Abstract
Objective
To determine the roles of nitric oxide synthase (NOS) and NADPH oxidase in the impairment of capillary blood flow in sepsis and in the reversal of this impairment by ascorbate.
Design
Prospective, controlled laboratory study.
Setting
Animal laboratory in research institute.
Subjects
Adult male wild type (WT), nNOS−/−, iNOS−/−, eNOS−/−, and gp91phox−/−mice.
Interventions
Sepsis was induced by feces injection into peritoneum (FIP). A bolus of ascorbate or NADPH oxidase inhibitor apocynin was injected intravenously at 6 hrs post-FIP. Alternatively, NOS cofactor (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4) or nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP) was superfused on the surface of extensor digitorum longus muscle.
Measurements and Main Results
Capillary blood flow impairment and NOS activity in extensor digitorum longus muscle were measured by intravital microscopy and by enzymatic assay, respectively. Sepsis at 6 hrs impaired flow in WT mice. Apocynin, and knockout of gp91phox but not of any NOS isoforms, rescued this impairment. Constitutive NOS activity was unaffected by sepsis, but it was abolished by nNOS knockout (iNOS activity was negligible in all mice). Ascorbate rapidly (10 mins) rescued impaired flow in WT, nNOS−/−, iNOS−/− but not eNOS−/− mice. Ascorbate also improved survival of WT mice after FIP. BH4 and SNAP rescued flow in WT mice, while BH4 failed to rescue it in eNOS−/− mice.
Conclusion
Capillary blood flow impairment in septic skeletal muscle requires NADPH oxidase but not NOS, and it is rapidly reversed by ascorbate and BH4 through an eNOS-dependent mechanism.
Keywords: sepsis, microcirculation, nitric oxide synthase, NADPH oxidase, ascorbate, tetrahydrobiopterin, skeletal muscle, blood pressure
Sepsis is the development of a systemic inflammatory response in the presence of infection. The response is characterized by several circulatory disorders, including impaired red blood cell flow in the microcirculation (1, 2). Despite fluid resuscitation, adequate arterial blood oxygenation and cardiac output in septic patients, this impairment is evidenced by the decreased density of perfused capillaries and increased proportion of non-perfused capillaries (3). The impairment leads to tissue hypoxia because the diffusion distance for oxygen increases (4). Tissue hypoxia may explain why microvascular dysfunction is a strong predictor of death and why one-third of severe sepsis patients die of organ failure even when shock is prevented (3, 5). Several studies have examined septic impairment of capillary blood flow without identifying definitively the principal underlying mechanism (2, 6–9).
Increased production of nitric oxide (NO) by nitric oxide synthase (NOS) has been implicated in sepsis-induced impairment of microvascular responsiveness (10–13) and capillary blood flow (9). However, pharmacologic studies of the roles of NOS isoforms have been confounded by the relative lack of specificity of the NOS inhibitors employed (14). Inducible NOS (iNOS) protein expression is up-regulated transiently during early stages of sepsis (15, 16) while, at later stages, neuronal NOS (nNOS) or its splice variants may be responsible for impaired microvascular responsiveness (10, 14, 17).
NADPH oxidase is the major source of superoxide in microvascular endothelial cells (18, 19). However, the effect of NADPH oxidase on capillary blood flow in sepsis is unknown. Antioxidants such as ascorbate improve outcome of clinical (20–22) and experimental sepsis (23–26). Ascorbate scavenges reactive oxygen species (ROS), inhibits protein expression of NADPH oxidase enzymes that are potential sources of ROS, and increases levels of NO derived from endothelial NOS (eNOS) (11, 19, 27).
Based on these facts, major objectives of the present study were to determine the roles of NOS and NADPH oxidase in the impairment of capillary blood flow in sepsis, and in the rapid reversal of this impairment by ascorbate. We used genetically modified mice with either isoform-specific NOS gene deletions or NADPH oxidase subunit gp91phox deletion.
METHODS
Animal Preparation
The experimental protocol was approved by the University of Western Ontario Council on Animal Care. Male wild type (WT), nNOS−/−, iNOS−/−, eNOS−/− and gp91phox−/− mice of C57BL/6 background (weight: 18–25 g, age: 1.5– 4 months) were obtained from Jackson Laboratory (Bar Harbor, ME). Sepsis was induced by feces injection into peritoneum (FIP) (28). Feces were collected from the cecum of a donor mouse, mixed with sterile saline at concentration 75 mg/mL, and then stored overnight at 4°C. The next day, the mixture was rewarmed to room temperature for 10 min, and an aliquot (50 mL/kg) was injected intraperitoneally. Next, sterile saline (1 mL) containing the analgesic buprenorphine (4 μg/mL) was injected subcutaneously, beginning immediately after FIP and then at 6 hrs intervals. Control mice were injected intraperitoneally with sterile saline (50 mL/kg), and also subcutaneously with 1 mL of saline and buprenorphine, beginning immediately after the intraperitoneal injection and then at 6 hrs intervals.
At 5.5 or 17.5 hrs post-FIP, mice were anesthetized with ketamine (80 mg/kg) plus xylazine (4 mg/kg) and kept anesthetized with supplemental doses as required, for up to 2 hrs. For intravital video microscopy, as we have described in detail (26), the surface of the right extensor digitorum longus (EDL) muscle was surgically exposed, covered by a glass coverslip, allowed a 30 min stabilization period, and then observed to i) visualize capillaries within a 0.61 × 0.87 mm video-recorded field of view (~0.1 mm depth of field) at the muscle surface, ii) measure the number of capillaries with moving and stationary red blood cells crossing a test line drawn on the video monitor, and iii) convert these counts to the densities of perfused and nonperfused capillaries per mm of test line, CDPER and CDSTAT, respectively. We recorded capillaries in five randomly chosen fields in each muscle and then computed the average CDPER and CDSTAT values per muscle. In several experiments, we also used the video flying spot technique (10) to measure the red blood cell velocity (VRBC) in randomly selected perfused capillaries at the EDL muscle surface. In a subset of mice, the right internal carotid artery was exposed to withdraw a 0.3 mL blood sample for measurement of plasma lactate (Yellow Springs Instruments analyzer) or ascorbate, which was quantified by HPLC-based electrochemical assay (25).
