Abstract
Purpose
To report spontaneous improvement of rod visual function in a patient with melanoma-associated retinopathy (MAR).
Methods
Electroretinography (ERG), and dark-adapted psychophysical thresholds were performed on a male patient with the MAR syndrome, during four visits over a period of almost 7 years.
Results
There was a spontaneous, subjective improvement in night vision and a decrease in the severity of photopsias of the patient’s left eye between the initial and most recent visits. Both the dark-adapted rod-isolated and maximal-flash b-wave ERG amplitude also improved his initial visit to the most recent visit. By comparison, the light-adapted brief-flash and flicker ERG amplitudes were initially within the range of normal in the left eye but showed a subsequent reduction in amplitude. The ERG response of the right eye was only measured at the most recent visit, but gave a dark-adapted response that was similar to that of the left eye on the initial visit, and a light-adapted response that was similar to that of the left eye on the most recent visit. Rod sensitivity was substantially better in the left eye than in the right eye by psychophysical testing, although the right eye did show a noticeable level of improvement in rod thresholds between the initial and most recent visits.
Conclusions
These findings document the potential for spontaneous improvement in electrophysiological and psychophysical rod function, as well as in subjective symptoms, in patients with the MAR syndrome.
Keywords: Electroretinography, melanoma-associated retinopathy, rod sensitivity, spontaneous improvement, visual thresholds
Melanoma-associated retinopathy (MAR) is a paraneoplastic retinal disorder that can occur in patients who have a cutaneous melanoma.1,2 Patients with the MAR syndrome often complain of nyctalopia (night blindness) and photopsias in the form of flickering, shimmering lights in their visual field.1–4 Retinal findings in these patients may include optic nerve pallor and retinal arteriolar attenuation, but generally the fundus has a normal appearance.1,2 The brief-flash electroretinogram (ERG) of the rod system of MAR patients shows a selective reduction in b-wave amplitude with a negative-appearing waveform1–4 similar to that observed in patients with some forms of congenital stationary night blindness.5 MAR is associated with the presence of serum autoantibodies that react to rod bipolar cells,6 which could be the cause of the retinal dysfunction observed in these patients. The brief-flash ERG waveform of the cone system is also abnormal in MAR patients,1,3,4 consistent with a reduced contribution from retinal ON bipolar cells.
There is no known treatment for the loss of visual function in patients with MAR. The use of corticosteroids has not been shown to be effective for measurably improving visual function, although the number of published reports on this topic is limited.2 To our knowledge, there are no previous reports of spontaneous improvement in either clinical symptoms or ERG findings in such patients. We report a notable degree of spontaneous improvement in the rod ERG response of the left eye in a patient with the MAR syndrome, with a corresponding subjective improvement in night vision and a reduction in photopsias. The patient’s right eye showed a small improvement in rod psychophysical thresholds, but the thresholds remained more elevated than in the left eye. By both electrophysiological and psychophysical measures, there was a marked asymmetry in rod system function between the left and right eyes.
Case Report
A 59-year-old man presented in September, 1999, with hazy vision in his right eye that had lasted for 2 to 3 months. Approximately one week later, his left eye also developed hazy vision. He additionally complained of nyctalopia, poor side vision, and constant photopsias. A general review of systems indicated a prior history for a malignant melanoma on his back, diagnosed in August, 1998. Previously obtained MRI, CT, and bone scans were negative for metastatic disease. A lymph node biopsy was positive in August, 1999, and the patient was started on interferon-a in September, 1999. This was subsequently discontinued in September, 2000. As noted in previous reports of this patient,4,7 his serum produced the specific immunolabeling of retinal bipolar cells that is typical of MAR.6
His visual acuity was correctable to 20/15 in both eyes. Ocular media were clear in each eye by slit-lamp examination and ocular pressures were normal. A fundus examination showed a normal-appearing optic disc, fovea, and retinal vessels. Goldmann visual fields showed a mild concentric restriction in each eye.
