Summary
Background
We previously demonstrated that exogenous application of bone morphogenetic protein 7 (BMP7) reduced 6-hydroxydopamine-mediated neurodegeneration in a rodent model of Parkinson’s disease. The purpose of this study is to examine the endogenous neurotrophic properties of BMP Receptor II in dopaminergic neurons of the nigrostriatal pathway.
Methods
Adult male BMPRII dominant negative (BMPRIIDN) mice and their wild type controls (WT) were placed in the activity chambers for 3 days to monitor locomotor activity. Animals were sacrificed for tyrosine hydroxylase (TH) immunostaining. A subgroup of BMPRIIDN and WT mice were injected with high doses of methamphetamine (MA) and were sacrificed for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) histochemistry at 4 days after injection.
Results
BMPRIIDN mice had lower locomotor activity than the WT. There is a significant decrease in TH neuronal number in substantia nigra compacta, TH fiber density in the substantia nigra reticulata, and TH immunoreactivity in striatum in the BMPRIIDN mice, suggesting that deficiency in endogenous BMP signaling reduces dopaminergic innervation and motor function in the nigrostriatal pathway. Administration of MA increased TUNEL labeling in the substantia nigra in the BMPRIIDN mice.
Conclusions
Endogenous BMPs have trophic effects on nigrostriatal dopaminergic neurons. Deficiency in BMP signaling increases vulnerability to insults induced by high doses of MA.
Keywords: Bone morphogenetic protein, BMPRII, methamphetamine, apoptosis
Introduction
Bone morphogenetic protein-7 (BMP7) is a trophic factor in the transforming growth factor (TGF)-β superfamily. Both BMP7 and its receptor are found in the nigrostriatal pathway in the CNS. BMP7 mRNA is strongly expressed in nigral area of adult rats [2]. Its receptor bone morphogenetic protein receptor type II (BMPR-II) is highly expressed in the dopaminergic neurons of substantia nigra and ventral tegmental area (VTA [9]).
The physiological function of BMP7 in nigrostriatal DA neurons has been studied in different laboratories. In rat mesencephalic cell cultures, BMP7 increased the number of tyrosine hydroxylase (TH) cells and dopamine uptake [7]. Intranigral delivery of BMP7 reduced motor deficits, prevented the loss of TH immunoreactivity in nigra, and restored dopamine (DA) release in striatum in 6-hydroxydopamine- lesioned rats [5]. BMP7 is also protective against other dopaminergic neurotoxins. Our recent studies show that BMP7, given exogenously, reduced methamphetamine (MA) -mediated toxicity in DA neuronal culture and in the substantial nigra in vivo [3]. These data suggest that BMP7 has trophic effects in the nigrostriatal DA pathway. The role of BMPRII in BMP7-mediated neuroprotection of the nigrostriatal system has not been determined.
A study by Althini et al. [1] found that mice homozygous for a truncated dominant-negative form of BMPRII (BMPRIIDN), targeted to the 3′UTR of the TH locus and expressed using an internal ribosomal entry sequence (IRES), exhibited reduced locomotor activity, low DA levels. There was reduced TH immunoreactivity in substantia nigra and striatum, as well as decreased norepinephrine (NE) levels in the sub-mandibular gland. However, some of the effects were due to a Neo cassette present in the targeting construct. Removal of the Neo cassette (Neo-) normalized NE content in the submandibular gland and partially restored DA content in nigra and striatum in these mice. These data suggested that the expression of the BMPRIIDN at the TH locus without the Neo cassette may affect dopamine production in the striatum, but the quantitative effects on locomotor activity or on the TH levels in the nigra, striatum, and VTA were not measured [1]. In this study, we examined the function of BMPRII using the BMPRIIDN mice without a Neo cassette. We found that BMPRIIDN mice had reduced striatal and nigral tyrosine hydroxylase immunoreactivity, decreased locomotor activity and increased sensitivity to methamphetamine toxicity. Our data indicate that deficiency in endogenous BMP signaling increases vulnerability to insults induced by the dopaminergic neurotoxin methamphetamine.
