Abstract
The central coiled coil of the essential spindle pole component Spc110p spans the distance between the central and inner plaques of the Saccharomyces cerevisiae spindle pole body (SPB). The carboxy terminus of Spc110p, which binds calmodulin, resides at the central plaque, and the amino terminus resides at the inner plaque from which nuclear microtubules originate. To dissect the functions of Spc110p, we created temperature-sensitive mutations in the amino and carboxy termini. Analysis of the temperature-sensitive spc110 mutations and intragenic complementation analysis of the spc110 alleles defined three functional regions of Spc110p. Region I is located at the amino terminus. Region II is located at the carboxy-terminal end of the coiled coil, and region III is the previously defined calmodulin-binding site. Overexpression of SPC98 suppresses the temperature sensitivity conferred by mutations in region I but not the phenotypes conferred by mutations in the other two regions, suggesting that the amino terminus of Spc110p is involved in an interaction with the γ-tubulin complex composed of Spc97p, Spc98p, and Tub4p. Mutations in region II lead to loss of SPB integrity during mitosis, suggesting that this region is required for the stable attachment of Spc110p to the central plaque. Our results strongly argue that Spc110p links the γ-tubulin complex to the central plaque of the SPB.
INTRODUCTION
In the yeast Saccharomyces cerevisiae, the spindle pole body (SPB) is a multilayered structure that functions as the microtubule-organizing center. Electron microscopy reveals that the central plaque or layer of the SPB lies in the plane of the nuclear envelope throughout the cell cycle. Cytoplasmic microtubules are organized by the electron-dense outer plaque, and nuclear microtubules are organized by the less electron-dense inner plaque. In addition to its functions in the proper nucleation and organization of microtubules, the SPB also functions in self-replication, which begins early in G1-S as spindle pole components assemble into a satellite structure on the cytoplasmic surface of the half-bridge. Coincident with bud emergence and the initiation of DNA synthesis, the satellite-bearing SPB gives rise to duplicated, paired side-by-side SPBs connected by a complete bridge. Separation of the duplicated SPBs after DNA synthesis results in the organization of the nuclear microtubules into a short complete spindle (Byers, 1981).
Microtubule nucleation at both the inner and outer plaques of the SPB appears to be regulated by a complex containing Spc98p, Spc97p, and Tub4p (the γ-tubulin homologue) (Sobel and Snyder, 1995; Marschall et al., 1996; Spang et al., 1996a; Knop et al., 1997). Spc98p, Spc97p, and Tub4p interact physically and functionally (Knop et al., 1997), and all three components localize to the inner and outer plaques (Rout and Kilmartin, 1990; Spang et al., 1996a; Knop et al., 1997). Spc110p is hypothesized to connect the γ-tubulin complex in the inner plaque to the central plaque (Kilmartin and Goh, 1996). The carboxy terminus of Spc110p, which contains a calmodulin-binding site (amino acids 897–917) (Geiser et al., 1993; Stirling et al., 1994; Spang et al., 1996b), is located at the central plaque of the SPB (Sundberg et al., 1996). The amino terminus of Spc110p is located at the inner plaque (Spang et al., 1996b). The long central coiled-coil rod of Spc110p spans between the two plaques (Kilmartin et al., 1993). The substructural localizations of the amino and carboxy termini of Spc110p support the hypothesis that Spc110p molecules form a parallel array in the SPB between the central and inner plaques.