The following studies were approved by an institutional review board at the University of Illinois at Chicago. Informed consent was obtained from the patient after the nature of the procedures had been explained. The examinations were conducted in accordance with Health Insurance Portability and Accountability Act regulations.
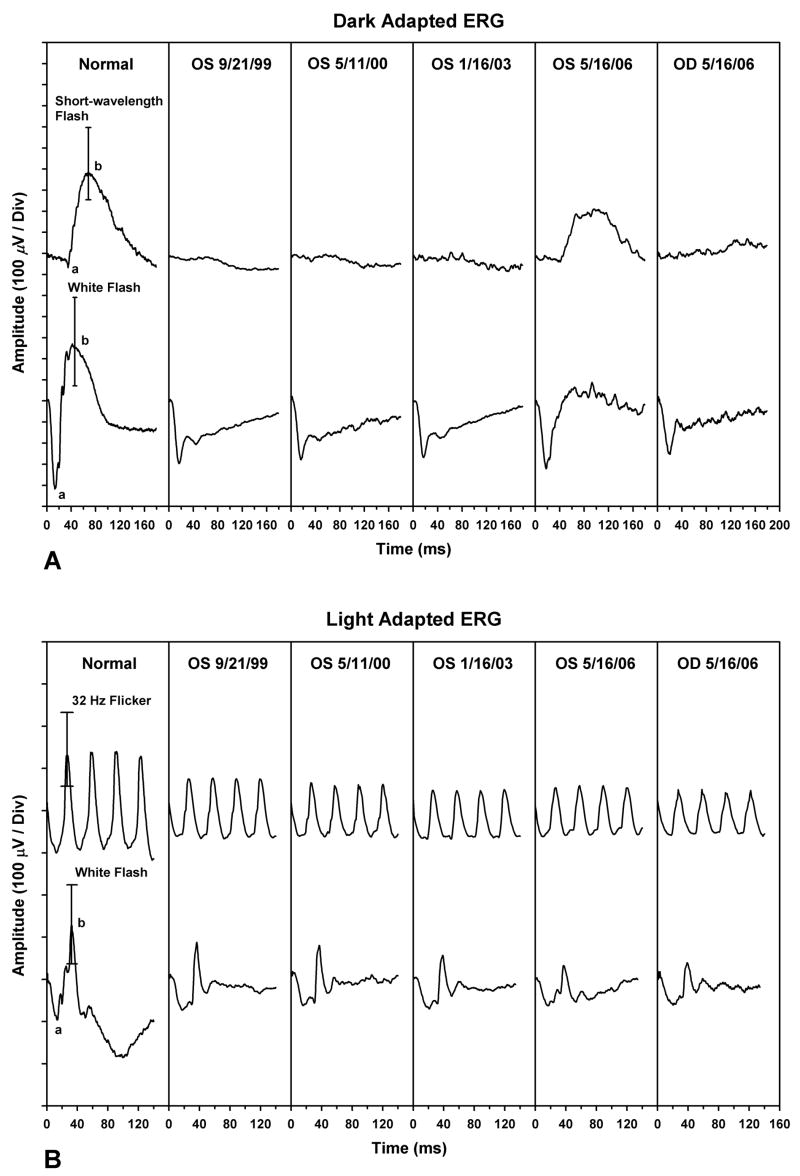
ERGs were performed using a technique described previously.8 ERGs were recorded with a unipolar Burian-Allen contact lens electrode. Stimuli were presented in a Nicolet Ganzfeld (Nicolet Biomedical Inc., Madison, WI) and signals were acquired with a Nicolet Viking IV system. As shown in Figure 1A, the dark-adapted ERG response of the patient’s left eye to a dim, rod-isolating, short-wavelength flash was nearly flat on 9/21/1999, the initial visit. The b-wave amplitude was only 15.6 μV, which was approximately 95% below the lower range of normal (see Table 1 for dark-adapted ERG amplitudes and implicit times). The dark-adapted maximal-flash ERG response of his left eye had a negative waveform, in which the a-wave amplitude was within normal limits, whereas the b-wave amplitude was markedly reduced. The light-adapted flicker ERG amplitude on this date was normal (Table 2). As reported previously,4 his light-adapted brief-flash ERG (Figure 1B) had the characteristic waveform shape seen in MAR patients.
