Abstract
Determining the biliary clearance of drugs in humans is very challenging because bile in not readily accessible due to the anatomy of the hepatobiliary tract. The collection of bile usually is limited to post-surgical patients with underlying hepatobiliary disease. In healthy subjects, feces typically are used as a surrogate to quantify the amount of drug excreted via non-urinary pathways. Nevertheless, it is very important to characterize hepatobiliary elimination because this is a potential site of drug interactions that might result in significant alterations in systemic or hepatic exposure. In addition to the determination of in vivo biliary clearance values of drugs, the availability of in vitro models that can predict the extent of biliary excretion of drugs in humans may be a powerful tool in the pre-clinical stages of drug development. In this review, recent advances in the most commonly used in vivo methods to estimate biliary excretion of drugs in humans are outlined. Additionally, in vitro models that can be employed to investigate the molecular processes involved in biliary excretion are discussed to present an updated picture of the new tools and techniques that are available to study the complex processes involved in hepatic drug transport.
Keywords: bile, excretion, biliary clearance, hepatic transport, hepatobiliary transport
INTRODUCTION
With the advent of combinatorial chemistry, an increasing number of new chemical entities that are more lipophilic and characterized by high molecular weight are being synthesized and screened during the drug discovery process.1 Drugs with these physicochemical characteristics often are associated with significant hepatic metabolism and/or excretion via the biliary route.2,3 The threshold molecular weight for drugs and metabolites to be excreted preferentially into bile varies between species, ranging from ~235 in rats up to 500–600 in humans. Biliary excretion of compounds can significantly impact the systemic exposure, pharmacological effect and toxicity of certain drugs. Drugs excreted into bile often undergo some degree of reabsorption along the gastrointestinal tract (e.g., mycophenolic acid4, warfarin5, and digoxin3). Enterohepatic recycling is also a very important physiological process for bile salt homeostasis. In humans, the intestinal reabsorption of bile salts is very efficient; more than 95% of the bile salt pool is reabsorbed in the distal ileum.6,7 So far, limited in vivo information has been available regarding the extent of biliary elimination of drugs and metabolites in humans, primarily due to the difficulty in obtaining bile samples from healthy human subjects. An accurate measure of biliary clearance and of the extent of biliary excretion of compounds would be extremely valuable in evaluating the contribution of biliary clearance to total systemic clearance, elucidating potential mechanisms of hepatobiliary toxicity, predicting drug-drug interactions, and identifying enterohepatic versus enteroenteric recirculation. Several clinical studies have shown that drug-drug interactions can result in changes in drug absorption due to inhibition of transport proteins [increased exposure to talinolol after P-glycoprotein (P-gp, gene symbol ABCB1) inhibition8, or inhibition of intestinal secretion resulting in improved therapeutic efficacy of paclitaxel9]. Important modifications in the pharmacokinetics/pharmacodynamics of digoxin, another P-gp substrate, have been observed with co-administration of other P-gp substrates/inhibitors, which are thought to cause interactions in intestinal10, biliary11,12 and/or renal13,14 levels. However, drug-drug interactions influencing biliary clearance of compounds have not been investigated systematically due to technical difficulties in performing these experiments in humans. The inhibition of hepatobiliary transport processes can affect the ability of the liver to extract bile salts from the bloodstream or, potentially more importantly, can affect the metabolism and biliary excretion of bile salts. Although mechanisms of hepatotoxicity are believed to be multifactorial, it has been demonstrated that inhibition of bile salt biliary excretion by drugs (e.g., troglitazone15 and bosentan16) can affect hepatocyte viability due to the accumulation and direct toxicity of detergent-like bile salts.17
The contribution of biliary excretion to the overall disposition and pharmacological effect of some drugs has been underestimated due to the lack of methodologies to accurately quantify the amount of drug subjected to enterohepatic recirculation, and a poor understanding of the processes involved in hepatic excretion and reabsorption of drugs from the canalicular space, bile ducts and intestine. Several animal models, primarily rodent, have been used to study genetic diseases affecting biliary clearance and drug-drug interactions. Mouse and rat isolated perfused liver experiments and bile duct cannulated animals are useful tools for studying biliary disposition of drugs in the early phases of drug development. The availability of rodent models genetically deficient in specific transport proteins has improved understanding of the complex molecular processes involved in excretion of endogenous and exogenous compounds into bile.18–22 However, accumulating evidence suggests that significant inter-species differences exist in the function and regulation of transport proteins, in addition to the already thoroughly characterized species differences in metabolism; these findings complicate the direct extrapolation of animal data to humans.23–25 Enterohepatic recirculation of drugs has been studied extensively in experimental animals where cannulation of the bile duct allows for interruption of bile flow into the intestine. The importance of enterohepatic recirculation also has been investigated with a similar study design in humans. The pharmacokinetic profiles of drugs have been compared in patients with bile duct obstruction, before and after removal of nasobiliary drainage. This design allows complete collection of bile and interruption of enterohepatic recirculation while the drainage is in place.26 However, since the subjects studied in this type of experimental design are affected by hepatobiliary disease, it is not possible to account for changes in the hepatobiliary tract that might have followed the resolution of bile duct obstruction.
Mechanisms for Excretion of Endogenous and Exogenous Compounds into Bile
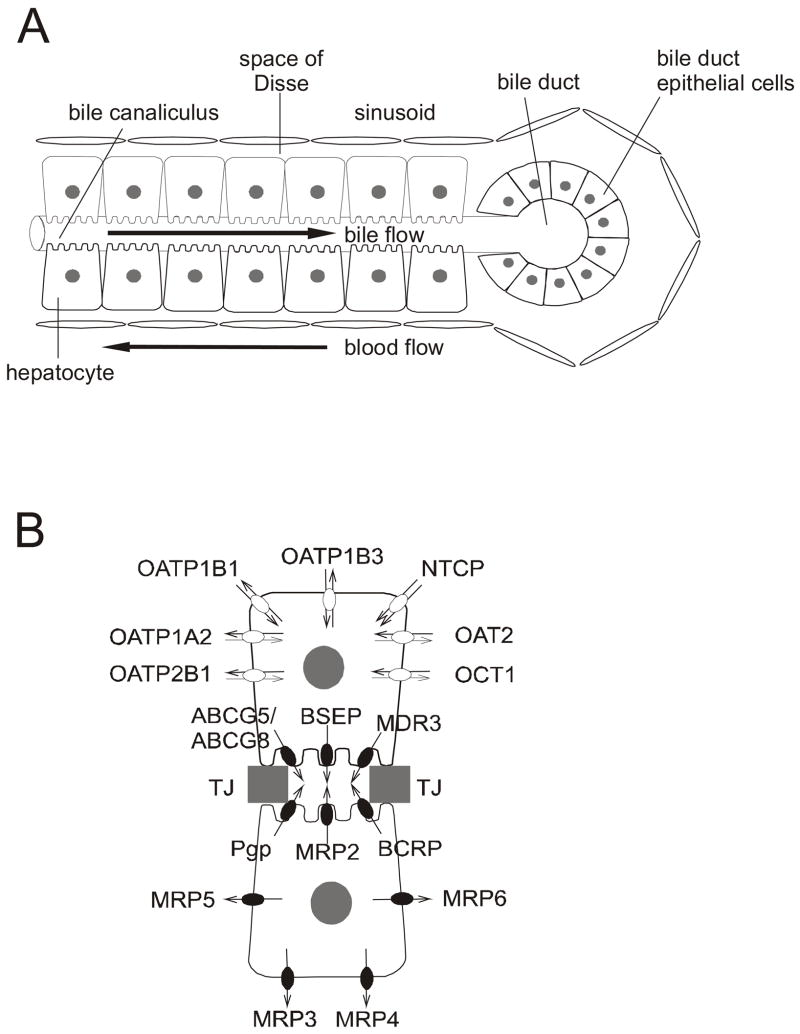
Several recent reviews have been published on the transporters involved in hepatobiliary disposition of drugs and bile salts.27–31 The liver (Figure 1) plays a central role in the biotransformation and removal of xenobiotics and endogenous compounds from the blood. While the majority of small, lipophilic compounds enter the hepatocyte from the Space of Disse on the basolateral membrane by simple passive diffusion, more polar and bulky molecules require transport systems to cross the sinusoidal membrane (Figure 2 A). Once inside the hepatocyte, compounds can be transported into bile either unchanged or as more hydrophilic metabolites after phase I and/or phase II biotransformations, or can be excreted into blood by basolateral transport proteins (Figure 2 B). The presence of several transport proteins on the canalicular domain of hepatocytes results in the excretion of solutes into the bile canaliculi, which are dilated intercellular spaces. One of the many functions of the liver is the synthesis of bile acids from cholesterol32. The major bile acids present in human bile are cholic and chenodeoxycholic acids conjugated with glycine and taurine.33 Bile salts are excreted into bile by the bile salt export pump (BSEP/ABCB11, Figure 2 B). Bile salts facilitate the absorption of nutrients in the intestine by emulsifying lipophilic molecules due to their surfactant characteristics.34 Once this function is served, bile salts are very efficiently reabsorbed into the cells of the distal ileum by the apical sodium-dependent bile salt transport protein (ASBT/IBAT/SLC10A2 35) and excreted across the basolateral membrane into portal blood by the recently identified organic solute transport proteins OST α and β.36,37 In addition to bile salt transport, hepatic transport proteins facilitate excretion of end-products of metabolism, such as bilirubin and/or xenobiotics, into bile for subsequent removal via the intestine, and thus act as an important clearance mechanism.