Methods
Animals and drug administration
Adult male BMPRIIDN homozygotes with no neo cassette (Neo-) and wildtype (WT) controls were provided from Uppsala University [1]. Some animals were injected subcutaneously with 4 doses (2 h apart) of MA (10 mg/kg s.c.) or saline (0.01 ml/10 g, s.c.). During injection period, animals were housed individually without bedding.
Behavioral measurement
Mice were individually placed in infrared-beam locomotor chambers equipped with food, water and bedding for 72 h (Accuscan activity monitor, Columbus, OH). The vertical sensors were situated 10 cm from the floor of the chamber. Motor activity was calculated using the number of beams broken by the animals.
TH immunostaining
Mice were anesthetized with chloral hydrate (400 mg/kg i.p.) and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (PB). Brain sections (25 μm) were rinsed in 0.1 M PB, blocked with 4% bovine serum albumin (BSA) and 0.3% Triton x-100 in 0.1 M PB. Sections were then incubated in a primary antibody solution (rabbit polyclonal anti-TH, 1:500, Chemicon, Temecula, CA) for 17–19 h at 4 °C. Sections were rinsed in 0.1 M PB and incubated in biotinylated horse anti-rabbit IgG (1:200; Vector Laboratories, Burlingame CA) for 1 h, followed by incubation for 1 h with avidin-biotin-horseradish peroxidase complex. Staining was developed with 2, 3′ diaminobenzidine tetrahydrochloride (0.5 mg/mL). Selective TH pixel density was obtained by subtracting the background density (in the parietal cortex) from the density in striatum.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
Animals were sacrificed 4 days after saline or MA administration and the brains were removed and sectioned (25 μm). A standard TUNEL procedure was performed as previously described [4]. Briefly, slide-mounted sections were rinsed in 0.5% Triton X-100 in 0.01 M PBS for 20 min at 80 °C. One hundred μL of the TUNEL reaction mixture was added onto each sample in a humidified chamber followed by 60 min incubation at 37 °C.
Statistics
Student’s t-test, 1 or 2-way ANOVA + post-hoc Newman-Keuls test were used for statistical comparison. Data are presented as mean + S.E.M.
Results
Locomotor activity
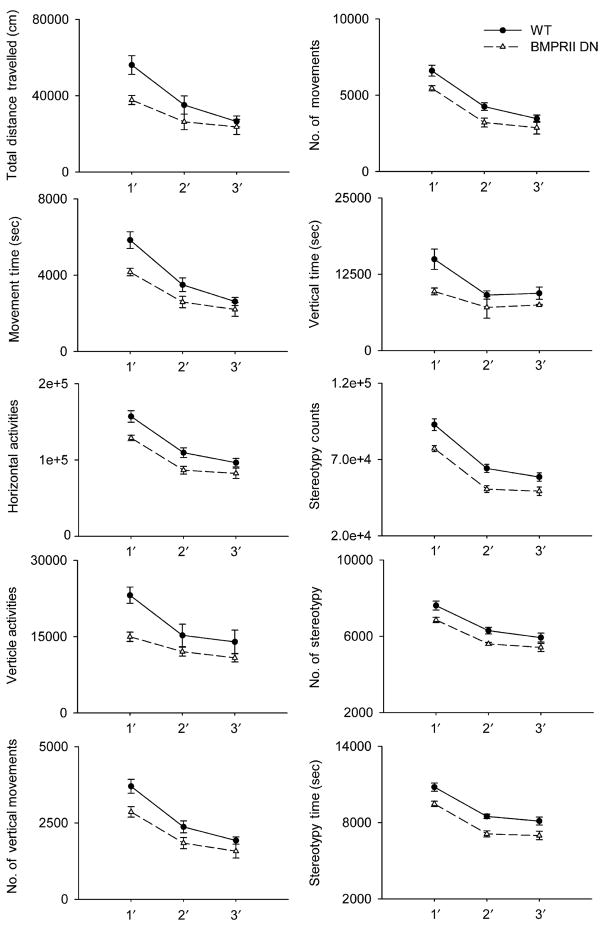
Twenty-five BMPRIIDN mice and 25 WT controls were individually placed in the activity chambers for 3 days. Ten different locomotor parameters were recorded daily (Fig. 1). The highest locomotor activity in both BMPRIIDN and WT was observed in the first day. The movement was reduced in the 2nd and 3rd days, indicating that animals had adapted to the new environment. There was no difference in the time spent for adaptation between the BMPRIIDN and WT mice. BMPRIIDN mice, compared to the WT mice, had a significant decrease in horizontal movement (i.e. total distance traveled, movement time, horizontal activity, number of movements, p<0.05, two way ANOVA), vertical movement (vertical activity, vertical time, number of vertical movements, p<0.05, two-way ANOVA), and stereotypic behaviors (stereotype time, # of stereotypy, stereotypy counts, p<0.05, two-way ANOVA) (Fig. 1).