Figure 1.
Results of the full-field electroretinograms (ERGs). (A) Dark-adapted ERG responses to a short-wavelength (blue) flash (top row) and white flash (bottom row) for (left to right): a representative normal subject (error bars indicate the normal range of b-wave amplitude); the patient’s left eye from 9/21/99, 5/11/00, 1/16/03, and 5/16/06; and the patient’s right eye from 5/16/06. (B) Light-adapted ERG responses to 32-Hz white flicker (top row) and a white flash (bottom row) for (left to right): a representative normal subject [error bars indicate the normal range for peak-to-trough amplitude (top) and b-wave amplitude (bottom)]; the patient’s left eye from 9/21/99, 5/11/00, 1/16/03, and 5/16/06 and the patient’s right eye from 5/16/06.
Table 1.
Amplitudes (Amp) and Implicit Times (IT) of the Dark Adapted ERG for the Patient’s Left Eye at Each of the Visits.
| Short-Wavelength Flash b-Wave | White Flash a-Wave | White Flash b-Wave | b/a-Wave Ratio | ||||
|---|---|---|---|---|---|---|---|
| Date | Amp (μV) | IT (ms) | Amp (μV) | IT (ms) | Amp (μV) | IT (ms) | |
| 9/21/1999 | 15.6 | 59.6 | 302.1 | 16.0 | 128.9 | 29.6 | 0.4 |
| 5/11/2000 | 33.3 | 55.6 | 286.5 | 16.0 | 115.9 | 30.4 | 0.4 |
| 1/16/2003 | 28.7 | 61.2 | 256.6 | 16.8 | 114.6 | 32.8 | 0.5 |
| 5/16/2006 | 224.0 | 83.6 | 317.7 | 16.8 | 382.8 | 63.2 | 1.2 |
| Lower Normal | 273.0 | 56.4 | 282.5 | 12.0 | 460.9 | 32.6 | 1.3 |
| Upper Normal | 684.0 | 72.8 | 537.1 | 20.8 | 908.2 | 57.8 | 2.2 |
Table 2.
Amplitudes (Amp) and Implicit Times (IT) of the Light Adapted ERG for the Patient’s Left Eye at Each of the Visits.
| White Flash a-Wave | White Flash b-Wave | 32-Hz White Flicker | b/a-Wave Ratio | ||||
|---|---|---|---|---|---|---|---|
| Date | Amp(μV) | IT(ms) | Amp(μV) | IT(ms) | Amp(μV) | IT(ms) | |
| 9/21/1999 | 76.8 | 17.2 | 160.2 | 36.0 | 139.6 | 29.6 | 2.1 |
| 5/11/2000 | 79.4 | 14.8 | 140.6 | 36.0 | 124.5 | 31.0 | 1.8 |
| 1/16/2003 | 72.9 | 20.4 | 126.3 | 38.0 | 111.8 | 32.0 | 1.7 |
| 5/16/2006 | 66.4 | 15.6 | 100.3 | 36.8 | 116.3 | 31.3 | 1.5 |
| Lower Normal | 58.6 | 11.6 | 132.8 | 30.8 | 130.5 | 22.3 | 1.4 |
| Upper Normal | 137.7 | 16.4 | 320.3 | 39.6 | 353.9 | 31.6 | 3.4 |
The patient returned for follow-up visits on 5/11/2000 and 1/16/2003. The b-wave amplitudes of the dark-adapted ERG responses to the short-wavelength and maximal-flash stimuli were 33.3 μV and 115.9 μV on 5/11/2000 and 28.7 μV and 114.6 μV on 1/16/2003. These amplitudes were reduced approximately 90% and 75%, respectively, below the lower limit of normal (Table 1). The light-adapted brief-flash b-wave amplitudes on 5/11/2000 and 1/16/2003 were both approximately 15% below the amplitude on the initial visit (Table 2). The light-adapted 32-Hz flicker ERG response was subnormal in amplitude on 5/11/2000, whereas both the light-adapted 32-Hz flicker amplitude and the brief-flash b-wave amplitude were subnormal on 1/16/2003 (Table 2).