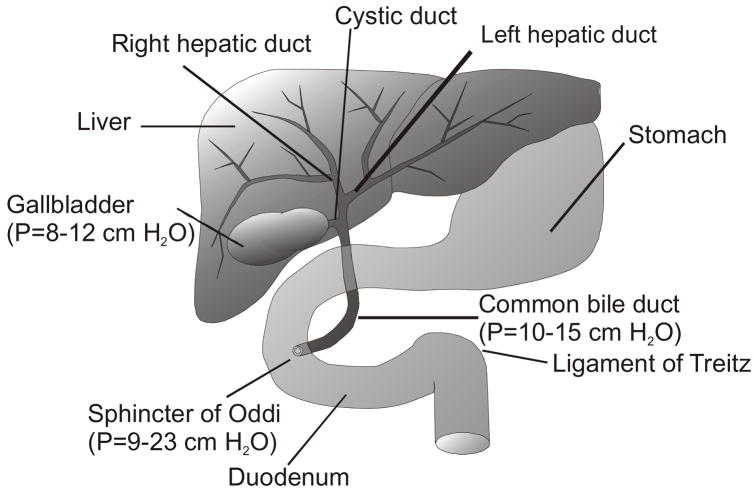
Figure 1.
Macro-anatomy of the hepatobiliary system: bile flow into the gallbladder is facilitated by lower internal pressure when compared to the rest of the biliary tract (pressure values are in parenthesis40). Water and solutes are reabsorbed within the gallbladder.
Figure 2. Micro-anatomy of the hepatobiliary tract.
(A) At the cellular level, hepatocytes are organized in cords and bathed by sinusoidal blood from the basolateral side; the canalicular membranes form the bile canaliculi. Bile flows in the opposite direction to blood and drains into bile ducts
(B) An enlarged view of two hepatocytes from Figure A. Transport proteins are involved in the uptake and excretion of endogenous substances and xenobiotics into blood and bile. Filled symbols represent unidirectional primary active transport by ATP-binding cassette (ABC) transport proteins (Pgp, ABCB1; BSEP, ABCB11; BCRP, ABCG2; MRP2, ABCC2; MRP3, ABCC3; MRP4, ABCC4; MRP5, ABCC5; MRP6, ABCC6; ABCG5, ABCG5; ABCG8, ABCG8). Open symbols represent transport by members of the solute carrier protein families including the SLC family (OAT2, SLC22A7; OCT1, SLC22A1; NTCP, SLC10A1) and the SLCO family (OATP1B1, SLCO1B1; OATP1B3, SLCO1B3; OATP1A2, SLC01A2; OATP2B1, SLCO2B1). Each functional hepatocyte contains all the transport proteins depicted in this figure. Adjacent hepatocytes form tight junctions (TJ) to seal the canalicular domain from the basolateral domain.
Bile Formation and Excretion
Bile formation includes uptake of bile components from the sinusoidal blood and secretion of these substances into the canalicular space. Water and other small ions also diffuse into bile due to the differential osmotic pressure between bile and the hepatocyte cytoplasm. Subsequently, bile is drained into the biliary tree along the liver lobules and reaches the hepatic ducts. In humans, a fraction of this hepatic bile is secreted continuously into the duodenum while the majority of the ~800 mL of bile secreted per day is concentrated and stored in the gallbladder (Figure 1). Bile freely flows into the gallbladder, because this organ has a much lower internal pressure than the intra-hepatic ducts, common bile duct and the Sphincter of Oddi (Figure 1). The gallbladder fills to its maximum capacity (40–70 mL) in approximately 6 hours, while re-absorbing water and electrolytes from hepatic bile.3,38–40 After ingestion of a meal, fats, proteins and acid reach the duodenum and endogenous neurohormones such as cholecystokinin (CCK) and secretin are released by endocrine cells in the small intestine.41 CCK, acting on the CCK-A type receptors of smooth muscle fibers, contracts the gallbladder and relaxes the Sphincter of Oddi. A similar effect can be obtained by intravenous infusion of physiological amounts of CCK analogs (e.g., CCK-8).42,43 CCK blood concentrations are decreased to minimal values during fasting, which promotes contraction of the Sphincter of Oddi and relaxation of the gallbladder walls, facilitating storage of bile in the gallbladder. CCK also delays gastric emptying in order to coordinate the digestive postprandial process. In the blood of humans and animals, CCK-33, 8 and 58 are the predominant active forms of this polypeptide 44 generated by differential post-translational modifications of the same gene; functional activity is dependent upon the last seven amino acids at the carboxyl terminus. In addition to the role of CCK in gallbladder contraction, CCK also functions as a neurotransmitter in the cerebral cortex to regulate satiety, and on the peripheral nerves of the GI tract to regulate bowel movement.45,46
Methods Used to Determine In Vivo Biliary Clearance
Several preclinical tools involving in vivo or ex vivo rodent models can be utilized to evaluate the extent of biliary excretion of drugs and the interaction of xenobiotics with bile salt transport proteins. These tools employ animal species with well characterized differences from humans, making the prediction/extrapolation to humans less accurate.47 Determination of the extent of biliary excretion can be attained in human subjects with different methodologies as described in detail below. However, only a few studies have been performed in vivo in humans to evaluate biliary clearance of drugs, and little is known about the contribution of biliary clearance to the overall systemic clearance for most drugs. Table I summarizes those studies published in the literature along with the techniques used for the determination of the amount of drug and metabolites excreted into bile.
Table I.
Summary of human studies that report the amount of parent drug and/or metabolites excreted via the biliary route. The percentage of the dose excreted into bile and/or feces as parent drug or total radioactivity is/are summarized where possible. The administration route and methods used to obtain this information, the particular population of subjects studied, and systemic and biliary clearance values, if published, also are listed. Antibiotics are a therapeutic class that has been studied in particular detail in terms of biliary excretion and distribution within the gallbladder tissue. In addition to the studies reported here, the reader is referred to comprehensive reviews on this topic.71,129–131
| Therapeutic Class | Compound | Administration route | Population studied | Bile collection methodology (number of subjects) | % of dose excreted in bile or feces (collection interval) | Clearance parameters | Reference |
|---|---|---|---|---|---|---|---|
| Antineoplastic Agent | Elsamitrucin | IV | Cancer patients | Indwelling biliary catheter (n=1) | ~22 unchanged (51 hr) | Cltotal=10–19 L/h/m2 | 132 |
| Irinotecan | IV (14C) | Cancer patients | Feces (n=7); T-tube bile and feces (n=1) | 54–75 total in feces, 30 total in bile; 32.3 unchanged, 14 total in feces | Cltotal=13.5 L/h/m2 |
67
133 |
|
| Taxol | IV | Cancer patients | Feces (n=5) | 5 and 71.1 unchanged and total (4–5 days) | N.D. | 134 | |
| Vincristine | aIV (3H) | Cancer patients | T-Tube bile and feces (n=1) | a49.6 total in bile and4.2 in feces (72 hr), 46.5 of bile radioactivity at 2 hr as unchanged | bCltotal=189 mL/min/m2 |
a, 55
b, 135 |
|
| Hepatobiliary Imaging Agent | Tc-99m Mebrofenin | IV | Healthy volunteers | Duodenal aspiration (n=4) | 67.1/84.2 unchanged/corrected by EF (3 hr) | Cltotal=17.3
Clbiliary=12.5 in vivo Clbiliary =16.1 mL/min/Kg |
83 |
| Cardiac Imaging Agent | Tc-99m Sestamibi | IV | Healthy volunteers | Duodenal aspiration (n=7) | 14.7/21.5 unchanged/corrected by EF (3 hr) | Cltotal=25.8
Clbiliary=3.9 in vivo Clbiliary=6.0 mL/min/Kg |
136 |
| NSAID | Aspirin | PO and IV | Patients | T-tube bile (n=3); nasobiliary tube bile (n=2) | 0.02–1.89 primarily conjugated (24 hr) | N.D. | 50 |
| Diclofenac | PO | Patients | Percutaneous transhepatic cholangiodrainage (n=2) | 4.6 total (24 hr), 1.1 unchanged and active phase II metabolites | N.D. | 51 | |
| Indomethacin | PO | Patients | Percutaneous transhepatic cholangiodrainage (n=3) | 0.23–15 (24 hr) | N.D. | 51 | |
| Ibuprofen | PO | Patients | Percutaneous transhepatic cholangiodrainage (n=3) | 0.82 (24 hr), 0.15 unchanged and active phase II metabolites | N.D. | 51 | |
| Analgesic, Antipyretic | Paracetamol/Acetaminophen | PO | cPatients; dhealthy volunteers | cT-tube bile (n=6); dduodenal aspiration for 15 min post CCK administration(n=10) | c2.6 total and c0.57 unchanged; d7.3 total | N.D. |
c,52
d, 137 |
| Antihyperlipidemic | Ezetimibe | PO (14C) | Healthy males | Feces (n=8) | 78 mostly unchanged (10 days) | N.D. | 138 |
| Fluvastatin | IV/PO (3H) | Healthy volunteers | Feces (n=6) | 86–92 total (120 hr), 1.8 of total radioactivity as unchanged | Cltotal=0.97 L/hr/Kg | 64 | |
| Pravastatin | IV | Healthy volunteers | Feces | 34 total and 23 unchanged | Cltotal=13.5 mL/min/Kg | 139 | |
| Rosuvastatin | PO (14C) | Healthy volunteers | Feces (n=6) | 90 total at 10 days (~70 at 72hr); 78.8 as unchanged (10 days) | N.D. | 68 | |
| Simvastatin | PO (14C) | Patients | T-tube bile and feces (n=4) | 25 total in bile and 20 in feces | N.D. | 53 | |