Fig. 1.
Locomotor activity of BMPRIIDN and WT mice. Animals were individually placed in the activity chambers for 3 days. Ten different locomotor parameters were recorded daily. BMPRIIDN mice (dashed tracing) had a significant decrease in horizontal (total distance traveled, movement time, horizontal activity, number of movement, p<0.01, two-way ANOVA), vertical movement (vertical activity, vertical time, number of vertical movement, p<0.05, two-way ANOVA), and stereotypy (stereotype time, # of stereotypy, stereotypy counts, p<0.05, two-way ANOVA) than the wild type mice (solid tracing)
TH immunoreactivity
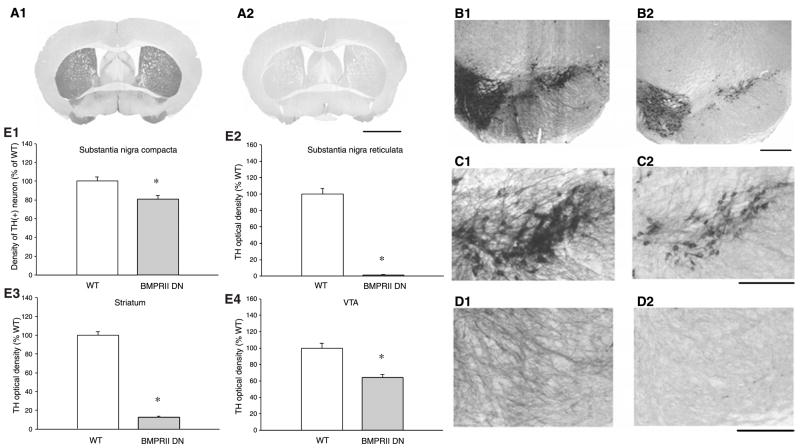
A total of 5 BMPRIIDN and 5 WT mice (or 10 samples for each group; left and right hemispheres from each section) were used for tyrosine hydroxylase (TH) immunostaining. TH immunoreactivity (THir) was quantitatively analyzed in 4 regions: THir optical density in striatum (WT: Fig. 2A1; BMPRIIDN: Fig. 2A2), THir optical density in VTA (WT: Fig. 2B1; BMPRIIDN: Fig. 2B2), THir neuron density in SNpc (WT: Fig. 2C1; BMPRIIDN: Fig. 2C2), and THir fiber density in SNpr (WT: Fig. 2D1; BMPRIIDN Fig. 2D2). In all these regions, BMPRIIDN mice had significantly less THir than the WT. THir optical density in striatum and VTA (Fig. 2E3, 2E4) of BMPRIIDN mice was reduced to 13% (p<0.001, t test) and 64% (p<0.001, t test), respectively, as compared to the WT controls. Similarly, TH fiber density in SNpr (Fig. 2E2) was significantly reduced to 1% (p<0.001, t-test, Fig. 2E2) and TH neuron density in SNpc was reduced to 81% (p = 0.004, t-test, Fig. 2E1) in the BMPRIIDN.