At the patient’s most recent visit (5/16/2006), he reported that there had been a noticeable improvement in night vision and a decrease in the severity of the photopsias of his left eye since his previous visit (1/16/2003). The b-wave amplitudes of the dark-adapted ERG responses to the short-wavelength and maximal-flash stimuli had increased to values that were now only 15% below the normal range (Table 1). In comparison, the light-adapted brief-flash ERG response of the left eye (Figure 1B) showed a progressive decrease in b-wave amplitude since the initial visit.
Longitudinal ERG data were not available for the patient’s right eye, but on 5/16/2006, the dark-adapted ERG responses from that eye were smaller than those of the left eye and were, in fact, similar to those of the left eye on his initial visit (Figure 1A). In comparison, the light-adapted ERG responses of the right eye were similar to those of the left eye on 5/16/2006, and were considerably smaller than those of the left eye on the initial visit.
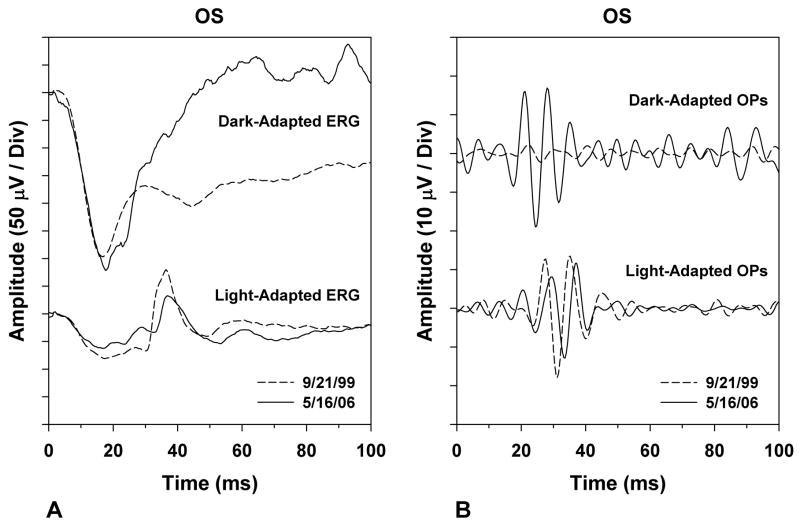
A comparison of the patient’s ERG responses of his left eye on the initial and most recent visits is presented in Figure 2A and a comparison of the corresponding oscillatory potentials (OPs) is shown in Figure 2B. The b-wave of the dark-adapted maximal-flash ERG of the patient with MAR increased substantially in amplitude between the two dates, and the later portion of the a-wave also increased in amplitude (Figure 2A). There was a corresponding increase in the amplitude of the dark-adapted OPs (Figure 2B). In comparison, the light-adapted ERG decreased in overall amplitude between the two dates (Figure 2A), and the light-adapted OPs were reduced in amplitude and delayed in implicit time at the later visit (Figure 2B).
Figure 2.
Comparison of the patient’s electroretinogram (ERG) responses from 9/21/99 (dashed traces) and 5/16/06 (solid traces). (A) Dark-adapted (top) and light-adapted (bottom) ERG responses. (B) Dark-adapted (top) and light-adapted (bottom) oscillatory potentials (OPs), derived by digitally band-pass filtering the corresponding ERG waveforms from 100 to 200 Hz.