| Antihistamine | Desloratadine | PO (14C) | Healthy volunteers | Feces | 47 primarily metabolites | N.D. | 140 |
| Ebastine | PO | Healthy volunteers | Feces | 6 total (24hr), 28 total (312 hr) | N.D. | 141 | |
| Fexofenadine | PO (14C) | Healthy volunteers | Feces | 80 total (~95 of radioactivity as unchanged) | Cl/F=1.10 L/hr/Kg | 140,141 | |
| Levocetrizine | PO (14C) | Healthy volunteers | Feces | 13 total and 86 unchanged | N.D. | 140 | |
| Mizolastine | PO (14C) | Healthy volunteers | Feces | 84–95 primarily glucuronidated | Cl/F=0.69 L/hr/Kg | 140,141 | |
| Antihypertensive | Eplerenone | PO (14C) | Healthy volunteers | Feces (n=8) | 32.0 primarily metabolites (96 hr) | N.D. | 63 |
| Ramipril | PO | Patients | T-tube bile (n=8) | ~17 total and 0.1 unchanged (24 hr) | N.D. | 54 | |
| Spironolactone | PO (3H) | Healthy males | Feces (n=5) | 22.7 total (5 days) | N.D. | 66 | |
| Valsartan | IV | Patients | Biliary-enteric diversion (n=1) | 89 mostly unchanged | N.D. | 142 | |
| Antidiabetic | Rosiglitazone | PO/IV (14C) | Healthy volunteers | Feces (n=4) | 21.6 from PO, 25.2from IV (21 days)mostly metabolites | N.D. | 143 |
| Immuno Suppressant/Modulator | Leflunomide | PO (14C) | Healthy volunteers | Feces (n=4) | 48 (28 days) as methylhydroxy-metabolite (M1) | Cltotal for M1=0.051 L/h | 144 |
| Immunosuppressant | Mycophenolic acid | PO | Healthy volunteers; patients | Feces (n=4); T-tube bile (n=17) | 5.5 in feces; 16.5–26 in bile as glucuronide conjugate, <0.1 unchanged in bile | Cltotal=8.5–11.6 L/min | 4 |
| Tacrolimus | eIV/PO (14C) fIV | eHealthy males; fpatients | eFeces (n=6) | e0.29 (IV) and 0.12 (PO) unchanged, e5.4 (IV) and 92.6 (PO) total (11 days) | fCltotal= 0.3–5.4 L/hr/Kg |
e, 69
f, 145 |
|
| Gastrointestinal Stimulant | Metoclopramide | PO | Patients | Nasobiliary tube (n=10), repeated with restored enterohepatic recirculation | <1 as unchanged and conjugated metabolites | Cl/F= 0.5 L/hr/Kg | 146 |
| Antiarrhythmic | Digoxin | PO | gHealthy Males; hpatients | Intestinal perfusion(n=6) | gCltotal=255 Clbile= g72–163 or h79–343 or I58–142 mL/min |
g, 147
h, 12 I, 11 |
|
| Antipsychotic | Quetiapine | PO | Feces | 21 total, < 1 of total radioactivity as unchanged (168 hr) | Cl/F=86–105 L/hr | 148 | |
| Skeletal Muscle Relaxant | Cyclobenzaprine | IV and PO (14C) | Healthy volunteers | Feces (n=5, PO) (n=3, IV) | 13.5 (PO) and 15.1 (IV) primarily as glucuronide (120 hr) | N.D. | 149 |
| Antifungal | Ketoconazole | PO (3H) | Healthy males | Feces (n=3) | 57 total, 20–65 of total radioactivity as unchanged (4 days) | N.D. | 150 |
| Benzodiazepine | Lormetazepam | PO | Patients | Nasobiliary drainage bile (n=5) | 0.3–2.8 total (24 hr) | Cltotal=1.9–6.2 mL/min/Kg | 26 |
| Analgesic Opioid | Methadone | PO (acute, intermediate, chronic administration) | Post-addict volunteers | Feces (n=12) | 3.0, 7.4, 20.1 total and 0.14, 0.47, 1.2 unchanged (acute, intermediate, chronic, respectively) | N.D. | 151 |
| Anti-asthma | Montelukast | PO (14C) | Healthy volunteers | Duodenal secretions suction at 2–8 and 8–12 hr post dose (n=6) | 3–20 total | N.D. | 152 |
| Antiretroviral | Lopinavir | PO (14C in combination with ritonavir) | Healthy volunteers | Feces (n=5) | 82.6 total, 19.8 unchanged (0–8 days) | N.D. | 153 |
| Ritonavir | PO (14C) | Healthy males | Feces (n=5) | 86.3 total, 33.8 unchanged (6 days) | N.D. | 154 | |
| Antibiotic | Azlocillin | IV | Healthy volunteers | Duodenal perfusion (n=5) | 3.4 as unchanged | Cltotal=173–375 | 71 |
| Ceftriaxone | IV | Healthy males | Duodenal perfusion for 6–8 hr (n=5) | 11–65 total | Clbile=1.4–25.9 mL/min
Cltotal=9–20 Clbile=1–13 mL/min |
155 | |
| Mezlocillin | IV | Healthy volunteers | Duodenal perfusion(n=5) | 29.9 unchanged | Cltotal=187–477
Clbile=45.8–122.8 mL/min |
71 | |
| Piperacillin | IV | jCholecystectomy patients; khealthy volunteers | jT-tube (n=5); kduodenal aspiration (n=3) | j0.7 as total (12 hr); k0.41/1.1 unchanged/corrected by EF (6 hr) |
jCltotal=276
jClbile=1.74 mL/min kCltotal=2.9 kClbiliary=0.012 k in vivo Clbiliary=0.032 mL/min/Kg |
j, 48
k, 84 |
|
| Quinupristin/Dalfopristin | IV | Healthy volunteers | Feces (n=24) | 74.4–77.5 total, <15 unchanged | Cltotal=0.83–0.87
Cltotal=0.85–0.82 L/hr/Kg |
156 | |
| Temocillin | IV | Healthy volunteers | Duodenal perfusion(n=6) | 2.2 unchanged (6 hr) | N.D. | 70 | |
| Trovafloxacin | PO (14C) | Healthy volunteers | Feces (n=4) | 63.3 total, 43.2 of total radioactivity as unchanged | N.D. | 157 |
IV= intravenous administration
PO= oral administration
EF= gallbladder ejection fraction
in vivo Clbiliary= biliary clearance corrected for EF
I. Patients as Study Subjects
Historically, there has been little success in the development of valid and reliable techniques to quantify biliary excretion of drugs or endogenous compounds in healthy human volunteers. Such data is obtained more easily from patients diagnosed with diseases of the gallbladder and biliary tract who require medical procedures that allow measurements of drug concentrations in bile. Usually these patients suffer from cholestasis and/or occlusion of the biliary or cystic ducts, and some may have undergone liver transplantation.
i. Cholecystectomy
Several groups have investigated gallbladder bile concentrations and disposition of drugs into bile by administering the compound of interest to patients prior to cholecystectomy. Drug concentrations in the gallbladder bile and tissue after removal are quantified and compared to the plasma concentrations. Although this approach only allows the determination of drug content in bile and tissue at a single time point, it is still very useful and has been employed to establish whether an antibiotic reaches the necessary therapeutic concentrations to treat bacterial infections of the gallbladder and biliary tract.48,49
ii. T-tube
Patients that require a temporary bile shunt (T-tube) that diverts part of the bile flowing from the liver to a transcutaneous port for external collection have been employed commonly to study biliary excretion of drugs. T-tubes are used primarily in patients with cholangitis, distal bile duct obstruction or edema, and often are inserted after cholecystectomy and choledochotomy. T-tubes allow for partial collection of liver bile, and provide an estimate of the biliary excretion of administered drugs during the time that the shunt is in place.50–55 A limitation of this technique is that bile collection in these patients is not complete. Furthermore, the composition of bile may be very different in patients due to underlying hepatobiliary disease.56
iii. Nasobiliary Drainage
Patients with bile duct stenosis can be treated with the temporary insertion of a nasobiliary tube. This patient population has been recruited less frequently for drug disposition studies, probably due to the fact that this procedure is relatively new. This intervention theoretically allows for complete collection of bile while the tube is in place. The nasobiliary drainage interrupts delivery of bile into the duodenum and can be used to evaluate the influence of enterohepatic recirculation on the plasma concentration-time profile for the drug of interest. In these studies, the same patients have been used as their own control when the stricture has resolved and the tube has been removed thus restoring the enterohepatic recycling process.26,50
Less commonly, bile has been collected from patients equipped with other types of drainage devices such as biliary-enteric diversions or percutaneous transhepatic cholangiodrainage.51 Although the details of the surgical procedures are beyond the scope of this review, all these techniques allow free access to partial or complete collection of hepatic bile from patients.
In recent years laparoscopic cholecystectomy with duct exploration and endoscopic retrograde cholangiopancreatography (ERCP) have largely replaced more invasive methods for removing common duct stones. As a result, many fewer patients with T-tubes have been available to enroll in studies examining the biliary excretion of drugs. The relatively few patients who are treated with nasobiliary drainage are seldom well enough or maintain the tube long enough to be suitable study candidates.