Fig. 2.
BMPRIIDN mice exhibit decreased TH-immunoreactivity in nigra and striatum. Photomicrographs were taken from WT and BMPRIIDN mice. THir is reduced in striatum (WT: A1 vs. BMPRIIDN: A2), VTA (WT: B1 vs. BMPRIIDN: B2), SNpc (WT: C1 vs. BMPRIIDN: C2) in the BMPRIIDN mouse. Less THir fibers were also found in the SNpr in this animal (WT: D1 vs. BMPRIIDN: D2). Calibration: A: 2000 μm; B: 500 μm; C, D: 250 μm. (E) TH immunoreactivity is reduced in BMPRIIDN mice. Averaged THir was taken from 5 WT (clear bar) and 5 BMPRIIDN (dark bar) mice. THir and TH neuronal density were normalized by comparison to the mean of WT controls. In all regions, BMPRIIDN mice had significantly less THir than the WT (*p<0.05, t-test)
Sensitivity to high doses of MA
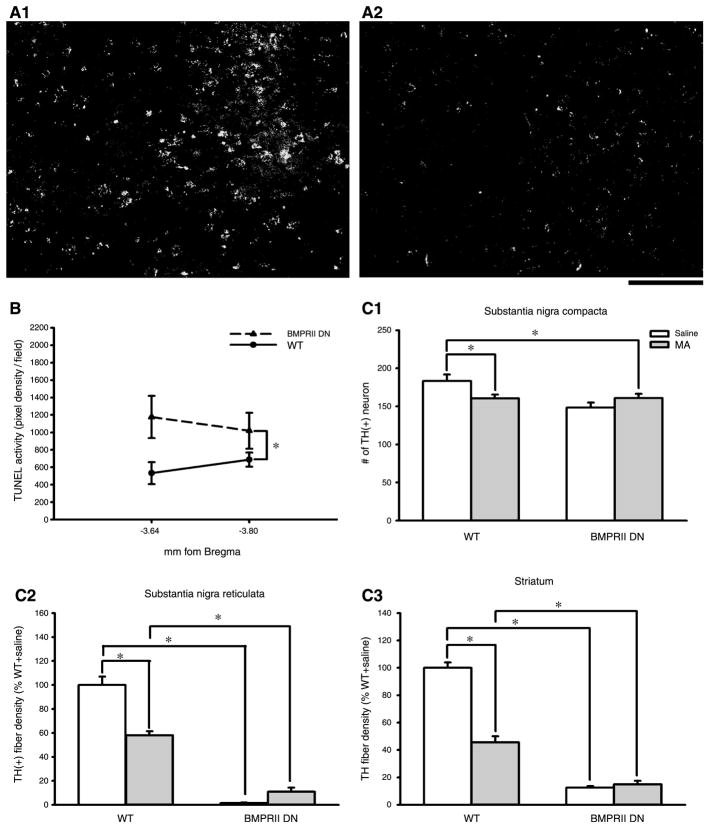
TUNEL labeling: BMPRIIDN and WT mice were sacrificed for TUNEL labeling on the 4th day after MA or saline administration. TUNEL was examined in 2 regions in SNpc (AP to bregma: −3.64 and −3.80 mm). We found that saline administration did not induce TUNEL in BMPRIIDN and WT. MA injection enhanced the density of TUNEL activity in both BMPRIIDN (n = 5) and WT animals (n = 7, Fig. 3). There was an enhancement of TUNEL in the BMPRIIDN mice (BMPRIIDN: Fig. 3, A1 vs. WT: Fig. 3, A2). The optical density of TUNEL was further analyzed in all animals. MA injection significantly increased TUNEL labeling in the BMPRIIDN, compared to the WT (Fig. 3B, p<0.05, two way ANOVA).