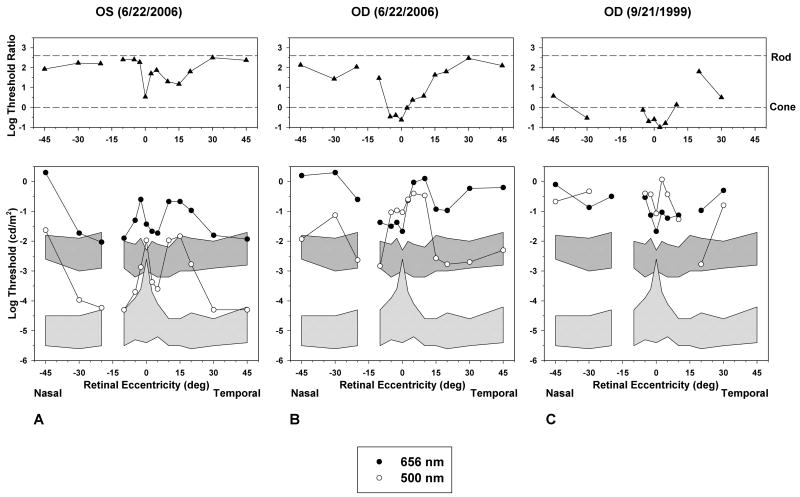
The presence of measurable rod system function in the patient’s left eye was confirmed by dark-adapted threshold testing on 6/22/06, using a procedure described previously.9 Dark-adapted thresholds were measured by means of a Tübinger perimeter (Oculus, Germany) using 500-nm and 656-nm test stimuli with a diameter of 1.7° and a duration of 500 ms. Thresholds were measured at 14 retinal locations, between 45° nasal and 45° temporal from fixation, along the horizontal meridian of the visual field.
The dark-adapted thresholds of the patient’s left eye on 6/22/2006 are shown in Figure 3A. For all measurements, thresholds are plotted in photopic units, so that if thresholds are equal for both wavelengths of test stimulus (log threshold ratio of 0; Figure 3A, top), thresholds are cone-mediated for both test stimuli. A log threshold ratio of approximately 2.5 indicates that thresholds are rod-mediated for both wavelengths of test stimulus. Log threshold ratios between these two values indicate that thresholds are rod-mediated for the 500-nm test flash and cone-mediated for the 656-nm test flash.
Figure 3.
Dark-adapted psychophysical threshold measurements (bottom) using 500-nm (open circles) and 656-nm (filled circles) test stimuli across the horizontal meridian of the visual field, and log threshold ratios (filled triangles) for the two test stimuli (top). The upper and lower shaded regions in the bottom plots represent ±2 standard deviations (SD) from the normal mean for the 656-nm and 500-nm test flashes, respectively. The horizontal dashed lines in the top plots represent the log threshold ratios expected if thresholds were rod-mediated (upper) or cone-mediated (lower) for both test stimuli. Log threshold ratios between the two dashed lines indicate that thresholds were rod-mediated for the 500-nm test flash and cone-mediated for the 656-nm test flash. (A) Threshold measurements from the patient’s left eye on 6/22/2006. (B) Threshold measurements from the patient’s right eye on 6/22/2006. (C) Threshold measurements from the patient’s right eye on 9/21/1999.
As shown in Figure 3A, thresholds for the patient’s left eye on 6/22/2006 were rod-mediated for the 500-nm test flash at peripheral retinal locations, and approached the normal range at some eccentricities, although thresholds were generally elevated. Thresholds for the 500-nm test stimulus were also rod-mediated at peripheral locations in the right eye on 6/22/2006 (Figure 3B), but they were considerably more elevated than in the left eye (Figure 3A). Nevertheless, the dark-adapted thresholds of the patient’s right eye were lower on 6/22/2006 (Figure 3B) than on the patient’s initial visit on 9/21/1999 (Figure 3C). On this initial visit, his dark-adapted thresholds for the 500-nm test stimulus were elevated by approximately 4 log units above the upper limit of normal and were cone-mediated at nearly all locations tested.