II. Healthy Volunteers as Study Subjects
One of the major limitations of the techniques described above is the use of subjects with significant hepatobiliary disease. Disease status may significantly influence transport protein expression, function, localization and/or bile flow, thereby altering drug excretion and reabsorption patterns.57,58 It is well known that biliary obstruction decreases the expression of BSEP and MRP2 (ABCC2) in hepatocytes, and increases P-gp, MDR3 (ABCB4) and MRP3 (ABCC3) expression.59,60 Moreover, cholestatic syndromes may be associated with genetic polymorphisms in transport proteins.61,62 More relevant information may be obtained by enrolling healthy volunteers as study subjects. In this case, the physiology of the hepatobiliary tract is intact and healthy. However, the collection of bile from the gallbladder or upon immediate secretion into the intestine is more challenging, due to the difficulty of accessing the gallbladder and the anatomy of the human hepatobiliary tract.
i. Fecal Recovery
The approach most commonly used in the determination of biliary excretion as a route for drug elimination is the quantification of the drug of interest in feces. Often these studies are performed with a radiolabeled compound in order to increase the selectivity and the recovery of drug and unknown metabolites, resulting in good mass balance.63–69 More recently, sensitive analytical techniques such as HPLC coupled with mass spectrometry have been applied widely to the analysis of biological matrices to identify the excretion routes of parent drugs and metabolites. Several drawbacks are associated with the quantification of drugs in feces. Most importantly, this method does not distinguish between biliary excretion and intestinal secretion processes. For orally administered drugs, this method does not distinguish between unabsorbed drug and that secreted by the liver and/or gut back into the intestinal lumen, thus, the percentage of the dose in feces does not necessarily reflect the dose excreted into bile. Moreover, relatively unstable drugs, or compounds that are transformed into unstable metabolites, may not be recovered in feces due to the long exposure to the intestinal contents and colonic flora. Finally, drugs subjected to entero-hepatic or entero-enteric recycling, such as glucuronide conjugates, can be reabsorbed in the colon upon intestinal degradation by β-glucuronidases of the microbial flora, and will not be recovered in the feces.
ii. Aspiration of Duodenal Fluids
An ideal methodology would provide information on the amount of parent drug and metabolites excreted into bile, and would be flexible enough to allow for studies examining potential drug-drug interactions. This type of information can be obtained by sampling duodenal fluids to collect bile upon discharge from the biliary tract into the small intestine.11,12,70,71 Duodenal bile is representative of gallbladder bile in terms of bile composition, although it is more dilute.72 The use of oroenteric tubes to withdraw pancreatico-biliary secretions from the duodenum is a common practice in the medical field, and has been used to study the biliary excretion of endogenous compounds such as radiolabeled bile acids.35,73 Some of these investigations have utilized occlusive balloons to facilitate more complete bile collection, along with the perfusion of a marker to evaluate leakage of bile or duodenal fluids distal to the balloon seal.74 Other groups have corrected for any incomplete bile collection by perfusing non-absorbable markers, such as polyethylene glycol or sulphobromophthalein, into the duodenum.71,75,76 Modified versions of these methodologies have been applied successfully to the determination of intestinal absorption and secretion of drugs, thus enhancing the knowledge regarding intestinal processes, which complements information acquired regarding biliary excretion of drugs.77–82 All the methods cited are particularly sophisticated, and the execution of these studies is not trivial. These clinical studies often require custom-made catheters, specialized personnel and equipment, and they need to be approved by local Institutional Review Boards and Research Ethics Committees. For these reasons, these methods are not used widely. Although a direct comparison of methods has not been published, it is reasonable to believe that the information obtained is, in many cases, an estimate of the amount of drug excreted via the biliary route. Nevertheless, many of the studies conducted in healthy human volunteers have resulted in incomplete and highly variable recovery of compounds excreted in bile. This is primarily due to the difficulty in obtaining and assessing the completeness of bile collection, and an inherent lack of control over gallbladder contraction. A novel method to increase the recovery of biliary secretions and correct for the degree of gallbladder contraction, with the intention of reducing the level of complexity of the procedure, has been developed recently by our group.83 This method assures the quantitative collection of bile secreted into the duodenum by temporary occlusion of the intestine with an inflatable balloon. At the same time, the administration of a hepatobiliary imaging agent (e.g. Tc-99m mebrofenin) is used to evaluate the degree of gallbladder contraction in response to pharmacological stimulation, and to detect any leakage of bile due to partial occlusion of the intestine. One of the major factors affecting the variability in the determination of biliary excretion in humans is related to differences in the amount of bile and drug delivered into the duodenum during the collection interval. Partial spontaneous contractions of the gallbladder during fasting are not uncommon, and can deliver over 25% of total gallbladder bile into the intestine. To obtain more complete gallbladder contraction, CCK-8 can be administered intravenously to pharmacologically stimulate gallbladder emptying. CCK-8 administered at a low dose (0.02 μg/Kg) infused slowly over 30–60 min has been reported to provide the most complete gallbladder contraction43; however, inter-individual response is variable, and possibly influenced by the collection procedure. Therefore, it is important to assess the degree of gallbladder contraction in each subject. The administration of trace amounts of a gamma-emitting probe that accumulates quickly in bile, such as Tc-99m mebrofenin, can assist in the determination of the overall gallbladder response, and indicate when intestinal occlusion is not complete. Alternatively, ultrasound may be used to determine the extent of gallbladder contraction together with perfusion of the occluded tract with a non-absorbable marker to identify leakages. A gallbladder ejection fraction (EF) can be calculated from the abdominal gamma images of the volunteers during gallbladder contraction, and this number can be incorporated as a correction factor when determining the amount of bile (containing drug) excreted into the duodenum and collected by aspiration.83 This method has been shown to correct reasonably well for the high variability in the gallbladder contraction which influences the amount of drug excreted into the duodenum.84 Quantification of parent drug and metabolites in bile aspirated directly from the duodenum rather than in feces can be extremely advantageous in the identification of unknown metabolites, uniquely formed in humans, that are unstable and would not be found after prolonged contact with intestinal enzymes (such as unstable glucuronides).
Finally, the possibility of repeating a pharmacokinetic study in the same volunteers with and without the balloon inflated enables an evaluation of the importance of biliary excretion in systemic drug exposure, and in studying the enterohepatic recirculation process.85 Biliary excretion and intestinal reabsorption may be one explanation for multiple peaks in the plasma concentration-time profiles of some drugs.86,87 Often, pharmacokinetic modeling of these data with a model that incorporates enterohepatic recycling has been used to explain these experimental observations.88 Unfortunately, this approach does not provide direct evidence for biliary excretion, and does not allow for precise quantification of the biliary clearance parameter. Experimentally, the pharmacokinetic disposition of drugs and glucuronide conjugates have been compared in patients before and, upon removal of nasobiliary drainage, after the restoration of enterohepatic recirculation.26 Other studies have interrupted enterohepatic recirculation with antibiotics, to eliminate the colonic flora, and/or cholestyramine, a resin that prevents the availability of lipophilic compounds for absorption in the gut lumen.4,89 Improved methods for studying drug disposition in healthy volunteers in the presence or absence of enterohepatic recirculation should enable the investigation of hepatobiliary disposition mechanisms in much more depth than what has been attempted to date.
Methods Used to Determine In vitro Biliary Excretion
Several in vitro models are available to investigate the processes governing biliary excretion and potential interactions of drugs with the hepatic uptake and excretion of bile salts and other endogenous and exogenous substances. These tools span from the whole hepatocyte to membrane vesicles prepared from cell lines transfected with specific transport proteins. Such techniques complement human and animal in vivo studies, and allow characterization of transport processes at the molecular level. Unfortunately, the results obtained with in vitro models are difficult to compare to human in vivo data. This is primarily due to the fact that the data generated in vitro are used to determine values for the intrinsic biliary clearance of a drug. In contrast, in vivo studies often are designed to obtain more crude estimates such as the percentage of the dose excreted into bile rather than pharmacokinetic parameters. The in vivo biliary clearance values in humans usually are not reported (Table I). Whole liver perfusion, a common technique in rodent models to obtain biliary clearance values, is rarely possible in humans due to limited availability of viable whole human livers.
i. Sandwich-Cultured Hepatocytes
Primary hepatocytes isolated from humans and animals are useful tools for studying hepatic metabolism and transport due to the expression of the full array of proteins involved in these processes. In conventional culture, a rapid decline in expression of both metabolizing enzymes and transport proteins occurs over time after cell isolation.90 In addition, collagenase digestion of liver, required for hepatocyte isolation results in the disruption of the canalicular membrane and consequently, the cellular internalization of transport proteins expressed at this membrane.91 A novel in vitro model system using primary hepatocytes from various species, including rat and human, cultured in a collagen-sandwich configuration (SC) has several distinct advantages over conventional hepatocyte culture. For example, SC hepatocytes develop functional and extensive bile canalicular networks, acquire and maintain normal cell polarity, and allow direct access to the hepatocyte and adjacent biliary compartment.92–96 Thus, hepatocytes maintained under these culture conditions offer a unique opportunity to study basolateral uptake and canalicular efflux transport processes. Using simple alterations in buffer components, accumulation of substrates of interest can be measured in hepatocytes alone, or in hepatocytes plus canalicular networks. This allows determination of the in vitro intrinsic biliary clearance. To date, most SC experiments have been conducted using rat hepatocytes. 93,96–100 Recently, SC human hepatocytes also have been characterized; uptake and efflux transport proteins are expressed, properly localized on the apical and basolateral membranes and functional.91,101 Since SC human hepatocytes also retain metabolic capabilities, this model allows for investigation of the interplay between many of the processes that take place in vivo. Additionally, these cells can be maintained in culture for several days allowing for the study of drug interactions involving induction mechanisms. Human hepatocytes are not readily available and are associated with high costs. Furthermore, each culture is performed with cells from a single donor reflecting the inherent variability among donors in protein expression, function, and canalicular network formation. SC human hepatocytes serve as a unique, comprehensive tool to investigate hepatobiliary transport mechanisms.
ii. Suspended Hepatocytes
Viability of primary hepatocytes can be maintained for several hours in suspension when supplemented with appropriate culture media and oxygen. Fresh or cryopreserved rat and human hepatocytes have been used extensively to determine the hepatic metabolic clearance of drugs. 102,103 Suspended hepatocytes are a useful tool to characterize hepatic uptake and metabolism processes and inhibition studies can be perfomed with this system. However, due to the limited viability of these cells, it is not possible to study induction processes.104–106 Additionally, internalization of canalicular transport proteins during collagenase perfusion limits the use of suspended hepatocytes to predict an overall value for biliary clearance when transport into bile is important.