The localization of calmodulin to the central plaque of the SPB (Sundberg et al., 1996) is dependent on the calmodulin-binding site of Spc110p (Spang et al., 1996b; Sundberg and Davis, unpublished results). The calmodulin-Spc110p interaction is required for proper chromosome segregation (Geiser et al., 1993; Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996). Characterization of the carboxy-terminal temperature-sensitive allele spc110–220, which has a mutation in the calmodulin-binding site, revealed that calmodulin binding to Spc110p is required for the proper assembly of spindle pole components. At the nonpermissive temperature, an aberrant aggregate of material, which contains Spc98p (formerly known as the 90-kDa spindle pole component) and Spc110–220p, forms in the nucleus and appears to nucleate microtubules. The aggregate has been named an intranuclear microtubule organizer (IMO). The IMO appears during nascent SPB formation and disappears after SPB separation. The defective Spc110–220p-calmodulin interaction prohibits the proper assembly and attachment of the carboxy terminus of Spc110–220p to the central plaque of the SPB. However, the amino terminus of Spc110–220p is apparently unaffected by the calmodulin-binding defect and remains capable of attracting nucleating components normally found at the inner plaque, thus leading to assembly of the IMO (Sundberg et al., 1996). Interestingly, the overexpression of SPC110 in wild-type cells is toxic and results in the formation of a large ordered spheroidal polymer that is composed of Spc110p and calmodulin (Kilmartin and Goh, 1996).
To further define the functions of Spc110p at the inner and central plaques of the SPB, we have created temperature-sensitive mutations in the amino and carboxy termini. Analysis of the amino-terminal and carboxy-terminal temperature-sensitive spc110 alleles suggests that three functional regions of Spc110p have been perturbed. Region III is the previously defined calmodulin-binding site and is involved in assembly of Spc110p at the SPB. Our results identify two new functional regions of Spc110p. Region I is located at the amino terminus of Spc110p and appears to be involved in an interaction with the γ-tubulin complex. Region II is located at the carboxy-terminal end of the central coiled coil and is involved in maintaining SPB integrity after spindle formation. Our results provide evidence that Spc110p attaches the γ-tubulin complex to the central plaque of the SPB.
MATERIALS AND METHODS
Media
SD complete, SD-uracil (Davis, 1992), SD-uracil low adenine (Sundberg et al., 1996), and YPD (Geiser et al., 1991) were described previously.
Plasmids
Plasmids used in this study are listed in Table 1.
Table 1.
Plasmids used in this study
| Plasmid | Parent plasmid | Relevant markersa | Source or reference |
|---|---|---|---|
| pGF29 | pRS306 | 2 μm origin (YEp24 EcoRI fragment) inserted at AatII site | G. Zhu |
| pHS26 | pTD29 | SPC110 LYS2 ADE3, 2 μm origin | Geiser et al., 1993 |
| pHS29 | pRS316 | SPC110 | Sundberg et al., 1996 |
| pHS32 | pHS29 | SPC110 with unique BamHI site toward 3′ end | Sundberg et al., 1996 |
| pHS33 | pHS31 | SPC110 with unique BamHI site toward 5′ end | This study |
| pHS37 | pHS29 | spc110 with V912E | This study |
| pHS38 | pHS29 | spc110 with C911R | Sundberg et al., 1996 |
| pHS39 | pHS32 | spc110-220b | Sundberg et al., 1996 |
| pHS40 | pRS306 | spc110-220 | Sundberg et al., 1996 |
| pHS41 | pGF29 | spc110-220 | Sundberg et al., 1996 |
| pHS42 | pHS33 | spc110-221c | This study |
| pHS43 | pRS306 | spc110-221 | This study |
| pHS44 | pHS33 | spc110-222c | This study |
| pHS45 | pRS306 | spc110-222 | This study |
| pHS48 | pHS32 | spc110-224b | This study |
| pHS49 | pRS306 | spc110-224 | This study |
| pHS50 | pHS32 | spc110-225b | This study |
| pHS51 | pRS306 | spc110-225 | This study |
| pHS52 | pHS32 | spc110-226b | This study |
| pHS53 | pRS306 | spc110-226 | This study |
| pHS80 | pHS39 | spc110 with spc110-220 and spc110-221 mutations | This study |
| pHS81 | pHS50 | spc110 with V912E and R934K | This study |
| pHS82 | pHS29 | spc110 with I828L | This study |
| pHS87 | pHS29 | spc110 with S853G | This study |
| pHS88 | pGF29 | SPC98 | This study |
| pHS91 | pGF29 | TUB4 | This study |
| pHS92 | pGF29 | SPC97 | This study |
| pRS306 | URA3, f1 origin | Sikorski and Hieter, 1989 | |
| pRS316 | CEN6 ARSH4 URA3, f1 origin | Sikorski and Hieter, 1989 | |
| pTD17 | YEp24 | CMD1 URA3, 2 μm origin | Zhu et al., 1993 |
Unless stated otherwise, all markers from the parent plasmid are present in the new construct.
