated with reproductive behavior, are of great interest because they can occur independent of sex steroid mediated signaling. 3α,5α-THP is secreted rapidly in the midbrain VTA in response to mating-relevant stimuli [3]. 3α,5α-THP's actions at the few intracellular PRs in the midbrain VTA are not essential for progestin-facilitated lordosis [5,8,9,14]. Rather, in the midbrain VTA, 3α,5α-THP facilitates lordosis in part by altering the inhibitory and excitatory GABAergic and glutamatergic inputs on dopamine cell bodies [5]. This gives rise to increases in dopaminergic signaling and downstream signal transduction processes, in the VTA and its connecting regions (striatum, cortex, and hippocampus). As such, our model system, that involves examining progestins' actions in the midbrain VTA to facilitate reproductive behaviors, is noteworthy because it is a unique site of “non-genomic” steroid actions associated with clear behavioral outcomes.
The effects in our model system of 3α,5α-THP in the VTA to facilitate reproductive behaviors also may provide insight into clinically relevant actions of 3α,5α-THP. There are rapid increases in 3α,5α-THP in the midbrain VTA in response to mating [3,33,61]. It has been proposed that such rapid increases in 3α,5α-THP due to physical and/or environmental challenges, as occurs in the midbrain VTA, may serve as a homeostatic mechanism to mediate (para)sympathetic activity [63]. It is very important to understand the effects and mechanisms of 3α,5α-THP given its role in the pathophysiology and/or treatment of stress-induced affective and neuropsychiatric disorders [64-68]. Indeed, 3α,5α-THP levels are reduced in cerebrospinal fluid of depressed men, compared to non-depressed controls [69]. Low levels of plasma 3α,5α-THP are associated with increased negative symptoms in schizophrenia [70]. Among men with depression, 3α,5α-THP concentrations are normalized, and depressive symptomology is ameliorated following treatment with the selective serotonin reuptake inhibitor, fluoxetine [69]. 3α,5α-THP is also increased by administration of antipsychotics, such as olanzapine and clozapine [18,71]. Thus, it is important to understand more about 3α,5α-THP given its potential role in the pathophysiology and/or treatment of stress-sensitive neuropsychiatric disorders.
In summary, results from this experiment demonstrate that 3α,5α-THP in the VTA can enhance anti-anxiety and social behaviors and may mediate biosynthesis of 3α,5α-THP in the hippocampus, diencephalon, and cortex. These findings suggest that an important role of 3α,5α-THP is perhaps mediating approach/avoidance behaviors and social interactions, which are typically disrupted in neuropsychiatric disorders. These are intriguing data suggesting that progestins modulate behaviors with important ethological relevance in regard to successful mating, as well as other behaviors [26,72,73].
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (MH06769801). The technical assistance of Jason Paris and John Roberts is greatly appreciated.
References
- 1.Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus of ovariectomized, estrogen-primed rats inhibits subsequent facilitation of estrous behavior by systemic progesterone. Brain Res. 1984;294:1–8. doi: 10.1016/0006-8993(84)91303-9. [DOI] [PubMed] [Google Scholar]
- 2.Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- 3.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 4.Luttge WG, Hughes JR. Intracerebral implantation of progesterone: re-examination of the brain sites responsible for facilitation of sexual receptivity in estrogen-primed ovariectomized rats. Physiol Behav. 1976;17:771–5. doi: 10.1016/0031-9384(76)90038-x. [DOI] [PubMed] [Google Scholar]
- 5.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α,5α-THP in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neurosciences. 2006;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Petralia SM. Mitochondrial benzodiazepine receptors in the ventral tegmental area modulate sexual behaviour of cycling or hormone-primed hamsters. J Neuroendocrinol. 2003;15:677–86. doi: 10.1046/j.1365-2826.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 8.Frye CA, Vongher JM. 3α,5α-THP in the midbrain ventral tegmental area of rats and hamsters is increased in exogenous hormonal states associated with estrous cyclicity and sexual receptivity. J Endocrinol Invest. 1999;22:455–64. doi: 10.1007/BF03343590. [DOI] [PubMed] [Google Scholar]
- 9.Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Frye CA, Walf AA, Sumida K. Progestins' actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–18. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Petralia SM, Frye CA. In the ventral tegmental area, G-proteins and cAMP mediate the neurosteroid 3α,5α-THP's actions at dopamine type 1 receptors for lordosis of rats. Neuroendocrinology. 2003;80:233–43. doi: 10.1159/000082752. [DOI] [PubMed] [Google Scholar]
- 12.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S. Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav. 2000;66:887–92. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 15.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 16.Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84:279–86. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Seliga AM. Olanzapine's effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrinology. 2003;28:657–73. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 19.Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology (Berl) 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- 20.Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic–pituitary–adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–62. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 22.Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, et al. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–10. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- 23.Drugan RC, Paul SM, Crawley JN. Decreased forebrain [35S]TBPS binding and increased [3H]muscimol binding in rats that do not develop stress-induced behavioral depression. Brain Res. 1993;631:270–6. doi: 10.1016/0006-8993(93)91545-4. [DOI] [PubMed] [Google Scholar]
- 24.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. PNAS. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 26.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JF, Teitelbaum P. Further analysis of sensory inattention following lateral hypothalamic damage in rats. J Comp Physiol Psychol. 1974;86:375–95. doi: 10.1037/h0035941. [DOI] [PubMed] [Google Scholar]