TH immunoreactivity: Five BMPRIIDN and 5 WT mice were injected with MA (10 mg/kg, ×4). MA-mediated changes in THir were examined at the 4th day after injection. MA significantly reduced THir in the striatum and SNpr in the WT mice. Since BMPRIIDN mice had very initial low basal TH innervation in striatum and nigra (Fig. 2E1–E3), administration of MA did not further attenuate the density of TH innervation in these regions. There was no difference in THir in the striatum and nigra with and without MA treatment in the BMPRIIDN mice (p>0.05, one-way ANOVA, Fig. 3C1–C3).
Fig. 3.
High doses of MA altered TUNEL and THir in nigrostriatal pathway. BMPRIIDN (A1) and WT (A2) mice were sacrificed on the 4th day after MA administration (10 mg/kg, ×4). MA injection induced TUNEL labeling. There is an enhancement of TUNEL activity in SNpc (−3.64 mm to bregma) in the BMPRIIDN (A1), compared to the WT (A2). Calibration: 50 μm. (B) The optical density of TUNEL labeling was averaged in 2 regions in SNpc (AP to bregma: −3.64 and −3.80 mm). MA injection significantly increased TUNEL density in the BMPRIIDN mice (p< 0.05, 2-way ANOVA). (C1–C3) THir in nigra and striatum after MA injection in BMPRIIDN and WT mice. THir was examined at 4th day after MA injection. MA significantly reduced THir in striatum and SNpr in the WT mice. There is no difference in THir in striatum and nigra with and without MA treatment in the BMPRIIDN mice (*p<0.05, 1-way ANOVA)
Discussion
In this study, we examined the trophic effects of BMPRII using dominant negative mice. We found that BMPRIIDN mice had reduced horizontal, vertical and stereotypic behaviors as well as less tyrosine hydroxylase expression in nigrostriatal pathway than the wild type controls. Previous reports have indicated that BMPRIIDN mice had less dopamine in nigra and striatum than the wild type [1]. Since the dominant-negative properties of the truncated BMPRII on BMP signaling were previously confirmed in cell culture (Althini et al. unpublished data), the change in dopaminergic function found in this and previous studies may be attributed to a deficiency in endogenous BMP signaling in the BMPRIIDN mice.
Our quantitative analysis indicates that TH neuronal fibers were more sensitive than cell bodies to the presence of the BMPRIIDN. TH fiber density in SNpr and striatum in the BMPRIIDN mice was reduced to 1 and 13% of the controls, respectively, whereas TH neuronal density was decreased only to 81%. The selective effect of BMPRIIDN on the THir terminals is supported by finding that a BMPRII agonist selectively promotes dendritic growth from rat sympathetic neurons [8]. These data suggest that BMPRII has stronger trophic influence on DA neuronal fibers than on cell bodies of the midbrain DA neurons.
We have recently found that BMP7 is protective against dopaminergic neurotoxins in the nigrostriatal dopaminergic system. BMP7, given intracerebral, reduced 6-hydroxydopamine or MA-mediated loss of THir and behavioral deficits [3, 5]. BMP7−/+ mice, which have less BMP7 expression, are more sensitive to toxic effects of MA [3]. In this study, the sensitivity to high doses of MA was examined in WT and homozygous BMPRIIDN mice. Administration of MA reduced locomotor activity and THir in WT mice. In contrast, MA did not reduce the already low THir in striatum or nigra in BMPRIIDN mice. Although the locomotor activity could be suppressed by MA in the BMPRIIDN mice, no difference in horizontal, vertical and stereotypy behaviors between BMPRIIDN and WT mice was found after MA injection (data not shown). These data suggest that MA did not further reduce TH innervation or movement in the BMPRIIDN mice. The lack of response of nigrostriatal TH expression to MA may be attributed to a “floor effect”, i.e. the near-complete loss of dopaminergic fibers in BMPRIIDN mice; THir was suppressed by more than 85% in striatum and SNpr in the absence of MA in these animals.