Plasmid does not contain unique BamHI site found in parent plasmid.
Plasmid does not contain unique NcoI and BamHI sites found in parent plasmid.
To create a plasmid with a unique BamHI site in the SPC110 coding region, plasmid pHS31 (Friedman et al., 1996) was linearized by partial digestion with BclI, and the ends were filled in with Klenow. Then a BamHI octomer linker was ligated to the blunt ends. Digestion with BamHI removed the excess linker, and the plasmid ends were ligated together. Restriction enzyme analysis identified a plasmid (named pHS33) with a unique BamHI site replacing the BclI site at the 5′ end of SPC110.
Plasmids pHS39, pHS48, pHS50, and pHS52 are the isolates of spc110–220, spc110–224, spc110–225, and spc110–226 identified in the screen for temperature-sensitive mutations in the carboxy terminus of SPC110. Note that the BamHI site in the coding region of SPC110 of the parent plasmid pHS32 is no longer present in pHS39, pHS48, pHS50, and pHS52.
Plasmids pHS42 and pHS44 are the isolates of spc110–221 and spc110–222 identified in the screen for temperature-sensitive mutations in the amino terminus of SPC110. Note that the NcoI and BamHI sites in SPC110 of the parent plasmid pHS33 are no longer present in pHS42 and pHS44.
The spc110 integrating vectors pHS40 (spc110–220), pHS49 (spc110–224), pHS51 (spc110–225), pHS53 (spc110–226), pHS43 (spc110–221), and pHS45 (spc110–222) were created by replacing the 4.6-kilobase (kb) AlwNI fragment of pHS39, pHS48, pHS50, pHS52, pHS42, and pHS44, respectively, with the 2.6-kb AlwNI fragment of pRS306 (Sikorski and Hieter, 1989).
To create an spc110 plasmid (pHS80) that contained the amino-terminal mutations of spc110–221 and the carboxy-terminal mutations of spc110–220, the 1.7-kb SacI fragment of pHS39 was replaced with the 1.7-kb SacI fragment of pHS42.
Site-directed mutagenesis (Kunkel et al., 1987) was used to introduce the V912E mutation into SPC110 in plasmid pHS29 to create pHS37. Plasmid pHS81 encoding SPC110 with both the V912E and R934K mutations was created by replacing the 2.5-kb HindIII fragment of pHS50 with the 2.5-kb HindIII fragment of pHS29. Plasmid pHS82 encoding SPC110 with the I828L mutation was created by replacing the 2.5-kb HindIII fragment of pHS29 with the 2.5-kb HindIII fragment of pHS50. Plasmid pHS87 encoding SPC110 with the S853G mutation was created by replacing the 2.5-kb HindIII fragment of pHS29 with the 2.5-kb HindIII fragment of pHS39.
Plasmid pHS88, which encodes SPC98, was created by filling the ends of the 3.3-kb NdeI fragment of pDF50 (gift of D. Friedman) with Klenow and then ligating the fragment into the SmaI site of pGF29. Plasmid pHS91 was created by ligating the SacI/XhoI fragment of pTS477 (gift of T. Stearns) which encodes TUB4 into the SacI/XhoI sites of pGF29. Plasmid pHS92 was created by ligating the ClaI/SacII fragment of pTN9 (gift of T. Nguyen) which encodes SPC97 into the ClaI/SacII sites of pGF29.
Strains
Strains used in this study are listed in Table 2. Strains HSY8, HSY9, and HSY91 were derived from CRY1 by integrating the spc110–224, spc110–225, and spc110–226 genes at the SPC110 locus by a two-step gene replacement (Boeke et al., 1987) using plasmids pHS49, pHS51, and pHS53, respectively, linearized with MluI. Strains HSY14 and HSY15 were derived from CRY1 by integrating the spc110–221 and spc110–222 genes at the SPC110 locus by a two-step gene replacement (Boeke et al., 1987) using plasmids pHS43 and pHS45, respectively, linearized with SnaBI. Integration was checked by Southern blot analysis. Presence of mutations was confirmed by PCR amplification and sequencing.