A bolus of ascorbate (10 or 200 mg/kg freshly dissolved in 0.1 mL of sterile saline), or a 0.1 mL bolus of sterile saline, was administered by a penile intravenous injection at 6 hrs post-FIP. In the majority of experiments, the bolus was injected immediately after the EDL muscle capillaries were initially viewed at 6 hrs post-FIP. Because this injection could not be done without altering the position of the mouse, repositioning and viewing of the same capillaries could be done only at 4 –10 mins after the injection. Regarding the choice of ascorbate doses, the higher dose prevented arteriolar hypocontractility in septic mice (11) and the lower dose approximated a dose given to human patients (29). In separate mice, we also injected intravenously a bolus of putative NADPH oxidase inhibitor apocynin (10 mg/kg in 0.1 mL of saline) at 6 hrs post-FIP. Alternatively, the NOS cofactor (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4, 0.1 μmol/L), the NO donor S-nitroso-N-acetylpenicillamine (SNAP, 5 μmol/L), decomposed SNAP (5 μmol/L), or physiologic saline vehicle was applied locally to the muscle surface at 6 hrs post-FIP, by temporarily lifting the coverslip and superfusing the surface with a 0.1 mL bolus. The physiologic saline (in mmol/L, 132 NaCl, 4.7 KCl, 2.2 CaCl2, 1.2 MgSO4, and 20 NaHCO3) was equilibrated with 5% CO2/95% N2 to maintain pH 7.4 and warmed to 33–34°C. Decomposed SNAP was prepared by incubating SNAP in physiologic saline overnight at 37°C (30).
To measure blood pressure, separate mice were anesthetized with ketamine/xylazine and the right internal carotid artery was cannulated with PE-10 tubing. As detailed by us (26), the cannula was tunneled subcutaneously to an incision in the back of the neck and attached to a swivel device, permitting access to water and food after recovery from anesthesia. The cannula was infused continuously with sterile saline (10 mL/h/kg) containing buprenorphine (33.3 μg/h/kg). Mean arterial pressure was measured in awake mice at 0 hr and during the 6–7 hrs post-FIP period.
NOS Activity Assay
EDL muscles were homogenized in five volumes (weight/volume) of homogenizing buffer (Tris-Cl 20 mmol/L, EGTA 1 mmol/L, EDTA 1 mmol/L, DTT 1 mmol/L, PMSF 1 mmol/L, leupeptin 20 μg/mL, and triton X-100 1%) and centrifuged (10,000 rpm for 20 min at 4°C). The centrifugation was repeated on the supernatant. The NOS enzyme activity in muscle homogenates was measured as described previously (31). The assay yielded measurements of the calcium-dependent constitutive NOS activity (i.e., eNOS + nNOS) and the calcium-independent iNOS activity in the presence of excess cofactors. Total protein in lysates was measured using the BioRad DC protein assay.
Statistical Analysis
Data are presented as mean ±SE; n indicates the number of mice. One EDL muscle per mouse was used. Data were analyzed by one- or two-way ANOVA followed by post hoc Student’s t-test with Bonferroni correction for multiple comparisons, unless otherwise stated. The level of significance was p < 0.05.
RESULTS
Model of Sepsis
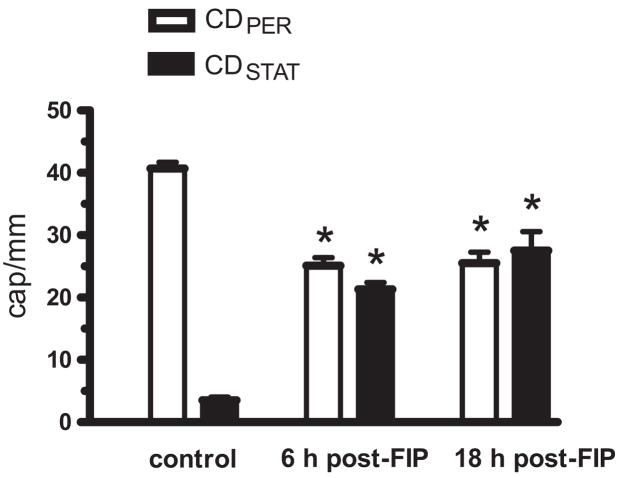
The FIP model of sepsis has been characterized according to several parameters (e.g., plasma cytokines, survival) (28). Consistent with this previous report, we observed in WT mice (n = 31) that survival was 100% at 6 hrs post-FIP, but only 97, 84 and 19% at 12, 18 and 24 hrs post-FIP, respectively. Plasma lactate increased from 0.7 ± 0.3 mmol/L at 0 hr to 2.1 ± 0.4 and to 3.1 ± 1.3 mmol/L at 6 and 18 hrs post-FIP, respectively (n = 5 or 6 mice per group, p < 0.05, Dunn’s multiple comparison test). At 6 hrs after intraperitoneal saline injection in control mice, CDPER and CDSTAT were 40.9 ± 0.9 and 3.5 ± 0.5 cap/mm (n = 4), respectively. Because these CD-PER and CDSTAT values did not differ from those of naive mice (i.e., not exposed to any intervention), the values for saline-injected and naive mice were pooled in the control group of Figure 1. CDPER decreases and CDSTAT increases were observed at both 6 and 18 hrs post-FIP (Fig. 1). Mice at 17.5 hrs post-FIP were difficult to prepare for intravital microscopy, because they could be easily overdosed and unintentionally euthanized with ketamine plus xylazine. Because capillary blood flow impairment was already fully developed at 6 hrs post-FIP, we studied the roles of NOS and NADPH oxidase at this time point.
Figure 1.
Effect of sepsis on capillary blood flow in wild type mouse extensor digitorum longus (EDL) muscle. Densities of capillaries per millimeter of test line, with moving red blood cells (CDPER) and with stationary red blood cells (CD-STAT), were measured in control mice, and in septic mice at 6 hrs and 18 hrs after feces injection into peritoneum (FIP; 3.75 g/kg suspended in 50 mL/kg saline). Sepsis impaired capillary blood flow at 6 hrs and 18 hrs post-FIP. *Significant difference from control (n = 11, 32 and 5 mice in control, 6 hrs and 18 hrs post-FIP groups, respectively; p < 0.05).
