Abstract
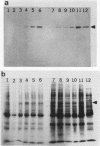
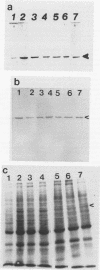
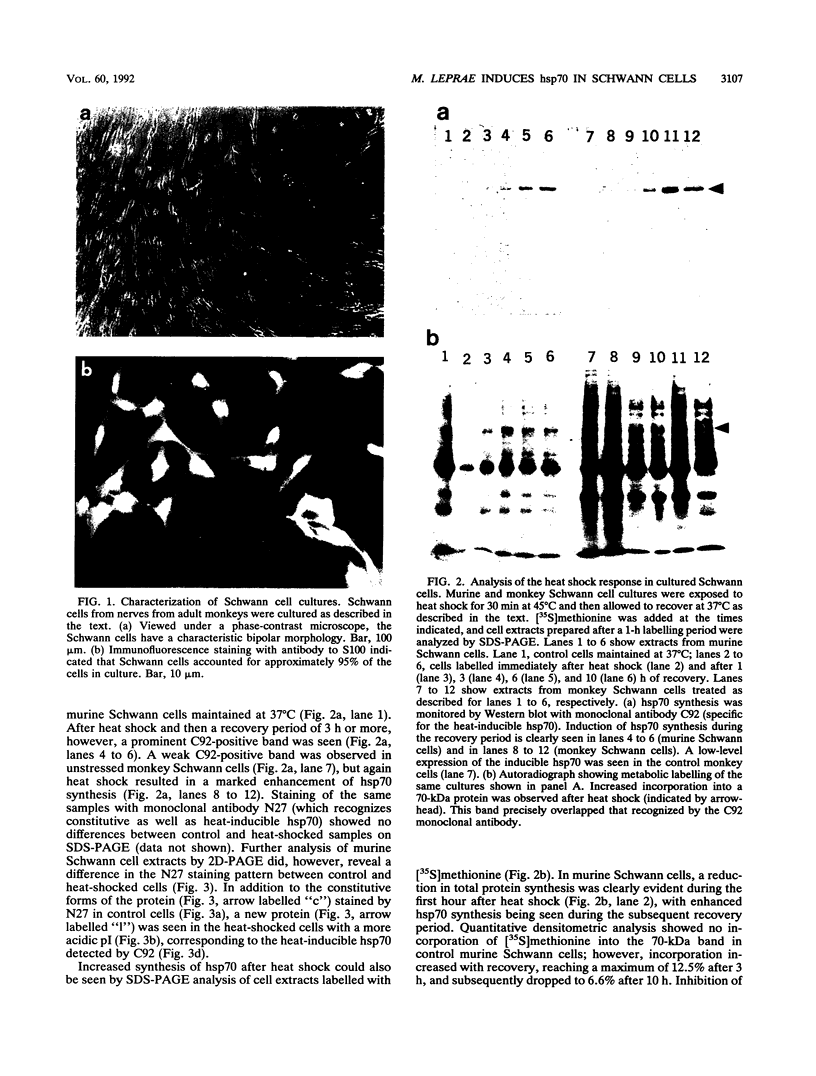
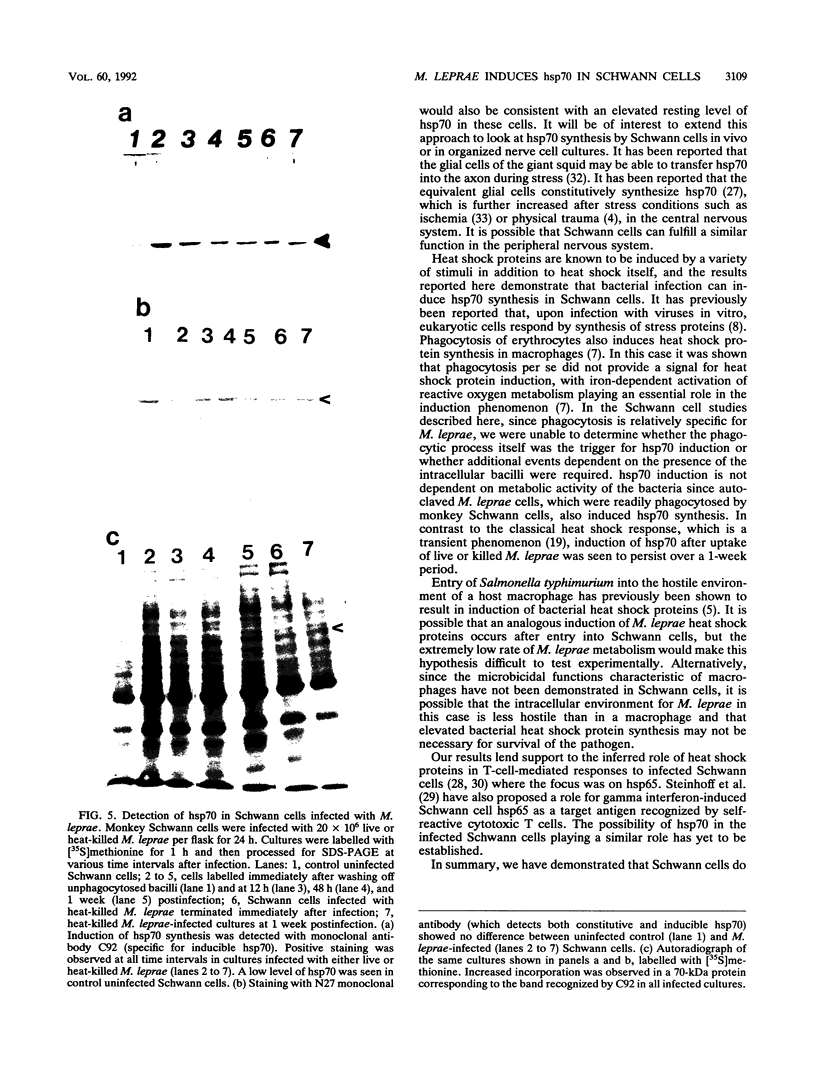
Induction of heat shock protein synthesis was monitored in murine and monkey Schwann cells exposed to elevated temperatures. Synthesis of the stress-inducible 70-kDa heat shock protein (hsp70) was detected in both murine and primate Schwann cells by metabolic labelling and by immunoblotting with a specific monoclonal antibody. hsp70 synthesis was also induced in Schwann cells after infection with Mycobacterium leprae and was detected from 24 h to 1 week postinfection. These results are discussed with respect to the possible role of heat shock proteins in immunopathological events associated with the clinical manifestations of leprosy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose E. J., Khanolkar S. R., Chulawalla R. G. A rapid test for bacillary resistance to dapsone. Lepr India. 1978 Apr;50(2):131–143. [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Dalakas M. C., Lawrence J. V., Carter L. S. Human schwann cells in tissue culture: histochemical and ultrastructural studies. Arch Neurol. 1980 Jun;37(6):329–337. doi: 10.1001/archneur.1980.00500550031001. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Brown I. R., Rush S., Ivy G. O. Induction of a heat shock gene at the site of tissue injury in the rat brain. Neuron. 1989 Jun;2(6):1559–1564. doi: 10.1016/0896-6273(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Cammer W., Bloom B. R., Norton W. T., Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. F., Shiraishi K., Hisanaga K., Sagar S. M., Mandabach M., Sharp F. R. Heat shock proteins as markers of neural injury. Brain Res Mol Brain Res. 1989 Jul;6(1):93–100. doi: 10.1016/0169-328x(89)90033-8. [DOI] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Harada K. The nature of mycobacterial acid-fastness. Stain Technol. 1976 Sep;51(5):255–260. doi: 10.3109/10520297609116714. [DOI] [PubMed] [Google Scholar]
- Iyer C. G. Predilection of M. leprae for nerves. Neurohistopathologic observations. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):634–645. [PubMed] [Google Scholar]