iii. Vectorial Transport Using Polarized Cell Lines
The Madin Darby Canine Kidney, strain II (MDCKII) cells are polarized cells that have been used extensively to express various combinations of recombinant uptake and efflux transport proteins. Initially, MDCKII cells transfected with different combinations of a single basolateral uptake and a single canalicular efflux pump were established.107–109 More recently, MDCKII cells transfected with a single efflux pump (MRP2/ABCC2) and three basolateral uptake transport proteins (OATP1B1/SLCO1B1, OATP1B3/SLCO1B3 and OATP2B1/SLCO2B1) have been established in an attempt to mimic vectorial transport processes in human liver.110 The combination of quadruple, double and single transfected cells is very useful for determining the contribution of individual transport proteins to vectorial transport processes. These cell lines can be transfected with transport proteins of interest, grown on a permeable membrane, and used to study the transcellular passage of selected substrates. Many model substrates have been studied using MDCK cells, and correlated with biliary clearance values obtained from rats in vivo.108 Although the polarized transfected cell systems are powerful tools for studying vectorial transport, they lack a full complement of transport proteins, metabolic enzymes and co-factors present in hepatocytes, and expression levels of transport proteins may not be comparable to hepatocytes in vivo. These issues must be considered in data interpretation and when extrapolating to in vivo hepatic or biliary clearance values. Nevertheless, transfected systems are simple to use and more readily available than primary human hepatocytes and are useful as a high-throughput screening tool.
iv. Whole Cells and Membrane Vesicles
Whole cell expression systems such as Xenopus laevis oocytes or immortalized cell lines transfected with the transporter of interest are ideal tools when functional studies of the kinetics of transport for individual transport proteins are needed.111 Such expression systems have been used extensively in the literature to demonstrate substrate specificity and for functional characterization of mutant proteins, such as genetic polymorphisms.112,113 Although oocytes are a transient expression system and need to be injected individually with cRNA of the protein of interest, oocytes often are used to study uptake processes. The commercial availability of this model facilitates their use for transport studies.114 These expression systems are more applicable to studying the effect of genetic diseases and the molecular mechanisms of transport than for the determination of hepatobiliary clearance.115 Because of technical difficulties in loading whole cells with a substrate of interest prior to measuring efflux, these models are more suitable for the study of uptake transport proteins. To circumvent this issue, the double transfected MDCKII cells have been used, or more commonly, efflux transport processes have been studied with inside-out membrane vesicles. Such vesicles can be prepared from primary hepatocytes or from cell lines transfected with a transport protein of interest. Basolateral and apical membrane vesicles prepared from polarized cells such as primary hepatocytes can be isolated through various centrifugation steps and membrane fractions enriched in basolateral or canalicular transporters can be used to investigate drug transport and transport inhibition.116,117 Canalicular vesicles are more commonly prepared from rat than human hepatocytes due to limited human tissue availability. However, several recent studies have used canalicular membrane vesicles isolated from human hepatocytes.117–119 Membrane vesicles prepared from primary hepatocytes have distinct advantages over transfected systems: the native membrane environment is in place during isolation from the other cellular components, and all the hepatic transport systems are present. Membrane vesicles can be prepared, stored frozen, and used as needed; however, isolation of basolateral and canalicular fractions is labor intensive and complete purity is never achieved.
Membrane vesicles prepared from cell lines transiently and stably expressing membrane proteins of interest have been used extensively to characterize polymorphisms, substrate specificity and structure-function relationships of efflux transport proteins.120–122 The expression systems used are many (insect cells: e.g., Sf9 cells 123,124; mammalian cells: e.g. HEK293 cell line 125) and expression of endogenous transporters may vary. The background activity of membrane vesicles must be accounted for by performing control experiments in untransfected cells; moreover, post-translational protein modifications may be different depending on the host cells and may affect the affinity of the transport protein for the substrate of interest.
CONCLUSIONS
Novel and important technologies for evaluation of biliary excretion and the related toxicological/pharmacological issues derived from drug-drug interactions at the hepatobiliary transport level are under development. Currently, several in vitro models are used as screening tools in drug discovery and early development to eliminate molecules that present challenges in this particular aspect of disposition.126–128 Comparison of results obtained using in vitro systems to data generated in vivo in humans would be extremely beneficial to substantiate in vitro observations. Unfortunately, information on in vivo biliary excretion of drugs is not readily available, and studies in volunteers will continue to be challenging and limited to few subjects. However, the findings of these studies can be extremely valuable, not only to explain results obtained using in vitro systems, but also to determine the importance of biliary excretion and enterohepatic recirculation in the overall disposition of drugs.
In the future, it is possible that a more systematic comparison between in vivo and in vitro hepatobiliary clearance will confirm the accuracy of in vitro scaled predictions, and allow the development of accurate models to predict drug disposition in humans with a higher degree of confidence. We believe that this combination of tools, together with extensive use of mathematical modeling and simulations, will not only improve our understanding of drug disposition, but will also help predict drug-drug interactions and/or the impact of genetic polymorphisms on drug exposure and toxicity.
Acknowledgments
The authors are grateful to Dr. William Heizer for helpful scientific discussions and to Mr. Robert Kelly for assistance with figure preparation. This work was supported by National Institutes of Health grant R01 GM41935 and grant RR00046 from the GCRC program of the Division of Research Resources. Giulia Ghibellini is an American Foundation for Pharmaceutical Education predoctoral fellow. Dr. Elaine M. Leslie is the recipient of a Canadian Institutes of Health Research postdoctoral fellowship.
References
- 1.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 2.Fleck C, Braunlich H. Factors determining the relationship between renal and hepatic excretion of xenobiotics. Arzneimittelforschung. 1990;40(8):942–946. [PubMed] [Google Scholar]
- 3.Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41(10):751–790. doi: 10.2165/00003088-200241100-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34(6):429–455. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jahnchen E, Meinertz T, Gilfrich HJ, Kersting F, Groth U. Enhanced elimination of warfarin during treatment with cholestyramine. Br J Clin Pharmacol. 1978;5(5):437–440. doi: 10.1111/j.1365-2125.1978.tb01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43(2):342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126(1):322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz UI, Gramatte T, Krappweis J, Oertel R, Kirch W. P-glycoprotein inhibitor erythromycin increases oral bioavailability of talinolol in humans. Int J Clin Pharmacol Ther. 2000;38(4):161–167. doi: 10.5414/cpp38161. [DOI] [PubMed] [Google Scholar]
- 9.Chico I, Kang MH, Bergan R, Abraham J, Bakke S, Meadows B, Rutt A, Robey R, Choyke P, Merino M, Goldspiel B, Smith T, Steinberg S, Figg WD, Fojo T, Bates S. Phase I study of infusional paclitaxel in combination with the P-glycoprotein antagonist PSC 833. J Clin Oncol. 2001;19(3):832–842. doi: 10.1200/JCO.2001.19.3.832. [DOI] [PubMed] [Google Scholar]
- 10.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66(4):338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 11.Angelin B, Arvidsson A, Dahlqvist R, Hedman A, Schenck-Gustafsson K. Quinidine reduces biliary clearance of digoxin in man. Eur J Clin Invest. 1987;17(3):262–265. doi: 10.1111/j.1365-2362.1987.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 12.Hedman A, Angelin B, Arvidsson A, Beck O, Dahlqvist R, Nilsson B, Olsson M, Schenck-Gustafsson K. Digoxin-verapamil interaction: reduction of biliary but not renal digoxin clearance in humans. Clin Pharmacol Ther. 1991;49(3):256–262. doi: 10.1038/clpt.1991.26. [DOI] [PubMed] [Google Scholar]
- 13.Hedman A, Angelin B, Arvidsson A, Dahlqvist R. Digoxin-interactions in man: spironolactone reduces renal but not biliary digoxin clearance. Eur J Clin Pharmacol. 1992;42(5):481–485. doi: 10.1007/BF00314854. [DOI] [PubMed] [Google Scholar]