There is evidence that MA induces neuronal degeneration through activation of programmed cell death [4, 6]. In this study, we found that MA induced TUNEL labeling in WT and BMRPIIDN mice. There was a significant enhancement of TUNEL after MA injection in the nigra of BMPRIIDN animals. A similar observation was made in BMP7+/− mice after MA injection [3]. Our recent studies have indicated that there is minimal co-localization of TUNEL and THir after MA treatment in VM dopaminergic neuronal cultures. It is possible that the TH expression is suppressed in injured cells as loss of phenotype often precedes cell death. Current observations the BMPRIIDN mice and recent observations of the BMP7+/− mice, taken together, suggest that attenuation of endogenous BMP signaling increases MA-mediated degeneration in the nigrostriatal system.
In this study, we used BMPRIIDN mice which contain no Neo cassette [1]. These animals showed some reduction of DA levels in SN and striatum [1]. Mice with knock-in truncated ALK-2, another BMP receptor, did not have deficits in DA production. These data suggest that BMPRII is more important than ALK-2 in BMP regulation of DA synthesis. The ALK2DN mice were constructed using the same strategy as the BMPRIIDN mice where in the mutant receptor is expressed from an IRES downstream of the TH coding sequence. The observation that the ALK2DN transgenic showed no effect on DA production suggests that the effects from the BMPRIIDN mutation on DA production are not the result of targeting the construct to the TH locus. Further characterization of the BMPRIIDN animals is needed to rule out the possibility that the IRES-BMPRIIDN sequence added to the TH mRNA alters the levels of THir.
In conclusion, we found that BMPRII is important for TH expression and function in nigrostriatal dopaminergic neurons. Deficiency in BMPR increases vulnerability to MA injury. Our data suggest that activation of BMPRII by endogenous BMPs may mediate through trophic effects on nigrostriatal dopaminergic neurons in adult animals.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute on Drug Abuse, National Institutes of Health, USA.
References
- 1.Althini S, Bengtsson H, Usoskin D, Söderström S, Kylberg A, Lindqvist E, Chuva de Sousa LS, Olson L, Lindeberg J, Ebendal T. Normal nigrostriatal innervation but dopamine dysfunction in mice carrying hypomorphic tyrosine hydroxylase alleles. J Neurosci Res. 2003;72:444–453. doi: 10.1002/jnr.10606. [DOI] [PubMed] [Google Scholar]
- 2.Chen HL, Lein PJ, Wang JY, Gash D, Hoffer BJ, Chiang YH. Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain Res. 2003;994:81–90. doi: 10.1016/j.brainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Chou J, Lu Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neurosci. 2007 doi: 10.1016/j.neuroscience.2007.10.044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 5.Harvey BK, Mark A, Chou J, Chen GJ, Hoffer BJ, Wang Y. Neurotrophic effects of bone morphogenetic protein-7 in a rat model of Parkinson’s disease. Brain Res. 2004;1022:88–95. doi: 10.1016/j.brainres.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 6.Koike K, Hashimoto K, Fukami G, Okamura N, Zhang L, Ohgake S, Koizumi H, Matsuzawa D, Kawamura N, Shimizu E, Iyo M. The immunophilin ligand FK506 protects against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Neuropharmacology. 2005;48:391–397. doi: 10.1016/j.neuropharm.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Koh HC, Chang MY, Park CH, Lee YS, Lee SH. Erythropoietin and bone morphogenetic protein 7 mediate ascorbate-induced dopaminergic differentiation from embryonic mesencephalic precursors. Neuroreport. 2003;14:1401–1404. doi: 10.1097/01.wnr.0000078542.07662.4b. [DOI] [PubMed] [Google Scholar]
- 8.Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 9.Soderstrom S, Bengtsson H, Ebendal T. Expression of serine/threonine kinase receptors including the bone morphogenetic factor type II receptor in the developing and adult rat brain. Cell Tissue Res. 1996;286:269–279. doi: 10.1007/s004410050697. [DOI] [PubMed] [Google Scholar]