- Kaplan G., Cohn Z. A. The immunobiology of leprosy. Int Rev Exp Pathol. 1986;28:45–78. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mehlert A., Young D. B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989 Feb;3(2):125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Welch W. J. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988 Apr;106(4):1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R., Mahadevan P. R., Antia N. H. Organized nerve culture. I. A technique to study the effect of M. leprae infection. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):183–188. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. M., Ross W. F. Nerve involvement in leprosy--pathology, differential diagnosis and principles of management. Lepr Rev. 1975 Sep;46(3):199–212. doi: 10.5935/0305-7518.19750022. [DOI] [PubMed] [Google Scholar]
- Scarpini E., Meola G., Baron P., Beretta S., Velicogna M., Scarlato G. S-100 protein and laminin: immunocytochemical markers for human Schwann cells in vitro. Exp Neurol. 1986 Jul;93(1):77–83. doi: 10.1016/0014-4886(86)90146-9. [DOI] [PubMed] [Google Scholar]
- Sprang G. K., Brown I. R. Selective induction of a heat shock gene in fibre tracts and cerebellar neurons of the rabbit brain detected by in situ hybridization. Brain Res. 1987 Dec;427(1):89–93. doi: 10.1016/0169-328x(87)90049-0. [DOI] [PubMed] [Google Scholar]
- Steinhoff U., Kaufmann S. H. Specific lysis by CD8+ T cells of Schwann cells expressing Mycobacterium leprae antigens. Eur J Immunol. 1988 Jun;18(6):969–972. doi: 10.1002/eji.1830180622. [DOI] [PubMed] [Google Scholar]
- Steinhoff U., Schoel B., Kaufmann S. H. Lysis of interferon-gamma activated Schwann cell by cross-reactive CD8+ alpha/beta T cells with specificity for the mycobacterial 65 kd heat shock protein. Int Immunol. 1990;2(3):279–284. doi: 10.1093/intimm/2.3.279. [DOI] [PubMed] [Google Scholar]
- Steinhoff U., Wand-Württenberger A., Bremerich A., Kaufmann S. H. Mycobacterium leprae renders Schwann cells and mononuclear phagocytes susceptible or resistant to killer cells. Infect Immun. 1991 Feb;59(2):684–688. doi: 10.1128/iai.59.2.684-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell M., Greenberg S. G., Lasek R. J. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986 Jan 15;363(1):161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- Vass K., Welch W. J., Nowak T. S., Jr Localization of 70-kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol. 1988;77(2):128–135. doi: 10.1007/BF00687422. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]