- 14.Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, Haefeli WE. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76(1):73–84. doi: 10.1016/j.clpt.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001;167(1):83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- 16.Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69(4):223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- 17.Rolo AP, Palmeira CM, Wallace KB. Interactions of combined bile acids on hepatocyte viability: cytoprotection or synergism. Toxicol Lett. 2002;126(3):197–203. doi: 10.1016/s0378-4274(01)00464-7. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KL. Hepatobiliary disposition of the metabolically stable opioid peptide [D-Pen2, D-Pen5]-enkephalin (DPDPE): pharmacokinetic consequences of the interplay between multiple transport systems. J Pharmacol Exp Ther. 2004;311(3):1203–1210. doi: 10.1124/jpet.104.070201. [DOI] [PubMed] [Google Scholar]
- 19.Patel NJ, Zamek-Gliszczynski MJ, Zhang P, Han YH, Jansen PL, Meier PJ, Stieger B, Brouwer KL. Phenobarbital alters hepatic Mrp2 function by direct and indirect interactions. Mol Pharmacol. 2003;64(1):154–159. doi: 10.1124/mol.64.1.154. [DOI] [PubMed] [Google Scholar]
- 20.Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO. Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology. 2005;42(5):1091–1098. doi: 10.1002/hep.20898. [DOI] [PubMed] [Google Scholar]
- 21.Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH, Borst P. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A. 2005;102(20):7274–7279. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nezasa K, Tian X, Zamek-Gliszczynski MJ, Patel NJ, Raub TJ, Brouwer KL. Altered Hepatobiliary Disposition of 5 (and 6)-carboxy-2′,7′ dichlorofluorescein in Abcg2 (Bcrp1) and Abcc2 (Mrp2) Knockout Mice. Drug Metab Dispos. 2006 doi: 10.1124/dmd.105.007922. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42(15):1331–1357. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka H, Konno K, Shiina T, Naganuma H, Nishimura K, Ito K, Suzuki H, Sugiyama Y. Species differences in the transport activity for organic anions across the bile canalicular membrane. J Pharmacol Exp Ther. 1999;290(3):1324–1330. [PubMed] [Google Scholar]
- 25.Zhang P, Swift BD, Tian X, Brouwer KR. Interspecies variability of transport protein functions in sandwich-cultured (B-ClearTM) rat, dog and human hepatocytes. Drug Metab Rev. 2005;37(supplement 2):307–307. [Google Scholar]
- 26.Hellstern A, Hildebrand M, Humpel M, Hellenbrecht D, Saller R, Madetzki C. Minimal biliary excretion and enterohepatic recirculation of lormetazepam in man as investigated by a new nasobiliary drainage technique. Int J Clin Pharmacol Ther Toxicol. 1990;28(6):256–261. [PubMed] [Google Scholar]
- 27.Faber KN, Müller M, Jansen PLM. Drug transport proteins in the liver. Adv Drug Deliv Rev. 2003;55(1):107–124. doi: 10.1016/s0169-409x(02)00173-4. [DOI] [PubMed] [Google Scholar]
- 28.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 29.Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35(4):305–317. doi: 10.1081/dmr-120026398. [DOI] [PubMed] [Google Scholar]
- 30.van Montfoort JE, Hagenbuch B, Groothuis GM, Koepsell H, Meier PJ, Meijer DK. Drug uptake systems in liver and kidney. Curr Drug Metab. 2003;4(3):185–211. doi: 10.2174/1389200033489460. [DOI] [PubMed] [Google Scholar]
- 31.Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21(5):719–735. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- 32.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433(2):397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40(3):539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm Res. 1996;13(1):163–167. doi: 10.1023/a:1016062224568. [DOI] [PubMed] [Google Scholar]
- 35.Galatola G, Jazrawi RP, Bridges C, Joseph AEA, Northfield TC. Direct measurement of first-pass ileal clearance of a bile acid in humans. Gastroenterology. 1991;100(4):1100–1105. doi: 10.1016/0016-5085(91)90288-v. [DOI] [PubMed] [Google Scholar]
- 36.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204(3):238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280(8):6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svanvik J. In: Hepatic Transport and Bile Secretion: Physiology and Pathophysiology. Tavoloni N, Berk PD, editors. Raven Press; New York: 1993. pp. 607–618. [Google Scholar]
- 39.LaRusso NF. Gallbaldder and Bile Ducts. Vol. 6 Churchill Livingstone; Philadelphia: 1997. [Google Scholar]
- 40.Krishnamurthy GTKS. Nuclear Hepatology: a textbook of hepatobiliary diseases. Springer; Berlin, New York: 2000. [Google Scholar]
- 41.Jazrawi RP. Review article: measurement of gall-bladder motor function in health and disease. Aliment Pharmacol Ther. 2000;14(Suppl 2):27–31. doi: 10.1046/j.1365-2036.2000.014s2027.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamamura T, Takahashi T, Kusunoki M, Kantoh M, Seino Y, Utsunomiya J. Gallbladder dynamics and plasma cholecystokinin responses after meals, oral water, or sham feeding in healthy subjects. Am J Med Sci. 1988;295(2):102–107. doi: 10.1097/00000441-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Ziessman HA, Muenz LR, Agarwal AK, ZaZa AA. Normal values for sincalide cholescintigraphy: comparison of two methods. Radiology. 2001;221(2):404–410. doi: 10.1148/radiol.2212010154. [DOI] [PubMed] [Google Scholar]
- 44.Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab. 2001;86(1):251–258. doi: 10.1210/jcem.86.1.7148. [DOI] [PubMed] [Google Scholar]
- 45.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite--role of CCK. Physiol Behav. 2004;83(4):617–621. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 46.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269(5 Pt 1):G628–646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 47.Lave T, Portmann R, Schenker G, Gianni A, Guenzi A, Girometta MA, Schmitt M. Interspecies pharmacokinetic comparisons and allometric scaling of napsagatran, a low molecular weight thrombin inhibitor. J Pharm Pharmacol. 1999;51(1):85–91. doi: 10.1211/0022357991772006. [DOI] [PubMed] [Google Scholar]
- 48.Westphal JF, Brogard JM, Caro-Sampara F, Adloff M, Blickle JF, Monteil H, Jehl F. Assessment of biliary excretion of piperacillin-tazobactam in humans. Antimicrob Agents Chemother. 1997;41(8):1636–1640. doi: 10.1128/aac.41.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edmiston CE, Jr, Suarez EC, Walker AP, Demeure MP, Frantzides CT, Schulte WJ, Wilson SD. Penetration of ciprofloxacin and fleroxacin into biliary tract. Antimicrob Agents Chemother. 1996;40(3):787–791. doi: 10.1128/aac.40.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brune K, Nuernberg B, Schneider HT. Biliary elimination of aspirin after oral and intravenous administration in patients. Agents Actions Suppl. 1993;44:51–57. [PubMed] [Google Scholar]
- 51.Schneider HT, Nuernberg B, Dietzel K, Brune K. Biliary elimination of non-steroidal anti-inflammatory drugs in patients. Br J Clin Pharmacol. 1990;29(1):127–131. doi: 10.1111/j.1365-2125.1990.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegers CP, Loeser W, Gieselmann J, Oltmanns D. Biliary and renal excretion of paracetamol in man. Pharmacology. 1984;29(5):301–303. doi: 10.1159/000138026. [DOI] [PubMed] [Google Scholar]
- 53.Cheng H, Schwartz MS, Vickers S, Gilbert JD, Amin RD, Depuy B, Liu L, Rogers JD, Pond SM, Duncan CA, et al. Metabolic disposition of simvastatin in patients with T-tube drainage. Drug Metab Dispos. 1994;22(1):139–142. [PubMed] [Google Scholar]
- 54.Verho M, Luck C, Stelter WJ, Rangoonwala B, Bender N. Pharmacokinetics, metabolism and biliary and urinary excretion of oral ramipril in man. Curr Med Res Opin. 1995;13(5):264–273. doi: 10.1185/03007999509111551. [DOI] [PubMed] [Google Scholar]
- 55.Jackson DV, Jr, Castle MC, Bender RA. Biliary excretion of vincristine. Clin Pharmacol Ther. 1978;24(1):101–107. doi: 10.1002/cpt1978241101. [DOI] [PubMed] [Google Scholar]
- 56.Pattinson NR, Willis KE, Frampton CM. Comparative analysis of cholesterol transport in bile from patients with and without cholesterol gallstones. J Lipid Res. 1991;32(2):205–214. [PubMed] [Google Scholar]
- 57.Lecureur V, Courtois A, Payen L, Verhnet L, Guillouzo A, Fardel O. Expression and regulation of hepatic drug and bile acid transporters. Toxicology. 2000;153(1–3):203–219. doi: 10.1016/s0300-483x(00)00315-2. [DOI] [PubMed] [Google Scholar]
- 58.Keitel V, Nies AT, Brom M, Hummel-Eisenbeiss J, Spring H, Keppler D. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2) Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G165–G174. doi: 10.1152/ajpgi.00362.2002. [DOI] [PubMed] [Google Scholar]
- 59.Trauner M, Wagner M, Fickert P, Zollner G. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J Clin Gastroenterol. 2005;39(4 Suppl 2):S111–124. doi: 10.1097/01.mcg.0000155551.37266.26. [DOI] [PubMed] [Google Scholar]
- 60.Shoda J, Kano M, Oda K, Kamiya J, Nimura Y, Suzuki H, Sugiyama Y, Miyazaki H, Todoroki T, Stengelin S, Kramer W, Matsuzaki Y, Tanaka N. The expression levels of plasma membrane transporters in the cholestatic liver of patients undergoing biliary drainage and their association with the impairment of biliary secretory function. Am J Gastroenterol. 2001;96(12):3368–3378. doi: 10.1111/j.1572-0241.2001.05339.x. [DOI] [PubMed] [Google Scholar]
- 61.Pratt DS. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2005;21(3):270–274. doi: 10.1097/01.mog.0000159819.01403.39. [DOI] [PubMed] [Google Scholar]
- 62.Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol. 2005;39(4 Suppl 2):S103–110. doi: 10.1097/01.mcg.0000155550.29643.7b. [DOI] [PubMed] [Google Scholar]
- 63.Cook CS, Berry LM, Bible RH, Hribar JD, Hajdu E, Liu NW. Pharmacokinetics and metabolism of [14C]eplerenone after oral administration to humans. Drug Metab Dispos. 2003;31(11):1448–1455. doi: 10.1124/dmd.31.11.1448. [DOI] [PubMed] [Google Scholar]
- 64.Tse FL, Jaffe JM, Troendle A. Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J Clin Pharmacol. 1992;32(7):630–638. doi: 10.1002/j.1552-4604.1992.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 65.Anggard E, Gunne LM, Homstrand J, McMahon RE, Sandberg CG, Sullivan HR. Disposition of methadone in methadone maintenance. Clin Pharmacol Ther. 1975;17(3):258–266. doi: 10.1002/cpt1975173258. [DOI] [PubMed] [Google Scholar]