Effects of NOS and gp91phox Knockouts on Impaired Capillary Blood Flow in Sepsis
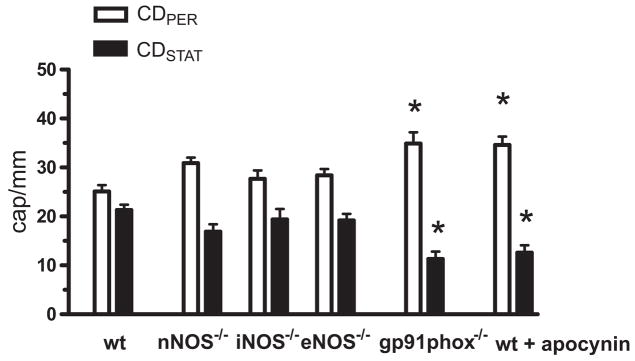
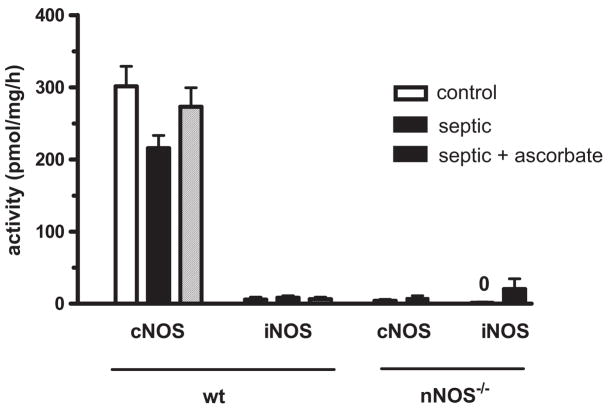
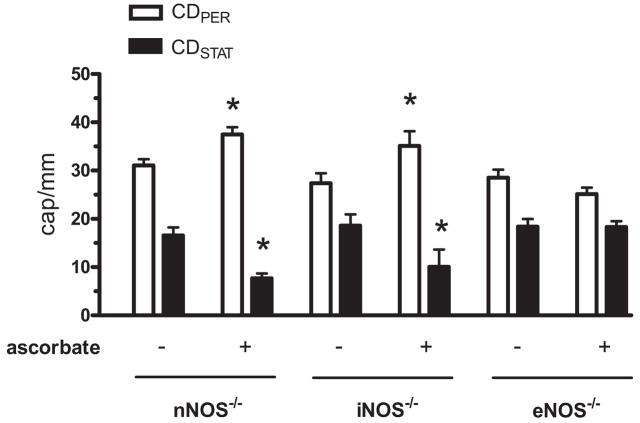
In control saline-injected nNOS−/−, iNOS−/−, eNOS−/− and gp91phox−/− mice, the CDPER and CDSTAT values (data not shown) were comparable with those of WT mice (Fig. 1, control). Regarding CDPER and CDSTAT values at 6 hrs post-FIP, there was no difference between WT and any of the NOS knockouts (Fig. 2). By contrast, genetic deletion of gp91phox resulted in a marked 50% reduction in the density of nonperfused capillaries and a 40% increase in the density of perfused capillaries (Fig. 2). Similar to this effect, the NADPH oxidase inhibitor apocynin improved capillary blood flow in septic WT mice (Fig. 2). Figure 3 shows that the constitutive NOS activity (i.e., eNOS + nNOS) was dominated by nNOS (i.e., genetic deletion of nNOS dramatically reduced constitutive NOS activity), while iNOS activity was negligible, in both control and septic skeletal muscles. Sepsis at 6 hrs post-FIP did not affect constitutive NOS activity (Fig. 3). Together, Figures 2 and 3 indicate that although nNOS may be the dominant source of NO in mouse skeletal muscle, nNOS-derived NO contributes minimally to impaired capillary blood flow in sepsis, while NADPH oxidase-derived ROS play a major role this impairment.
Figure 2.
Effect of knockout of nNOS, iNOS and eNOS and gp91phox (NADPH oxidase subunit) on capillary blood flow in septic EDL muscle at 6 hrs post-FIP. Impairment of capillary blood flow was NOS-independent but gp91phox-dependent. Intravenous bolus injection of the NADPH oxidase inhibitor apocynin (10 mg/kg in 0.1 mL of saline) in wild type mice at 6 h post-FIP restored capillary blood flow at 7 h post-FIP. *Significant difference from wild type (wt) group (n = 37, 15, 13, 19, 6 and 6 mice in wt, nNOS−/−, iNOS−/−, eNOS−/−, gp91phox−/− and wt + apocynin groups, respectively; p < 0.05).
Figure 3.
NOS enzyme activities in EDL muscle in control and septic wild type and nNOS−/− mice, at 6–7 hrs post-FIP. In wild type mice, sepsis, or sepsis plus ascorbate injection at 6 hrs post-FIP, did not alter constitutive cNOS activity (i.e., total of eNOS + nNOS), while iNOS activity was negligible in the EDL muscle of all mice. Genetic deletion of nNOS dramatically reduced cNOS activity in control and septic muscles, indicating the constitutive NOS activity in wild type muscle was dominated by nNOS. n = 4 or 5 muscles from 4 or 5 mice per group.
Ascorbate Restores Capillary Blood Flow and Improves Survival after FIP
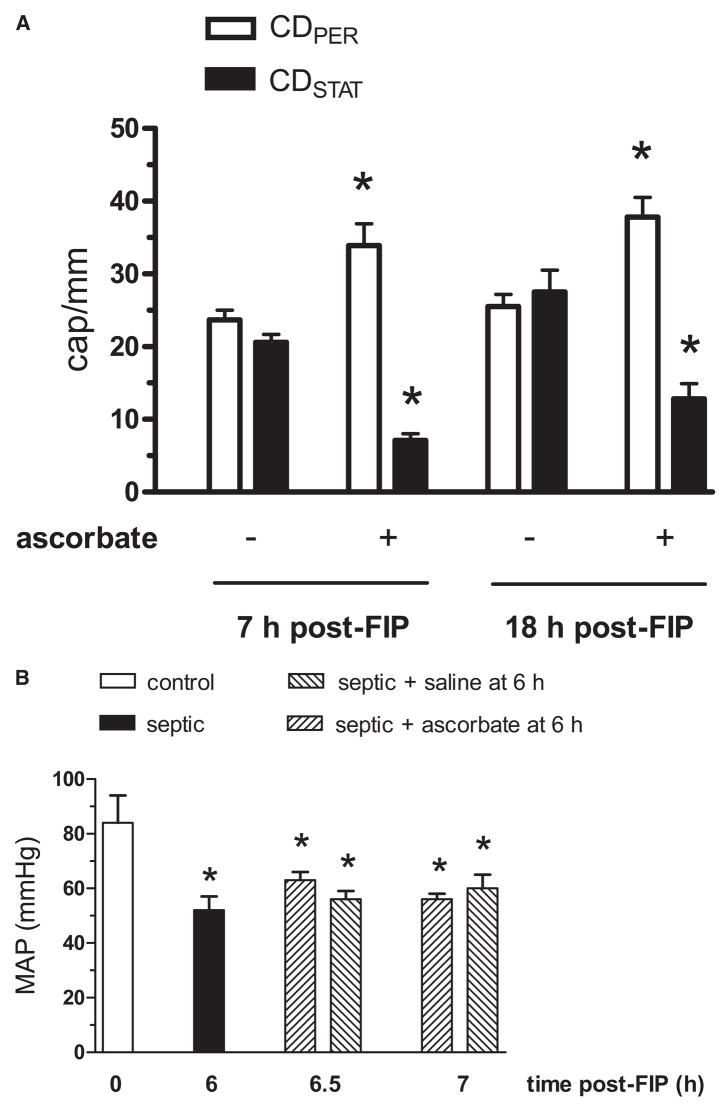
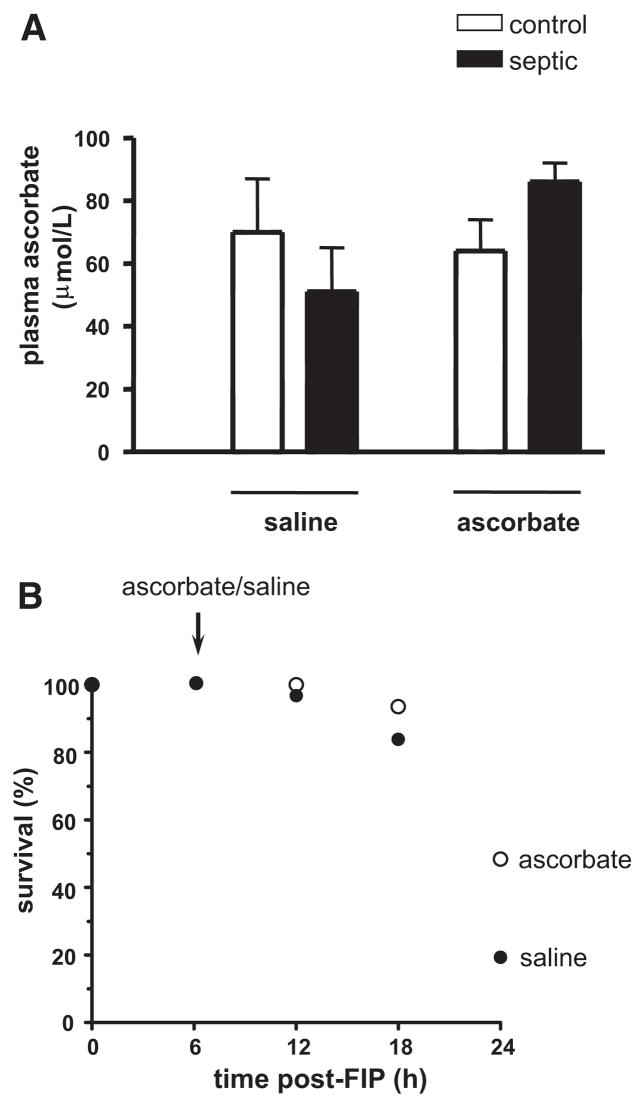