Discussion
To our knowledge, this is the first reported case of a patient with the MAR syndrome in which there was spontaneous improvement in rod system function, both subjectively and by the measurement of visual function. Subjectively, the patient reported a marked decrease in the severity of photopsias and an improvement in night vision in the left eye. The patient also showed an increase in the amplitude of the rod ERG response of the left eye. In comparison, the amplitude of the cone ERG response of the left eye became reduced over time. The rod psychophysical thresholds of the left eye were near-normal at a number of retinal loci at the most recent visit. The dark-adapted ERG responses of the patient’s right eye at the most recent visit were similar to those of the left eye at the initial visit, indicating that there was likely to have been little improvement in ERG rod system function in the right eye. Nevertheless, by psychophysical threshold testing, there was a noticeable overall improvement in rod sensitivity in the right eye between the initial and most recent visits, although there was a marked asymmetry between the rod thresholds of the right and left eyes.
Jacobzone and colleagues reported an improvement in the dark-adapted ERG response in one female patient with a presumed case of MAR.9 However, the published ERG waveform was not characteristic of MAR, and it is not apparent whether the authors used a high-intensity stimulus to evaluate a-wave as well as b-wave amplitudes. Furthermore, their patient was diagnosed with bilateral posterior uveitis and hyalitis, and treated with systemic corticosteroids. Subsequently, the uveitis completely cleared in the right eye, with improved ERG findings, and there was a decrease in vitreous cells in the left eye. Their patient also noted a subjective improvement in vision following the corticosteroid therapy. It is unclear as to whether the improvement in the ERG findings in the right eye was due to the resolved uveitis. Furthermore, it has been shown that the use of oral prednisone alone can result in increased ERG amplitudes.10
It is not apparent why our patient with MAR showed spontaneous improvement in his rod visual function, both subjectively and by the measurement of the ERG and absolute thresholds. It is also not clear why the improvement occurred asymmetrically between the two eyes. Nevertheless, the increase in rod system function suggests that other such patients have the potential to improve their rod visual function, even after a period of several years, if safe and effective treatments for the MAR syndrome were to become available.
Acknowledgments
This study was supported by The Foundation Fighting Blindness, Owings Mills, Maryland; The Grant Healthcare Foundation, Chicago, Illinois; NIH research grant EY08301 and NIH core grant EY01792, Bethesda, Maryland; and an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, New York.
Footnotes
The authors have no proprietary interest in this work.
References
- 1.Berson EL, Lessell S. Parneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106:307–311. doi: 10.1016/0002-9394(88)90366-2. [DOI] [PubMed] [Google Scholar]
- 2.Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21:173–187. doi: 10.1097/00041327-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alexander KR, Fishman GA, Peachey NS, Marchese AL, Tso MOM. On” response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci. 1992;33:477–483. [PubMed] [Google Scholar]
- 4.Alexander KR, Barnes CS, Fishman GA, Milam AH. Nature of the cone ON-pathway dysfunction in melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1189–1197. [PubMed] [Google Scholar]
- 5.Miyake Y, Yagasaki K, Horiguchi M, et al. Congenital stationary night blindness with negative electroretinogram: a new classification. Arch Ophthalmol. 1986;104:1013–1020. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- 6.Milam AH, Saari JC, Jacobson SG, et al. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:91–100. [PubMed] [Google Scholar]
- 7.Alexander KR, Barnes CS, Fishman GA, Pokorny J, Smith VC. Contrast-processing deficits in melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 2004;45:305–310. doi: 10.1167/iovs.03-0840. [DOI] [PubMed] [Google Scholar]
- 8.Peachey NS, Fishman GA, Derlacki DJ, Alexander KR. Rod and cone dysfunction in carriers of x-linked retinitis pigmentosa. Ophthalmology. 1988;95:677–685. doi: 10.1016/s0161-6420(88)33128-3. [DOI] [PubMed] [Google Scholar]
- 9.Jacobzone C, Cochard-Marianowski C, Kupfer I, et al. Corticosteroid treatment for melanoma-associated retinopathy. Effect on visual acuity and electrophysiologic findings. Arch Dermatol. 2004;140:1258–1261. doi: 10.1001/archderm.140.10.1258. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman TJ, Dawson WW, Fitzgerald CR. Part I: Electroretinographic changes in normal eyes during administration of prednisone. Ann Ophthalmol. 1973;7:757–765. [PubMed] [Google Scholar]