- 66.Karim A, Zagarella J, Hribar J, Dooley M. Spironolactone. I. Disposition and metabolism. Clin Pharmacol Ther. 1976;19(2):158–169. doi: 10.1002/cpt1976192158. [DOI] [PubMed] [Google Scholar]
- 67.Slatter JG, Schaaf LJ, Sams JP, Feenstra KL, Johnson MG, Bombardt PA, Cathcart KS, Verburg MT, Pearson LK, Compton LD, Miller LL, Baker DS, Pesheck CV, Lord RS., 3rd Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patients. Drug Metab Dispos. 2000;28(4):423–433. [PubMed] [Google Scholar]
- 68.Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822–2835. doi: 10.1016/s0149-2918(03)80336-3. [DOI] [PubMed] [Google Scholar]
- 69.Moller A, Iwasaki K, Kawamura A, Teramura Y, Shiraga T, Hata T, Schafer A, Undre NA. The disposition of 14C-labeled tacrolimus after intravenous and oral administration in healthy human subjects. Drug Metab Dispos. 1999;27(6):633–636. [PubMed] [Google Scholar]
- 70.Maudgal DP, Lanzini A, Northfield TC, Bridges C, Joseph AE. Quantification of temocillin biliary excretion and gallbladder bile concentration in healthy subjects. Drugs. 1985;29(Suppl 5):146–150. doi: 10.2165/00003495-198500295-00030. [DOI] [PubMed] [Google Scholar]
- 71.Gundert-Remy U, Frohnapfel F, Jourdan W, Weber E, Stiehl A. Estimation of biliary excretion of ureidopenicillins in healthy volunteers using marker dilution technique. Br J Clin Pharmacol. 1982;13(6):795–801. doi: 10.1111/j.1365-2125.1982.tb01868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janowitz P, Swobodnik W, Wechsler JG, Zöller A, Kuhn K, Ditschuneit H. Comparison of gall bladder bile and endoscopically obtained duodenal bile. Gut. 1990;31(12):1407–1410. doi: 10.1136/gut.31.12.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Northfield TC, Hofmann AF. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut. 1975;16(1):1–11. doi: 10.1136/gut.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryde M, Gustavsson S. Biliary excretion of olsalazine sodium in humans. Eur J Drug Metab Pharmacokinet. 1987;12(1):17–24. doi: 10.1007/BF03189857. [DOI] [PubMed] [Google Scholar]
- 75.Lanzini A, Pigozzi MG, Wuhrer A, Facchinetti D, Castellano M, Bettini L, Guerra UP, Beschi M, Muiesan G. Quantitative measurement of biliary excretion and of gall bladder concentration of drugs under physiological conditions in man. Gut. 1989;30(1):104–109. doi: 10.1136/gut.30.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caldwell JH, Cline CT. Biliary excretion of digoxin in man. Clin Pharmacol Ther. 1976;19(4):410–415. doi: 10.1002/cpt1976194410. [DOI] [PubMed] [Google Scholar]
- 77.von Richter O, Greiner B, Fromm MF, Fraser R, Omari T, Barclay ML, Dent J, Somogyi AA, Eichelbaum M. Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther. 2001;70(3):217–227. doi: 10.1067/mcp.2001.117937. [DOI] [PubMed] [Google Scholar]
- 78.Drescher S, Glaeser H, Murdter T, Hitzl M, Eichelbaum M, Fromm MF. P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther. 2003;73(3):223–231. doi: 10.1067/mcp.2003.27. [DOI] [PubMed] [Google Scholar]
- 79.Glaeser H, Drescher S, Hofmann U, Heinkele G, Somogyi AA, Eichelbaum M, Fromm MF. Impact of concentration and rate of intraluminal drug delivery on absorption and gut wall metabolism of verapamil in humans. Clin Pharmacol Ther. 2004;76(3):230–238. doi: 10.1016/j.clpt.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernas H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin Pharmacol Ther. 2003;74(5):423–436. doi: 10.1016/S0009-9236(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 81.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31(6):805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 82.Takamatsu N, Kim ON, Welage LS, Idkaidek NM, Hayashi Y, Barnett J, Yamamoto R, Lipka E, Lennernas H, Hussain A, Lesko L, Amidon GL. Human jejunal permeability of two polar drugs: cimetidine and ranitidine. Pharm Res. 2001;18(6):742–744. doi: 10.1023/a:1011020025338. [DOI] [PubMed] [Google Scholar]
- 83.Ghibellini G, Johnson BM, Kowalsky RJ, Heizer WD, Brouwer KL. A novel method for the determination of biliary clearance in humans. AAPS J. 2004;6(4):e33. doi: 10.1208/aapsj060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghibellini G, Vasist LS, Hill TE, Bridges AS, Heizer WD, Brouwer KL. Determination of Piperacillin Biliary Clearance in Humans and Identification of Its Metabolites in Bile and Urine. AAPS J. 2005;7(S2):M1293. [Google Scholar]
- 85.Reynolds KS, Song MH, Heizer WD, Burns CB, Sica DA, Brouwer KL. Effect of pancreatico-biliary secretions and GI transit time on the absorption and pharmacokinetic profile of ranitidine in humans. Pharm Res. 1998;15(8):1281–1285. doi: 10.1023/a:1011908412058. [DOI] [PubMed] [Google Scholar]
- 86.Colburn WA, Vane FM, Bugge CJ, Carter DE, Bressler R, Ehmann CW. Pharmacokinetics of 14C-isotretinoin in healthy volunteers and volunteers with biliary T-tube drainage. Drug Metab Dispos. 1985;13(3):327–332. [PubMed] [Google Scholar]
- 87.Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther. 2001;23(6):871–885. doi: 10.1016/s0149-2918(01)80075-8. [DOI] [PubMed] [Google Scholar]
- 88.Hoglund P, Ohlin M. Effect modelling for drugs undergoing enterohepatic circulation. Eur J Drug Metab Pharmacokinet. 1993;18(4):333–338. doi: 10.1007/BF03190182. [DOI] [PubMed] [Google Scholar]
- 89.Herman RJ, Van Pham JD, Szakacs CB. Disposition of lorazepam in human beings: enterohepatic recirculation and first-pass effect. Clin Pharmacol Ther. 1989;46(1):18–25. doi: 10.1038/clpt.1989.101. [DOI] [PubMed] [Google Scholar]
- 90.LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. Pharm Biotechnol. 1996;8:121–159. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- 91.Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KL. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm Res. 2004;21(7):1294–1302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- 92.Hamilton GA, Jolley SL, Gilbert D, Coon DJ, Barros S, LeCluyse EL. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306(1):85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- 93.Annaert PP, Turncliff RZ, Booth CL, Thakker DR, Brouwer KL. P-glycoprotein-mediated in vitro biliary excretion in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2001;29(10):1277–1283. [PubMed] [Google Scholar]
- 94.Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KL. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 1999;289(3):1592–1599. [PubMed] [Google Scholar]
- 95.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3(2):174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 96.Turncliff RZ, Meier PJ, Brouwer KL. Effect of dexamethasone treatment on the expression and function of transport proteins in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2004;32(8):834–839. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999;277(1 Pt 1):G12–21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- 98.Liu X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15(10):1533–1539. doi: 10.1023/a:1011994831139. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KL. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999;27(6):637–644. [PubMed] [Google Scholar]
- 100.Chandra P, Lecluyse EL, Brouwer KL. Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. In Vitro Cell Dev Biol Anim. 2001;37(6):380–385. doi: 10.1007/BF02577575. [DOI] [PubMed] [Google Scholar]
- 101.Kostrubsky VE, Strom SC, Hanson J, Urda E, Rose K, Burliegh J, Zocharski P, Cai H, Sinclair JF, Sahi J. Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci. 2003;76(1):220–228. doi: 10.1093/toxsci/kfg217. [DOI] [PubMed] [Google Scholar]
- 102.McGinnity DF, Soars MG, Urbanowicz RA, Riley RJ. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos. 2004;32(11):1247–1253. doi: 10.1124/dmd.104.000026. [DOI] [PubMed] [Google Scholar]
- 103.Ito K, Houston JB. Comparison of the use of liver models for predicting drug clearance using in vitro kinetic data from hepatic microsomes and isolated hepatocytes. Pharm Res. 2004;21(5):785–792. doi: 10.1023/b:pham.0000026429.12114.7d. [DOI] [PubMed] [Google Scholar]
- 104.Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KL. Multiple transport systems mediate the hepatic uptake and biliary excretion of the metabolically stable opioid peptide [D-penicillamine2,5]enkephalin. Drug Metab Dispos. 2005;33(2):287–293. doi: 10.1124/dmd.104.001420. [DOI] [PubMed] [Google Scholar]
- 105.Kemp DC, Zamek-Gliszczynski MJ, Brouwer KL. Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol Sci. 2005;83(2):207–214. doi: 10.1093/toxsci/kfi020. [DOI] [PubMed] [Google Scholar]
- 106.Blanchard N, Alexandre E, Abadie C, Lave T, Heyd B, Mantion G, Jaeck D, Richert L, Coassolo P. Comparison of clearance predictions using primary cultures and suspensions of human hepatocytes. Xenobiotica. 2005;35(1):1–15. doi: 10.1080/00498250400021820. [DOI] [PubMed] [Google Scholar]
- 107.Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol. 2001;60(5):934–943. doi: 10.1124/mol.60.5.934. [DOI] [PubMed] [Google Scholar]
- 108.Sasaki M, Suzuki H, Aoki J, Ito K, Meier PJ, Sugiyama Y. Prediction of in vivo biliary clearance from the in vitro transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II monolayer expressing both rat organic anion transporting polypeptide 4 and multidrug resistance associated protein 2. Mol Pharmacol. 2004;66(3):450–459. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 109.Matsushima S, Maeda K, Kondo C, Hirano M, Sasaki M, Suzuki H, Sugiyama Y. Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein. J Pharmacol Exp Ther. 2005;314(3):1059–1067. doi: 10.1124/jpet.105.085589. [DOI] [PubMed] [Google Scholar]