Because of the apparent major role of ROS in the impairment, we carried out experiments addressing the protective role of the antioxidant ascorbate. In the first experiment, we asked if a 10 mg/kg ascorbate bolus injected at 6 hrs post-FIP acutely reverses the capillary blood flow impairment. Remarkably, at 10 min postinjection, we observed a 40% increase in CDPER and a 60% reduction in CDSTAT (CDPER = 32.8 ± 1.9 and CDSTAT = 9.0 ± 3.3 cap/mm, n = 4) when compared with preinjection values. Here, capillaries with stationary RBCs became reperfused with RBCs, which moved at a rate comparable with that in capillaries already perfused, without any apparent hyperemia. Figure 4A shows that the restorative effect of ascorbate persisted when capillary flow was observed at 60 min postinjection (i.e., 7 hrs post-FIP) and again at 18 hrs post-FIP. Hyperemia was not observed at either time point. Consistently, the mean arterial blood pressure remained unchanged during the 60 mins postinjection period (Fig. 4B). Figure 5A shows that the ascorbate bolus at 6 hrs post-FIP did not significantly alter plasma ascorbate concentration measured at 7 hrs post-FIP. Importantly, the ascorbate bolus improved survival of mice at 24 hrs post-FIP from 19 to 50% (Fig. 5B). This finding agrees with the reported increased survival to 65% when ascorbate bolus is given at the onset of sepsis (cecal ligation and puncture model, CLP) (24).
Figure 4.
Effect of ascorbate bolus intravenous injection (10 mg/kg freshly dissolved in 0.1 mL of saline) on capillary blood flow in EDL muscle and on the mean arterial blood pressure (MAP) in septic wild type mice. Panel A: Ascorbate was injected at 6 hrs post-FIP and subsequently CDPER and CDSTAT values were measured 7 hrs and 18 hrs post-FIP. Ascorbate reversed impaired capillary blood flow seen at 7 hrs and 18 hrs post-FIP. *Significant difference from respective nonascorbate group (i.e., mice given intravenous 0.1 mL saline bolus, or no bolus, at 6 hrs post-FIP). n = 32 and 9 mice in nonascorbate and ascorbate groups, respectively, at 7 hrs post-FIP, and 5 and 4 mice in nonascorbate and ascorbate groups at 18 hrs post-FIP; p < 0.05. Panel B: MAP at 0 h (control) and 6 hrs post-FIP (septic) was measured in separate groups of 5 and 10 awake mice, respectively. At 6 hrs, the 10 mice were randomly assigned to two groups of 5, which were injected with a 0.1 mL bolus of either ascorbate (10 mg/kg) or saline vehicle. MAP in these two groups was measured at 6.5 hrs and 7 hrs post-FIP. Sepsis reduced MAP at 6 hrs post-FIP. Neither ascorbate nor saline vehicle altered MAP at 6.5 hrs or 7 hrs post-FIP, compared with 6 hrs post-FIP. *Significant difference from control (p < 0.05).
Figure 5.
Effect of ascorbate bolus (10 mg/kg, given at 6 hrs post-FIP) on plasma ascorbate concentration at 7 hrs post-FIP and on survival at 24 hrs, in wild type mice. Panel A: Control and septic mice were injected iv with saline or ascorbate at 6 hrs, and blood samples were collected at 7 hrs post-FIP. Analysis by two-way ANOVA revealed no significant effect of sepsis or ascorbate on plasma ascorbate concentration at 7 hrs (n = 5 and 6 for saline injected control and septic mice, and 5 and 7 for ascorbate injected control and septic mice, respectively). Panel B: Septic mice were monitored every 6 hrs for 24 hrs. Based on Kaplan-Meier survival analysis, survival of mice given ascorbate at 6 hrs post-FIP (n = 32) was significantly higher (p < 0.05) than that of mice given saline (n = 31).