Table 2.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| CRY1 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | R. Fuller |
| JGY46 | MATa/MATα ade2-1oc/ade2-loc can1-100/can1-100 his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 trp1-1/trp1-1 ura3-1/ura3-1 | Geiser et al., 1991 |
| HSY2-1C (pHS26) | MATα ade2-1oc ade3Δ can1-100 his3-11,15 leu2-3,112 lys2Δ::HIS3 spc110Δ::TRP1 trp1-1 ura3-1, carrying plasmid pHS26 | Geiser et al., 1993 |
| HSY8 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-224 trp1-1 ura3-1 | This study |
| HSY9 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-225 trp1-1 ura3-1 | This study |
| HSY10 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-220 trp1-1 ura3-1 | Sundberg et al., 1996 |
| HSY11 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-220/SPC110 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY14 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-221 trp1-1 ura3-1 | This study |
| HSY15 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-222 trp1-1 ura3-1 | This study |
| HSY18 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-221/SPC110 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY20 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-220/spc110-220 trp1-1/trp1-1 ura3-1/ura3-1 | Sundberg et al., 1996 |
| HSY21 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-221/spc110-221 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY28 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-224/SPC110 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY29 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-225/SPC110 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY31 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-220/spc110-221 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY38 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-224/spc110-220 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY39 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-225/spc110-220 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY41 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-221/spc110-224 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY42 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-221/spc110-225 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY46 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-224/spc110-224 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY47 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-221/spc110-224 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY48 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-225/spc110-225 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY55-2B | MAT? ade2-1oc can1-100 his3-11,15 leu2-3,112 mad1Δ::URA3 spc110-221 trp1-1 ura3-1 | This study |
| HSY57 | MATa/MATα ade2-1oc/ade2-1oc ADE3/ade3Δ can1-100/can1-100 his3-11,15/HIS3 leu2-3,112/leu2-3,112 spc110-226/SPC110 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY78 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/ leu2-3,112 spc110-226/spc110-220 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY79 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-226/spc110-221 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY81 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-226/spc110-224 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY82 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-226/spc110-225 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY83 | MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ADE3 can1-100/can1-100 HIS3/his3-11,15 leu2-3,112/leu2-3,112 spc110-226/spc110-226 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| HSY91 | MATa ade2-1oc can1-100 his3-11,15 leu2-3,112 spc110-226 trp1-1 ura3-1 | This study |
| TDY436 | MATα ade2-1oc can1-100 his3-11,15 leu2-3,112 mad1Δ::URA3 trp1-1 ura3-1 | M. Flory |
Isolation of Temperature-sensitive Mutations in SPC110
Temperature-sensitive mutations in SPC110 were created by combining a PCR mutagenesis technique (Muhlrad et al., 1992) with a plasmid shuffle strategy (Davis, 1990; Geiser et al., 1991) as described previously (Sundberg et al., 1996). The mutagenesis was targeted to the amino- and carboxy-terminal regions of Spc110p. The results of the carboxy-terminal screen have been published previously (Sundberg et al., 1996). For the amino-terminal screen, the region of interest of SPC110 (base pairs 51–1056) was amplified under mutagenic PCR conditions (described in Sundberg et al., 1996). Then plasmid pHS33 was digested with NcoI and BamHI to create a 667-base pair gap in the amino terminus of SPC110. The gapped pHS33 plasmid contains homology to both ends of the mutagenized PCR product. Mutagenized PCR product (1 μg) was cotransformed with 100 ng of gel-purified gapped NcoI-BamHI pHS33 plasmid and 20 μl of sheared salmon sperm DNA (20 mg/ml) into the plasmid shuffle indicator strain HSY2-1C (pHS26) (Geiser et al., 1993). Homologous recombination between the gapped pHS33 plasmid ends and the mutagenized DNA ends repairs the gapped plasmid, introducing mutations into SPC110. The transformants were plated at 37°C, selecting for the repaired plasmid on SD-uracil low adenine medium. The indicator strain HSY2–1C (pHS26) was constructed such that colonies carrying a version of plasmid pHS33 in which the SPC110 gene contained a temperature-sensitive mutation would remain solid red at 37°C and sector white at 21°C. Of the 22,000 colonies screened at 37°C for amino-terminal mutations, 2,600 solid red colonies were isolated and patched at 37°C and 21°C to check for sectoring. Two colonies remained red at 37°C and sectored at 21°C. The repaired SPC110 plasmid was rescued from both colonies, and both plasmids conferred temperature sensitivity when retransformed into the plasmid shuffle indicator strain.
