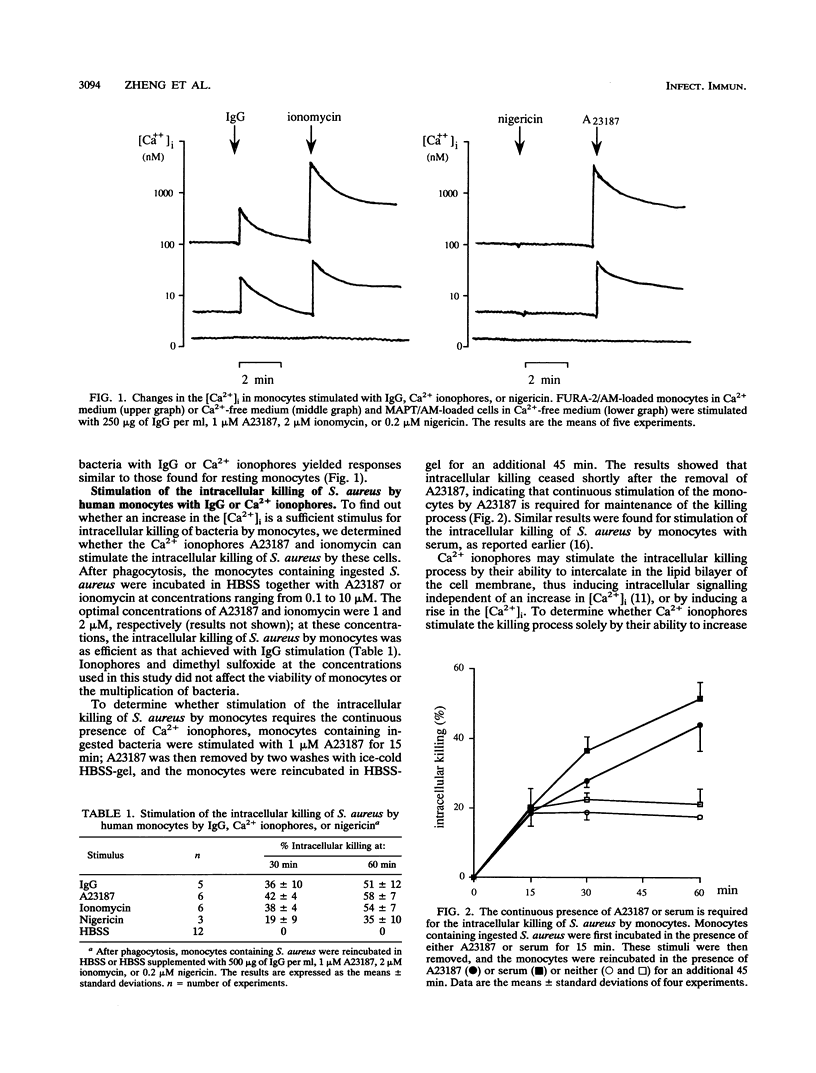
Abstract
Earlier studies have shown that the intracellular killing of Staphylococcus aureus by human monocytes requires continuous stimulation by serum factors, e.g., immunoglobulin G (IgG). In the present study, we demonstrate that IgG, at concentrations that stimulate the intracellular killing of S. aureus, induces a transient increase in the intracellular free calcium concentration ([Ca2+]i) in monocytes. The Ca2+ ionophores A23187 and ionomycin stimulate the killing process as efficiently as IgG does and initiate O2- production in resting monocytes but not in monocytes containing bacteria. The Ca2+ ionophore-stimulated killing process was markedly inhibited by the NADPH oxidase inhibitor diphenyleneiodonium bisulfate, which indicates that these ionophores stimulate oxygen-dependent bactericidal mechanisms. Reduction of the [Ca2+]i to values below 1 nM, obtained by loading monocytes with MAPT/AM (1,2-bis-5-methyl-aminophenoxylethane-N,N,N',N'-tetraacetoxymet hyl acetate) in the absence of extracellular Ca2+, rendered the cells unresponsive to IgG or Ca2+ ionophore stimulation of the intracellular killing of S. aureus, but the response could be restored by reincubating these cells in the presence of extracellular Ca2+. It is concluded that cytosolic free Ca2+ is essential for the IgG-stimulated intracellular killing of S. aureus by human monocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Macrophage activation for intracellular killing as induced by calcium ionophore. Correlation with biologic and biochemical events. J Immunol. 1991 Jan 1;146(1):217–223. [PubMed] [Google Scholar]
- Della Bianca V., Grzeskowiak M., Rossi F. Studies on molecular regulation of phagocytosis and activation of the NADPH oxidase in neutrophils. IgG- and C3b-mediated ingestion and associated respiratory burst independent of phospholipid turnover and Ca2+ transients. J Immunol. 1990 Feb 15;144(4):1411–1417. [PubMed] [Google Scholar]
- Di Virgilio F., Meyer B. C., Greenberg S., Silverstein S. C. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988 Mar;106(3):657–666. doi: 10.1083/jcb.106.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Mayer S. J., Jones O. T. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988 May 1;251(3):887–891. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. H., Pabst M. J., Suzuki H., Guthrie L. A., Forehand J. R., Phillips W. A., Johnston R. B., Jr Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J Biol Chem. 1987 Sep 15;262(26):12589–12596. [PubMed] [Google Scholar]
- Fällman M., Lew D. P., Stendahl O., Andersson T. Receptor-mediated phagocytosis in human neutrophils is associated with increased formation of inositol phosphates and diacylglycerol. Elevation in cytosolic free calcium and formation of inositol phosphates can be dissociated from accumulation of diacylglycerol. J Clin Invest. 1989 Sep;84(3):886–891. doi: 10.1172/JCI114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990 May;110(5):1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels G. C., Gervaix A., Lew P. D., Verhoeven A. J. The chymotrypsin inhibitor carbobenzyloxy-leucine-tyrosine-chloromethylketone interferes with phospholipase D activation induced by formyl-methionyl-leucyl-phenylalanine in human neutrophils. J Biol Chem. 1991 Aug 25;266(24):15870–15875. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Haines K. A., Wilkenfeld C., Lundquist K. F., Weissmann G. Activation of the human neutrophil by calcium-mobilizing ligands. II. Correlation of calcium, diacyl glycerol, and phosphatidic acid generation with superoxide anion generation. J Biol Chem. 1988 Aug 15;263(23):11098–11105. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun. 1981 Sep;33(3):714–724. doi: 10.1128/iai.33.3.714-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by monocytes: regulation by immunoglobulin G and complement components C3/C3b and B/Bb. J Immunol. 1982 Jul;129(1):332–337. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. W., Maxfield F. R. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J Cell Biol. 1990 Jan;110(1):43–52. doi: 10.1083/jcb.110.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odin J. A., Edberg J. C., Painter C. J., Kimberly R. P., Unkeless J. C. Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science. 1991 Dec 20;254(5039):1785–1788. doi: 10.1126/science.1837175. [DOI] [PubMed] [Google Scholar]
- Walker B. A., Hagenlocker B. E., Stubbs E. B., Jr, Sandborg R. R., Agranoff B. W., Ward P. A. Signal transduction events and Fc gamma R engagement in human neutrophils stimulated with immune complexes. J Immunol. 1991 Jan 15;146(2):735–741. [PubMed] [Google Scholar]
- Young J. D., Ko S. S., Cohn Z. A. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: role in phagocytosis. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5430–5434. doi: 10.1073/pnas.81.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]