The next experiment examined the effect of 200 mg/kg ascorbate bolus injected at 6 hrs post-FIP. Here, the CDPER pre- and 60 mins postinjection values were 25.6 ± 1.5 and 37 ± 2.7 cap/mm, respectively, (n = 6, p < 0.05) while the corresponding CDSTAT values were 26.3 ± 1.9 and 14.6 ± 1.8 cap/mm (n = 6, p < 0.05). Further, pre- and 60 mins postinjection VRBC values were 152 ± 7 and 155 ± 9 mm/s, respectively (n = 36 capillaries in 6 mice, p > 0.05), indicating that even this larger dose of ascorbate did not result in hyperemia within the capillary bed. Because of the similarity of the restorative effects of both ascorbate doses, we focused on elucidation of the mechanism of the lower dose (i.e., clinically relevant dose).
The following experiment asked if the restorative effect of ascorbate depends on NOS. Although enzymatic assay showed that ascorbate did not alter septic constitutive NOS and iNOS activities in whole muscle homogenates (Fig. 3), a possible localized effect of ascorbate on NOS activity in cells within the microvasculature (i.e., occupying a small fraction of the skeletal muscle volume) might be undetectable by the assay. It has been shown that i) enhanced adhesion of leukocytes, platelets and RBCs to the endothelium occurs in experimental sepsis (32–34) (i.e., a possible mechanism for impairment of capillary blood flow), and ii) this enhanced adhesion can be attenuated by NO (35, 36). Because ascorbate increases levels of eNOS-derived NO in endothelial cells and platelets (27, 37–39), we reasoned that the restorative effect on capillary flow could be eNOS-dependent. Alternatively, NOS-independence would indicate a possible direct antioxidant effect of ascorbate against NADPH oxidase-derived ROS. Figure 6 demonstrates that the restorative effect was eNOS-dependent, because the lower ascorbate dose reversed septic capillary blood flow impairment in nNOS−/−, iNOS−/−, but not in eNOS−/− mice. The fact that the larger ascorbate dose also failed to reverse this impairment in eNOS−/− mice (data not shown) indicates that ascorbate did not exert other eNOS-independent restorative effects. Thus, the protective effect of ascorbate may be mediated by redox signaling involving eNOS, rather than by direct scavenging of ROS.
Figure 6.
Effect of ascorbate on capillary blood flow in EDL muscle in septic NOS knockout mice. At 6 hrs post-FIP, mice were given saline or ascorbate (10 mg/kg) bolus. Subsequently, CDPER and CDSTAT values were measured at 7 hrs post-FIP. For each NOS knockout genotype, capillary blood flow values for saline-injected and naive mice did not differ and therefore were pooled into one nonascorbate group (labeled as “−”). Ascorbate altered capillary blood flow in nNOS−/− and iNOS−/− but not in eNOS−/− mice. *Significant difference from respective nonascorbate group. n = 13 and 6 mice for nNOS−/− nonascorbate and ascorbate groups, n = 11 and 5 mice for iNOS−/− nonascorbate and ascorbate groups, and n = 14 and 8 mice for eNOS−/− nonascorbate and ascorbate groups, respectively; p < 0.05.
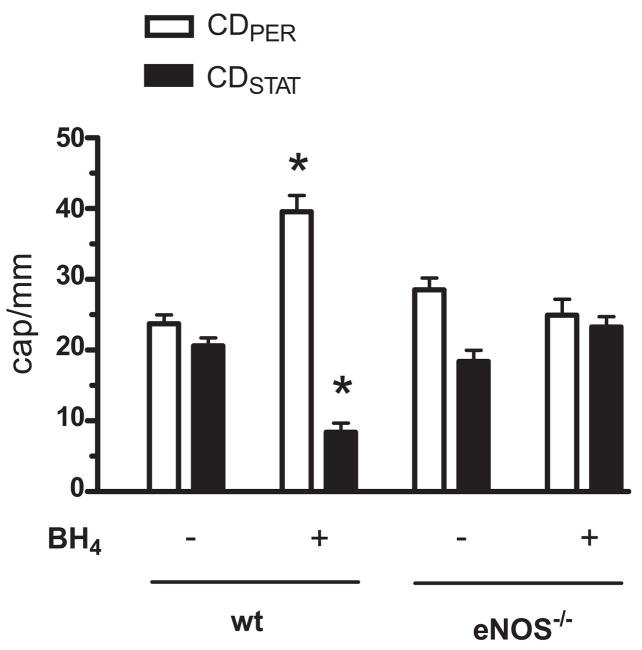
Experiments with endothelial cell cultures have shown that BH4 synthesis is increased by bacterial lipopolysaccharide (40) but BH4 concentration is decreased by ROS (41). Our finding that NADPH oxidase-derived ROS mediate septic impairment of capillary blood flow (Fig. 2) suggested that BH4 may be oxidized during sepsis and that the restorative effect of ascorbate may be achieved through the maintenance of BH4 in the reduced state required for it to function as a cofactor of eNOS (42, 43). We reasoned that if the eNOS-dependent reversal by ascorbate of the impairment is due to an increase in endogenous BH4 concentration, then exogenous supplementation of this cofactor at 6 hrs post-FIP will also restore capillary blood flow in WT but not eNOS−/− mice. In agreement with the reported hemodynamic improvement elicited by BH4 analogue in endotoxin-injected rats (44), we discovered that superfusion of the EDL muscle surface with BH4 during the 6–7 hrs post-FIP period indeed restored capillary flow in WT but not in eNOS−/− mice (Fig. 7). Superfusion with the vehicle had no effect on capillary blood flow in WT mice (data not shown). Thus, the restorative effects of ascorbate and BH4 required the presence of eNOS, indicating that this isoform was involved in the rescue of capillary blood flow in sepsis.
Figure 7.
Effect of BH4 on capillary blood flow in EDL muscle in septic wild type and eNOS knockout mice. Between 6 and 7 hrs post-FIP, the muscle surface was periodically superfused with a 0.1 mL bolus of physiologic saline vehicle or BH4 (0.1 μmol/L) and subsequently CDPER and CD-STAT values were measured at 7 hrs post-FIP. CDPER or CDSTAT in nonsuperfused and vehicle-superfused mice did not differ and therefore were pooled into one control group (labeled as “−”). BH4 altered capillary blood flow in WT but not in eNOS−/− mice. *Significant difference from control (n = 32 and 6 mice for WT control and BH4 groups, and n = 14 and 5 mice for eNOS−/− control and BH4 groups, respectively; p < 0.05).