Isolation of a Synchronous Population of G1 Daughters
Synchronous populations of G1 daughters were isolated by centrifugal elutriation as described previously (Sundberg et al., 1996). The landmark used to align data from different elutriation shift experiments is the midpoint of S phase, set as time t = 0 min in Figures 3, 4, and 6D. The rationale behind this method of alignment is described elsewhere (Sundberg et al., 1996).
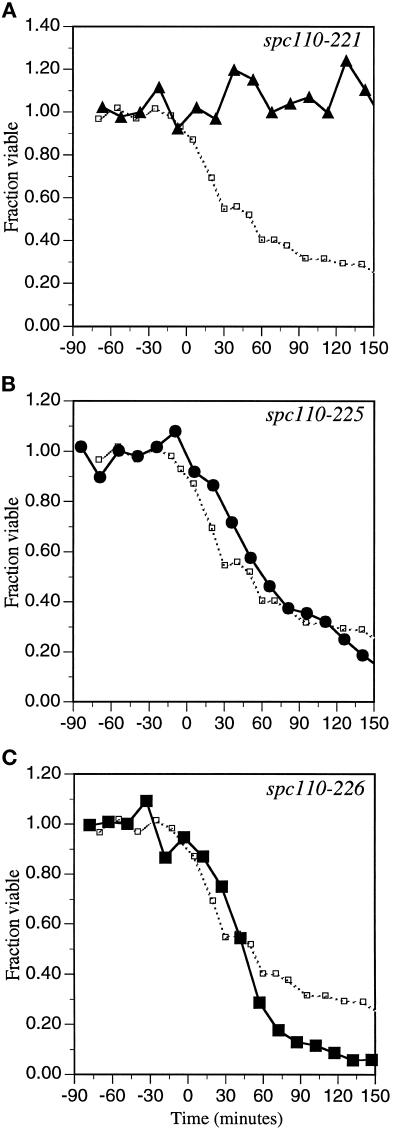
Figure 3.
The amino-terminal spc110–221 mutant remains viable. The carboxy-terminal mutants spc110–226, spc110–225, and spc110–220 suffer considerable lethality. spc110–220, spc110–221, spc110–226, and spc110–225 mutant cultures (strains HSY20, HSY21, HSY83, and HSY48, respectively) were synchronized in early G1 by elutriation as described in MATERIALS AND METHODS and released at 37°C. At regular intervals, aliquots were removed, sonicated, and titered for colony-forming units on YPD at 21°C. Time zero is the midpoint of S phase and is the landmark chosen to align the data from different elutriation experiments. The fraction of viable cells in the spc110–220 mutant culture (□) is shown in each panel. (A) ▴, fraction of spc110–221 cells viable when plated at the permissive temperature. (B) •, fraction of spc110–225 cells viable when plated at the permissive temperature. (C) ▪, fraction of spc110–226 cells viable when plated at the permissive temperature.
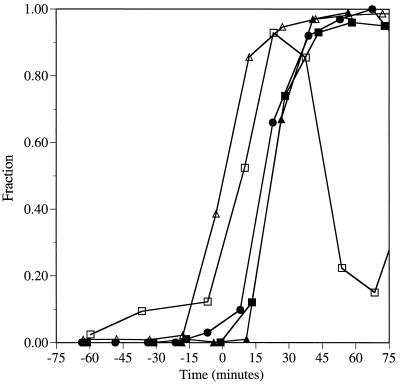
Figure 4.