- 110.Kopplow K, Letschert K, Konig J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol. 2005;68(4):1031–1038. doi: 10.1124/mol.105.014605. [DOI] [PubMed] [Google Scholar]
- 111.Pritchard JB, Miller DS. Expression systems for cloned xenobiotic transporters. Toxicol Appl Pharmacol. 2005;204(3):256–262. doi: 10.1016/j.taap.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 112.Urban TJ, Sebro R, Hurowitz EH, Leabman MK, Badagnani I, Lagpacan LL, Risch N, Giacomini KM. Functional genomics of membrane transporters in human populations. Genome Res. 2005 doi: 10.1101/gr.4356206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280(10):9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- 114.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005;315(3):1288–1297. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- 115.Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33(3):434–439. doi: 10.1124/dmd.104.001909. [DOI] [PubMed] [Google Scholar]
- 116.Zamek-Gliszczynski MJ, Brouwer KRL. In: Pharmaceutical profiling in drug discovery for lead selection. Borchardt RT, Kerns EH, Lipinski CA, Thakker DR, Wang B, editors. AAPS Press; Arlington: 2004. pp. 259–292. [Google Scholar]
- 117.Horikawa M, Kato Y, Tyson CA, Sugiyama Y. Potential cholestatic activity of various therapeutic agents assessed by bile canalicular membrane vesicles isolated from rats and humans. Drug Metab Pharmacokinet. 2003;18(1):16–22. doi: 10.2133/dmpk.18.16. [DOI] [PubMed] [Google Scholar]
- 118.Shilling AD, Azam F, Kao J, Leung L. Use of canalicular membrane vesicles (CMVs) from rats, dogs, monkeys and humans to assess drug transport across the canalicular membrane. J Pharmacol Toxicol Methods. 2005 doi: 10.1016/j.vascn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 119.Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, Ikeda T. Oatp1b1, Oatp1b3 and Mrp2 Are Involved in Hepatobiliary Transport of Olmesartan, a Novel Angiotensin Ii Blocker. Drug Metab Dispos. 2006 doi: 10.1124/dmd.105.008888. [DOI] [PubMed] [Google Scholar]
- 120.Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21(10):1895–1903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 121.Letourneau IJ, Deeley RG, Cole SP. Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1) Pharmacogenet Genomics. 2005;15(9):647–657. doi: 10.1097/01.fpc.0000173484.51807.48. [DOI] [PubMed] [Google Scholar]
- 122.Leslie EM, Bowers RJ, Deeley RG, Cole SP. Structural requirements for functional interaction of glutathione tripeptide analogs with the human multidrug resistance protein 1 (MRP1) J Pharmacol Exp Ther. 2003;304(2):643–653. doi: 10.1124/jpet.102.044073. [DOI] [PubMed] [Google Scholar]
- 123.Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2) J Biol Chem. 2003;278(26):23538–23544. doi: 10.1074/jbc.M303504200. [DOI] [PubMed] [Google Scholar]
- 124.Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277(50):47980–47990. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- 125.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59(5):1171–1180. doi: 10.1124/mol.59.5.1171. [DOI] [PubMed] [Google Scholar]
- 126.Song S, Suzuki H, Kawai R, Sugiyama Y. Effect of PSC 833, a P-glycoprotein modulator, on the disposition of vincristine and digoxin in rats. Drug Metab Dispos. 1999;27(6):689–694. [PubMed] [Google Scholar]
- 127.Watanabe T, Miyauchi S, Sawada Y, Iga T, Hanano M, Inaba M, Sugiyama Y. Kinetic analysis of hepatobiliary transport of vincristine in perfused rat liver. Possible roles of P-glycoprotein in biliary excretion of vincristine. J Hepatol. 1992;16(1–2):77–88. doi: 10.1016/s0168-8278(05)80098-4. [DOI] [PubMed] [Google Scholar]
- 128.Watanabe T, Suzuki H, Sawada Y, Naito M, Tsuruo T, Inaba M, Hanano M, Sugiyama Y. Induction of hepatic P-glycoprotein enhances biliary excretion of vincristine in rats. J Hepatol. 1995;23(4):440–448. doi: 10.1016/0168-8278(95)80203-7. [DOI] [PubMed] [Google Scholar]
- 129.Karachalios G, Charalabopoulos K. Biliary excretion of antimicrobial drugs. Chemotherapy. 2002;48(6):280–297. doi: 10.1159/000069712. [DOI] [PubMed] [Google Scholar]
- 130.Balant L, Dayer P, Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clinical Pharmacokinetics. 1985;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- 131.Brogard JM, Jehl F, Blickle JF, Dorner M, Arnaud JP, Monteil H. Biliary pharmacokinetic profile of piperacillin: experimental data and evaluation in man. Int J Clin Pharmacol Ther Toxicol. 1990;28(11):462–470. [PubMed] [Google Scholar]
- 132.Raber MN, Newman RA, Newman BM, Gaver RC, Schacter LP. Phase I trial and clinical pharmacology of elsamitrucin. Cancer Res. 1992;52(6):1406–1410. [PubMed] [Google Scholar]
- 133.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7(8):2182–2194. [PubMed] [Google Scholar]
- 134.Walle T, Walle UK, Kumar GN, Bhalla KN. Taxol metabolism and disposition in cancer patients. Drug Metab Dispos. 1995;23(4):506–512. [PubMed] [Google Scholar]
- 135.Gidding CE, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29(3):267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 136.Ghibellini G, Vasist LS, Heizer WD, Kowalsky RJ, Brouwer KL. Quantitation of Tc-99m Sestamibi Biliary Excretion in Humans. Clin Pharmacol Ther. 2006;79(2):PI-48. [Google Scholar]
- 137.Jayasinghe KS, Roberts CJ, Read AE. Is biliary excretion of paracetamol significant in man? Br J Clin Pharmacol. 1986;22(3):363–366. doi: 10.1111/j.1365-2125.1986.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467–494. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 139.Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39(6):397–412. doi: 10.2165/00003088-200039060-00002. [DOI] [PubMed] [Google Scholar]
- 140.Molimard M, Diquet B, Benedetti MS. Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. Fundam Clin Pharmacol. 2004;18(4):399–411. doi: 10.1111/j.1472-8206.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 141.Simons FE, Simons KJ. Clinical pharmacology of new histamine H1 receptor antagonists. Clin Pharmacokinet. 1999;36(5):329–352. doi: 10.2165/00003088-199936050-00003. [DOI] [PubMed] [Google Scholar]
- 142.Brookman LJ, Rolan PE, Benjamin IS, Palmer KR, Wyld PJ, Lloyd P, Flesch G, Waldmeier F, Sioufi A, Mullins F. Pharmacokinetics of valsartan in patients with liver disease. Clin Pharmacol Ther. 1997;62(3):272–278. doi: 10.1016/S0009-9236(97)90029-1. [DOI] [PubMed] [Google Scholar]
- 143.Cox PJ, Ryan DA, Hollis FJ, Harris AM, Miller AK, Vousden M, Cowley H. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28(7):772–780. [PubMed] [Google Scholar]
- 144.Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet. 2002;41(6):421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- 145.Wallemacq PE, Verbeeck RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet. 2001;40(4):283–295. doi: 10.2165/00003088-200140040-00004. [DOI] [PubMed] [Google Scholar]
- 146.Hellstern A, Hellenbrecht D, Saller R, Gatzen M, Achtert G, Brockmann P, Hausleiter HJ. Minimal biliary excretion and enterohepatic recirculation of metoclopramide in patients with extrahepatic cholestasis. Eur J Clin Pharmacol. 1993;45(5):415–418. doi: 10.1007/BF00315511. [DOI] [PubMed] [Google Scholar]
- 147.Hedman A, Angelin B, Arvidsson A, Dahlqvist R. No effect of probenecid on the renal and biliary clearances of digoxin in man. Br J Clin Pharmacol. 1991;32(1):63–67. doi: 10.1111/j.1365-2125.1991.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 149.Hucker HB, Stauffer SC, Balletto AJ, White SD, Zacchei AG, Arison BH. Physiological disposition and metabolism of cyclobenzaprine in the rat, dog, rhesus monkey, and man. Drug Metab Dispos. 1978;6(6):659–672. [PubMed] [Google Scholar]
- 150.Daneshmend TK, Warnock DW. Clinical pharmacokinetics of ketoconazole. Clin Pharmacokinet. 1988;14(1):13–34. doi: 10.2165/00003088-198814010-00002. [DOI] [PubMed] [Google Scholar]
- 151.Verebely K, Volavka J, Mule S, Resnick R. Methadone in man: pharmacokinetic and excretion studies in acute and chronic treatment. Clin Pharmacol Ther. 1975;18(2):180–190. doi: 10.1002/cpt1975182180. [DOI] [PubMed] [Google Scholar]
- 152.Balani SK, Xu X, Pratha V, Koss MA, Amin RD, Dufresne C, Miller RR, Arison BH, Doss GA, Chiba M, Freeman A, Holland SD, Schwartz JI, Lasseter KC, Gertz BJ, Isenberg JI, Rogers JD, Lin JH, Baillie TA. Metabolic profiles of montelukast sodium (Singulair), a potent cysteinyl leukotriene1 receptor antagonist, in human plasma and bile. Drug Metab Dispos. 1997;25(11):1282–1287. [PubMed] [Google Scholar]
- 153.Kumar GN, Jayanti VK, Johnson MK, Uchic J, Thomas S, Lee RD, Grabowski BA, Sham HL, Kempf DJ, Denissen JF, Marsh KC, Sun E, Roberts SA. Metabolism and disposition of the HIV-1 protease inhibitor lopinavir (ABT-378) given in combination with ritonavir in rats, dogs, and humans. Pharm Res. 2004;21(9):1622–1630. doi: 10.1023/b:pham.0000041457.64638.8d. [DOI] [PubMed] [Google Scholar]
- 154.Denissen JF, Grabowski BA, Johnson MK, Buko AM, Kempf DJ, Thomas SB, Surber BW. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab Dispos. 1997;25(4):489–501. [PubMed] [Google Scholar]
- 155.Arvidsson A, Alvan G, Angelin B, Borga O, Nord CE. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother. 1982;10(3):207–215. doi: 10.1093/jac/10.3.207. [DOI] [PubMed] [Google Scholar]
- 156.Bearden DT. Clinical pharmacokinetics of quinupristin/dalfopristin. Clin Pharmacokinet. 2004;43(4):239–252. doi: 10.2165/00003088-200443040-00003. [DOI] [PubMed] [Google Scholar]
- 157.Vincent J, Teng R, Dalvie DK, Friedman HL. Pharmacokinetics and metabolism of single oral doses of trovafloxacin. Am J Surg. 1998;176(6A Suppl):8S–13S. doi: 10.1016/s0002-9610(98)00213-x. [DOI] [PubMed] [Google Scholar]