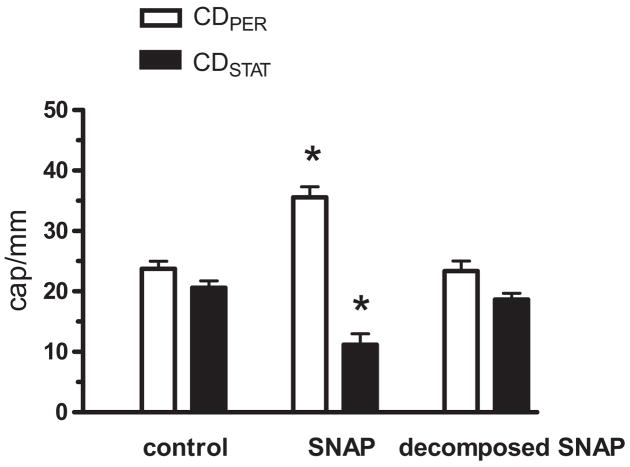
Finally, we tested if exogenous BH4 stimulates eNOS activity to increase NO production, and thus reverses capillary blood flow impairment in sepsis (35, 36). To mimic increased NO production, we locally applied the NO donor SNAP at 6 hrs post-FIP. Figure 8 shows that SNAP restored capillary blood flow while decomposed SNAP had no effect (CDPER and CDSTAT were measured at 15 min post-SNAP; i.e., time when the temporary vasodilatory effect of SNAP had already ended). Thus, despite the persistence of nNOS to produce NO in septic skeletal muscle (Fig. 3), supplemental NO may be required to rescue the capillary blood flow impairment. Experimental sepsis has been associated with a temporal window in which soluble guanylate cyclase is not functional (45). The ability of NO to restore capillary blood flow (Fig. 8) attests to the functionality of soluble guanylate cyclase in the present model of sepsis.
Figure 8.
Effect of exogenous NO donor SNAP on capillary blood flow in EDL muscle in septic wild type mice. At 6 hrs post-FIP, the muscle surface was superfused with a single 0.1 mL bolus of physiologic saline vehicle, SNAP (5 μmol/L) or decomposed SNAP (5 μmol/L), and the CDPER and CDSTAT values were determined 15 min later when the temporary vasodilatory effect of SNAP had already ended. CDPER or CDSTAT values in nonsuperfused and vehicle-superfused mice did not differ and therefore were pooled into one control group. SNAP increased CDPER and reduced CDSTAT during sepsis. *Significant difference from control (n = 32, 6, and 5 mice for control, SNAP and decomposed SNAP groups, respectively; p < 0.05).
DISCUSSION
Impaired blood flow within the capillary bed is a hallmark of sepsis (3, 46, 47). We studied this impairment in a model of severe sepsis (19% survival at 24 hrs) because, in patients with severe sepsis, capillary blood flow improves in survivors but fails to improve in nonsurvivors (3, 5). We report here three novel findings: i) knockout of any single NOS isoform did not alter septic capillary blood flow impairment, ii) knockout of the NADPH oxidase subunit gp91phox and inhibition of NADPH oxidase significantly reduced the impairment, and iii) intravenous bolus of the antioxidant ascorbate rapidly and lastingly (at least for 12 hrs) reversed the impairment through an eNOS-dependent mechanism.
To our knowledge, there are no reports of the effect of NOS or NADPH oxidase subunit knockout on capillary blood flow in septic tissue. The present lack of effect of NOS knockout on capillary blood flow seems to be at odds with our own reports that iNOS knockout prevents arteriolar hypocontractility (24) and that nNOS knockout prevents arteriolar hypovasodilatation in septic mouse skeletal muscle (10). However, restored arteriolar function in sepsis through iNOS/nNOS knockout may not necessarily lead to improved capillary blood flow. For example, increased adhesion between the endothelium and leukocytes, platelets or RBCs in sepsis (32–34) could precipitate capillary blood flow cessation, independent of arteriolar function. This independence of arteriolar versus capillary function was recently underscored by the report that impairment of capillary blood flow persisted or even worsened during vasopressor infusion in pigs exposed to FIP (48).
Consistent with other reports dealing with septic skeletal muscle (14, 16, 17), we show that nNOS is the dominant isozyme in this tissue. However, nNOS-derived NO evidently is not responsible for impairment of capillary blood flow. First, sepsis did not alter nNOS activity, yet it markedly elevated the number of nonperfused capillaries. Second, genetic deletion of nNOS dramatically reduced constitutive NOS activity but did not prevent impairment of flow. In this regard, any restoration of mitochondrial function and ATP concentration potentially achieved by suppressed NO production in sepsis (49) would also be unlikely to affect this impairment.
Inhibition of NO production in skeletal muscle with the nonspecific NOS inhibitor L-NAME can stop RBC flow in the muscle capillaries (50). In view of the above-discussed dominance of nNOS isozyme, why did we not observe increased stoppage of flow in capillaries in both control and septic nNOS−/− mice? It is possible that blood flow in capillaries was facilitated by locally produced NO, generated by eNOS or iNOS in cells within or near the capillary wall (35, 36). NOS activity in the small volume of these cells would not be detected by the present enzymatic assay performed in whole muscle homogenates. Nevertheless, Figure 2 indicates that this possible source of local NO was unlikely to account for the capillary blood flow impairment since no effect of eNOS or iNOS knockout was seen.
The protective effects of gp91phox knockout and apocynin (Fig. 2) indicate that NADPH oxidase-derived ROS are chiefly responsible for the capillary blood flow impairment in sepsis. Sepsis activates NADPH oxidase in microvascular endothelial cells and thus increases superoxide production in these cells (19). Ascorbate inhibits this activation and ROS production by preventing sepsis-induced p47phox protein up-regulation (19). The present findings that septic capillary blood flow impairment is NADPH oxidase-dependent, and is reversible by ascorbate, are consistent with these reports.
What accounts for the reversal by ascorbate? The absence of increased RBC velocity in capillaries after ascorbate injection indicates that the restoration of blood flow is not achieved through a vasodilatory effect of ascorbate, but rather through re-establishing blood flow at the capillary/venular level. It has been shown that enhanced adhesion of leukocytes, platelets and RBCs to the endothelium occurs in sepsis (32–34) and that ROS-stimulated adhesion of leukocytes and platelets to the endothelium impedes microcirculatory blood flow (32, 51–53). Because eNOS is expressed in both endothelial cells and platelets (39), it is likely that the eNOS-dependent reversal effect of ascorbate depends on one or both of these cell types. However, detailed examination of eNOS-dependent endothelial/platelet interaction in capillaries (54) was beyond the objectives of the present study.
The present findings that BH4 reverses the impairment eNOS-dependently and that exogenous NO also restores blood flow, and our preliminary observation that ascorbate does not dislodge adhering leukocytes in septic venules, support the following mechanism. In the presence of sepsis-induced ROS increase (11, 19), BH4 becomes oxidized and thereby uncouples eNOS to synthesize superoxide rather than NO (55). At this point, genetic deletion of eNOS does not prevent the impairment of capillary blood flow, as eNOS is already “dysfunctional.” Ascorbate prevents BH4 oxidation, increases BH4 content in platelets and endothelial cells, elevates their eNOS-derived NO (27, 37, 42), reduces platelet aggregability (38, 39) and thus restores capillary blood flow. In addition to the BH4 effect, ascorbate also releases NO from adducts [e.g., by ascorbate’s reduction of S-nitrosothiols (56)] that are synthesized from eNOS-derived NO and stored in vascular tissue.