- 28.Schindler CJ, Slamberova R, Vathy I. Bicuculline seizure susceptibility and nigral GABAA α1 receptor mRNA is altered in adult prenatally morphine-exposed females. Psychoneuroendocrinology. 2003;28:348–63. doi: 10.1016/s0306-4530(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes ME, Frye CA. Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia. 2004;45:1531–8. doi: 10.1111/j.0013-9580.2004.16504.x. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–95. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- 31.Long JA, Evans HM. Oestrous cycle in the rat and its associated phenomena. Memoirs of The University of California. 1922;6:1–146. [Google Scholar]
- 32.Feder HH. Hormones and sexual behavior. Annu Rev Psychol. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-diol. J Neuroendocrinol. 1999;11:839–47. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 34.Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: an autoradiographic analysis. Endocrinology. 1982;111:1581–6. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- 35.Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–30. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–8. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 37.File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- 38.Baum M. Paradoxical effect of alcohol on the resistance to extinction of an avoidance response in rats. J Comp Physiol Psychol. 1969;69:238–40. doi: 10.1037/h0028188. [DOI] [PubMed] [Google Scholar]
- 39.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 40.Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–61. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–85. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 42.Gans S, Erskine MS. Effects of neonatal testosterone treatment on pacing behaviors and development of a conditioned place preference. Horm Behav. 2003;44:354–64. doi: 10.1016/s0018-506x(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 43.McClintock MK, Adler NT. The role of the female during copulation in wild and domestic Norway rats (Rattus norvegicus) Behaviour. 1978;67:68–96. [Google Scholar]
- 44.Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–8. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- 45.Gray JA. A theory of anxiety: the role of the limbic system. Encephale. 1983;9:161B–6B. [PubMed] [Google Scholar]
- 46.Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3α-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–9. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 47.Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, et al. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–6. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- 48.Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–70. [PubMed] [Google Scholar]
- 49.Rodbard D, Hutt DM, International Atomic Energy Agency . Symposium on radioimmunoassay and related procedures in medicine. Uniput; New York: 1974. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting; pp. 209–23. [Google Scholar]
- 50.Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion in the rat estrous cycle temporally related to gonadotropins and steroid levels found in peripheral plasma. Endocrinology. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Guasti A, Picazo O. Changes in burying behavior during the estrous cycle: effect of estrogen and progesterone. Psychoneuroendocrinology. 1992;17:681–9. doi: 10.1016/0306-4530(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 52.Komisaruk BR, Diakow C. Lordosis reflex intensity in rats in relation to the estrous cycle, ovariectomy, estrogen administration and mating behavior. Endocrinology. 1973;93:548–57. doi: 10.1210/endo-93-3-548. [DOI] [PubMed] [Google Scholar]
- 53.Gans SE, Stamper JL, Butler T, McClintock MK. Endocrine basis for two types of individual differences in lordosis reflex intensity. Horm Behav. 1995;29:367–91. doi: 10.1006/hbeh.1995.1026. [DOI] [PubMed] [Google Scholar]
- 54.McClintock MK. Social control of the ovarian cycle and the function of estrous synchrony. Am Zool. 1981;21:243–56. [Google Scholar]
- 55.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J Neuroendocrinol. 2005;17:545–52. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–8. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 57.Roselli CE, Snipes CA. Progesterone 5α-reductase in mouse brain. Brain Res. 1984;305:197–202. doi: 10.1016/0006-8993(84)90425-6. [DOI] [PubMed] [Google Scholar]
- 58.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 59.Frye CA, Leadbetter EA. 5α-Reduced progesterone metabolites are essential in hamster VTA for sexual receptivity. Life Sci. 1994;54:653–9. doi: 10.1016/0024-3205(94)00548-6. [DOI] [PubMed] [Google Scholar]
- 60.Petralia SM, Walf AA, Frye CA. In the ventral tegmental area, progestins' membrane-mediated actions for lordosis of hamsters and rats involve protein kinase A. Neuroendocrinology. 2006;84:405–14. doi: 10.1159/000100510. [DOI] [PubMed] [Google Scholar]
- 61.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel SR, Grant KA. Neurosteroids and behavior. Integr Rev Neurobiol. 2001;46:321–48. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- 64.Grobin AC, Gizerian S, Lieberman JA, Morrow AL. Perinatal allopregnanolone influences prefrontal cortex structure, connectivity and behavior in adult rats. Neuroscience. 2006;138:809–19. doi: 10.1016/j.neuroscience.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–73. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 66.Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–5. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 67.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 68.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 69.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- 71.Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanza-pine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–4. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- 72.Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of behavioral neurobiology: sexual differentiation. Plenum; New York: 1992. pp. 85–128. [Google Scholar]
- 73.Lee TM, McClintock MK. Female rats in a laboratory display seasonal variation in fecundity. J Reprod Fertil. 1986;77:51–9. doi: 10.1530/jrf.0.0770051. [DOI] [PubMed] [Google Scholar]