Astonishingly, the restorative effect of a single 10 mg/kg ascorbate bolus lasted at least 12 hrs (Fig. 4A), despite normalization of ascorbate plasma level 1 hr postinjection (Fig. 5A). This restoration is consistent with the reported restorative effect lasting 18 –24 hrs following a 76 mg/kg ascorbate bolus injection into CLP rats (26). Microvascular endothelial cells of skeletal muscle origin accumulate a high level of intracellular ascorbate (up to 16 mmol/L) that persists longer than does extracellular ascorbate (57). Thus, the eNOS and NO adducts in microvascular endothelial cells may be exposed for prolonged periods to millimolar concentrations of ascorbate that cause them to produce NO. Millimolar concentrations of ascorbate are also high enough to prevent the interaction of superoxide and NO (58), which is another way ascorbate accumulated in microvascular endothelial cells may restore NO-dependent capillary blood flow during sepsis.
The decreased arterial pressure in FIP mice was not rescued by ascorbate at 1 hr after injection of the vitamin (Fig. 4B). Impaired vascular (24, 11) and myocardial function (59) in sepsis cause hypotension. In CLP mice, a 200 mg/kg ascorbate injection at 3 hrs post-CLP rescued the arteriolar vasoconstrictor response but not hypotension at 6 hrs post-CLP, indicating that the initial target of ascorbate protection was microvascular rather than myocardial function (24). However, in CLP rats, 76 mg/kg ascorbate injection at 24 hrs post-CLP prevented hypotension at 48 hrs post-CLP (26). Thus, ascorbate bolus may not restore blood pressure rapidly, but rather over the longer period of 24 hrs when myocardial function may recover.
In conclusion, we used WT, nNOS−/−, iNOS−/−, eNOS−/−, and gp91phox−/− mice to address the mechanism of impaired capillary blood flow in a model of severe sepsis. The impairment required NADPH oxidase but not NOS. Further, the impairment was rapidly and persistently reversed by ascorbate through an eNOS-dependent mechanism. We suggest that ascorbate bolus administration may be beneficial as an adjunct therapy in septic patients who retain sufficient eNOS protein expression, and whose survival depends on restoring capillary blood flow.
Acknowledgments
We thank Dr. David Schuster for measuring ascorbate.
Supported in part, by grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario (awarded to K.T. and J.X.W.), by the Division of Vascular Surgery, London Health Sciences Centre (salary support to K.T.), Lawson Health Research Institute, and by the National Institutes of Health (NIH/NCCAM 1R01AT003643-01A2) (to J.X.W.).
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Bone RC. Gram-negative sepsis. Background, clinical features, and intervention. Chest. 1991;100:802– 808. doi: 10.1378/chest.100.3.802. [DOI] [PubMed] [Google Scholar]
- 2.Lam C, Tyml K, Martin C, et al. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest. 1994;94:2077–2083. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakr Y, Dubois MJ, De BD, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 4.Goldman D, Bateman RM, Ellis CG. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: interpreting capillary oxygen transport data using a mathematical model. Am J Physiol Heart Circ Physiol. 2004;287:H2535–H2544. doi: 10.1152/ajpheart.00889.2003. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368 –1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Hangai-Hoger N, Nacharaju P, Manjula BN, et al. Microvascular effects following treatment with polyethylene glycol-albumin in lipopolysaccharide-induced endotoxemia. Crit Care Med. 2006;34:108 –117. doi: 10.1097/01.ccm.0000190623.97200.82. [DOI] [PubMed] [Google Scholar]
- 7.Amon M, Menger MD, Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-alpha-induced microcirculatory dysfunction and apoptotic cell death. FASEB J. 2003;17:175–185. doi: 10.1096/fj.02-0368com. [DOI] [PubMed] [Google Scholar]
- 8.Piper RD, Pitt-Hyde ML, Anderson LA, et al. Leukocyte activation and flow behavior in rat skeletal muscle in sepsis. Am J Respir Crit Care Med. 1998;157:129 –134. doi: 10.1164/ajrccm.157.1.9609012. [DOI] [PubMed] [Google Scholar]
- 9.Bateman RM, Jagger JE, Sharpe MD, et al. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am J Physiol Heart Circ Physiol. 2001;280:H2848 –H2856. doi: 10.1152/ajpheart.2001.280.6.H2848. [DOI] [PubMed] [Google Scholar]
- 10.Lidington D, Li F, Tyml K. Deletion of neuronal NOS prevents impaired vasodilation in septic mouse skeletal muscle. Cardiovasc Res. 2007;74:151–158. doi: 10.1016/j.cardiores.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R50 –R56. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg SM, Cunnion RE, Zimmerberg J. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to catecholamines in septic rats. Am J Physiol. 1993;264:H660 –H663. doi: 10.1152/ajpheart.1993.264.2.H660. [DOI] [PubMed] [Google Scholar]
- 13.Baker CH, Sutton ET. Arteriolar endothelium-dependent vasodilation occurs during endotoxin shock. Am J Physiol. 1993;264:H1118 –H1123. doi: 10.1152/ajpheart.1993.264.4.H1118. [DOI] [PubMed] [Google Scholar]
- 14.Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;278:H1480 –H1489. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- 15.Lirk P, Hoffmann G, Rieder J. Inducible nitric oxide synthase—time for reappraisal. Curr Drug Targets Inflamm Allergy. 2002;1:89 –108. doi: 10.2174/1568010023344913. [DOI] [PubMed] [Google Scholar]
- 16.el-Dwairi Q, Comtois A, Guo Y, et al. Endotoxin-induced skeletal muscle contractile dysfunction: contribution of nitric oxide synthases. Am J Physiol. 1998;274:C770 –C779. doi: 10.1152/ajpcell.1998.274.3.C770. [DOI] [PubMed] [Google Scholar]
- 17.McKinnon RL, Lidington D, Bolon M, et al. Reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle is mediated by nNOS-derived NO. Cardiovasc Res. 2006;69:236 –244. doi: 10.1016/j.cardiores.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Yu G, Peng T, Feng Q, et al. Abrupt reoxygenation of microvascular endothelial cells after hypoxia activates ERK1/2 and JNK1, leading to NADPH oxidase-dependent oxidant production. Microcirculation. 2007;14:125–136. doi: 10.1080/10739680601131218. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Schuster DP, Tyml K, et al. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med. 2007;42:124 –131. doi: 10.1016/j.freeradbiomed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Crimi E, Liguori A, Condorelli M, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth Analg. 2004;99:857– 863. doi: 10.1213/01.ANE.0000133144.60584.F6. [DOI] [PubMed] [Google Scholar]
- 21.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814 – 822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka H, Matsuda T, Miyagantani Y, et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326 –331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 23.Lehr HA, Germann G, McGregor GP, et al. Consensus meeting on “relevance of parenteral vitamin C in acute endothelial dependent pathophysiological conditions (EDPC) Eur J Med Res. 2006;11:516 –526. [PubMed] [Google Scholar]
- 24.Wu F, Wilson JX, Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic Biol Med. 2004;37:1282–1289. doi: 10.1016/j.freeradbiomed.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Armour J, Tyml K, Lidington D, et al. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol. 2001;90:795– 803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- 26.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med. 2005;33:1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Lee SI, Lee DH, et al. Ascorbic acid synthesis due to L-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem Biophys Res Commun. 2006;345:1657–1662. doi: 10.1016/j.bbrc.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 28.Stamme C, Bundschuh DS, Hartung T, et al. Temporal sequence of pulmonary and systemic inflammatory responses to graded polymicrobial peritonitis in mice. Infect Immun. 1999;67:5642–5650. doi: 10.1128/iai.67.11.5642-5650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider MP, Delles C, Schmidt BM, et al. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am J Hypertens. 2005;18:1111–1117. doi: 10.1016/j.amjhyper.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Lidington D, Ouellette Y, Li F, et al. Conducted vasoconstriction is reduced in a mouse model of sepsis. J Vasc Res. 2003;40:149 –158. doi: 10.1159/000070712. [DOI] [PubMed] [Google Scholar]
- 31.Scott JA, Machoun M, McCormack DG. Inducible nitric oxide synthase and vascular reactivity in rat thoracic aorta: effect of aminoguanidine. J Appl Physiol. 1996;80:271–277. doi: 10.1152/jappl.1996.80.1.271. [DOI] [PubMed] [Google Scholar]
- 32.Cerwinka WH, Cooper D, Krieglstein CF, et al. Superoxide mediates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am J Physiol Heart Circ Physiol. 2003;284:H535–H541. doi: 10.1152/ajpheart.00311.2002. [DOI] [PubMed] [Google Scholar]
- 33.Eichelbronner O, Sielenkamper A, Cepinskas G, et al. Endotoxin promotes adhesion of human erythrocytes to human vascular endothelial cells under conditions of flow. Crit Care Med. 2000;28:1865–1870. doi: 10.1097/00003246-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Singer G, Urakami H, Specian RD, et al. Platelet recruitment in the murine hepatic microvasculature during experimental sepsis: role of neutrophils. Microcirculation. 2006;13:89 –97. doi: 10.1080/10739680500466343. [DOI] [PubMed] [Google Scholar]
- 35.Cerwinka WH, Cooper D, Krieglstein CF, et al. Nitric oxide modulates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am J Physiol Heart Circ Physiol. 2002;282:H1111–H1117. doi: 10.1152/ajpheart.00391.2001. [DOI] [PubMed] [Google Scholar]
- 36.Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review: microvascular dysfunction in sepsis– hemodynamics, oxygen transport, and nitric oxide. Crit Care. 2003;7:359 –373. doi: 10.1186/cc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 38.Takajo Y, Ikeda H, Haramaki N, et al. Augmented oxidative stress of platelets in chronic smokers. Mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. J Am Coll Cardiol. 2001;38:1320 –1327. doi: 10.1016/s0735-1097(01)01583-2. [DOI] [PubMed] [Google Scholar]
- 39.Randriamboavonjy V, Fleming I. Endothelial nitric oxide synthase (eNOS) in platelets: how is it regulated and what is it doing there? Pharmacol Rep. 2005;57 (Suppl):59 – 65. [PubMed] [Google Scholar]
- 40.Kalivendi S, Hatakeyama K, Whitsett J, et al. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide—a role for GFRP? Free Radic Biol Med. 2005;38:481– 491. doi: 10.1016/j.freeradbiomed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708 –1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 43.Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546 –22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 44.Fitzal F, Redl H, Strohmaier W, et al. A 4-amino analogue of tetrahydrobiopterin attenuates endotoxin-induced hemodynamic alterations and organ injury in rats. Shock. 2002;18:158 –162. doi: 10.1097/00024382-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes D, da Silva-Santos JE, Duma D, et al. Nitric oxide-dependent reduction in soluble guanylate cyclase functionality accounts for early lipopolysaccharide-induced changes in vascular reactivity. Mol Pharmacol. 2006;69:983–990. doi: 10.1124/mol.105.015479. [DOI] [PubMed] [Google Scholar]
- 46.De BD, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403– 408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 47.Spronk PE, Ince C, Gardien MJ, et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 48.Krejci V, Hiltebrand LB, Sigurdsson GH. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit Care Med. 2006;34:1456 –1463. doi: 10.1097/01.CCM.0000215834.48023.57. [DOI] [PubMed] [Google Scholar]
- 49.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219 –223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell D, Tyml K. Nitric oxide release in rat skeletal muscle capillary. Am J Physiol. 1996;270:H1696 –H1703. doi: 10.1152/ajpheart.1996.270.5.H1696. [DOI] [PubMed] [Google Scholar]
- 51.Johnston B, Kanwar S, Kubes P. Hydrogen peroxide induces leukocyte rolling: modulation by endogenous antioxidant mechanisms including NO. Am J Physiol. 1996;271:H614 –H621. doi: 10.1152/ajpheart.1996.271.2.H614. [DOI] [PubMed] [Google Scholar]
- 52.Tran ED, Schmid-Schonbein GW. An in-vivo analysis of capillary stasis and endothelial apoptosis in a model of hypertension. Microcirculation. 2007;14:793– 804. doi: 10.1080/10739680701419992. [DOI] [PubMed] [Google Scholar]
- 53.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678 – 689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 54.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 55.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 56.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 57.Wilson JX, Dixon SJ, Yu J, et al. Ascorbate uptake by microvascular endothelial cells of rat skeletal muscle. Microcirculation. 1996;3:211–221. doi: 10.3109/10739689609148290. [DOI] [PubMed] [Google Scholar]
- 58.Jackson TS, Xu A, Vita JA, et al. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916 –922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 59.Piper RD, Li FY, Myers ML, et al. Effects of isoproterenol on myocardial structure and function in septic rats. J Appl Physiol. 1999;86:993–1001. doi: 10.1152/jappl.1999.86.3.993. [DOI] [PubMed] [Google Scholar]