Timing of spindle formation as measured by two dots of anti-98 kDa staining. Populations of cells in early G1 were obtained by centrifugal elutriation from strains JGY46 (wild type), HSY21 (spc110–221/spc110–221), HSY48 (spc110–225/spc110–225), and HSY83 (spc110–226/spc110–226) and shifted to 37°C. At regular intervals samples were removed for immunofluorescence and processed as described in MATERIALS AND METHODS. The cells were stained with anti-98 kDa antibodies (diluted 1:10; gift of J. Kilmartin). The secondary antibodies were rhodamine-conjugated affinity-purified sheep IgG anti-mouse Ig (20 μg/ml; Boehringer Mannheim Corp., Indianapolis, IN). Time zero is the midpoint of S phase. ▵, fraction of the cell population that has replicated at least half of its genome; □, fraction of wild-type cells with two dots of anti-98 kDa staining; ▴, fraction of spc110–221 cells with two dots of anti-98 kDa staining; ▪, fraction of spc110–226 cells with two dots of anti-98 kDa staining; •, fraction of spc110–225 cells with two dots of anti-98 kDa staining.
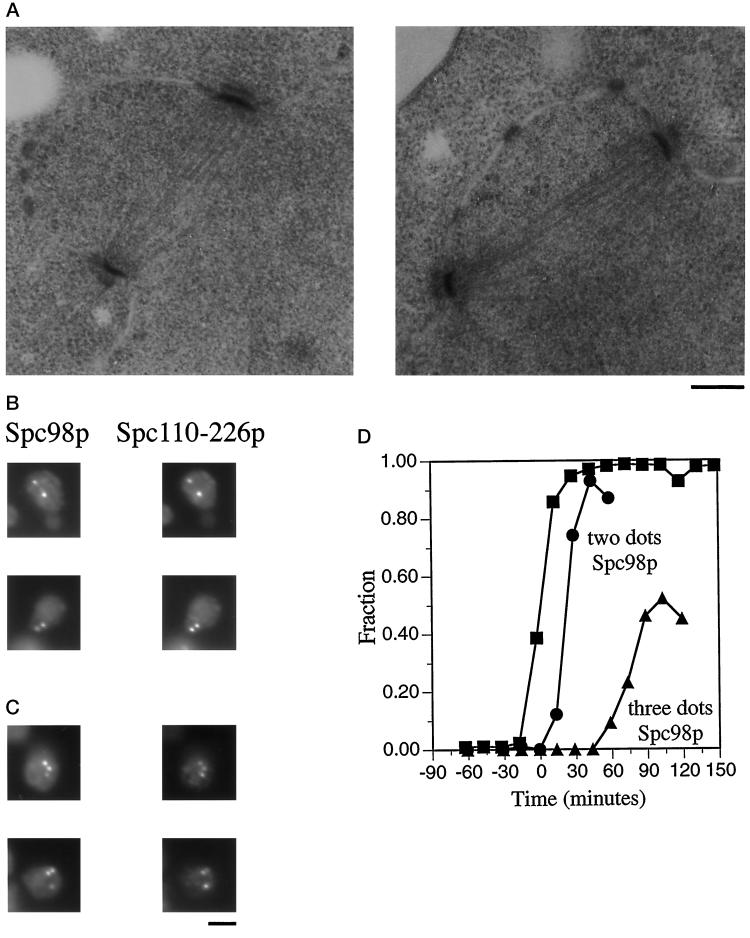
Figure 6.
spc110–226 forms normal spindles early, but spindle integrity is compromised later. A population of synchronous spc110–226 cells was obtained from strain HSY83 by centrifugal elutriation and shifted to 37°C. At regular intervals samples were removed and processed for either electron microscopy or immunofluorescence. t = 0 min is the midpoint of S phase. (A) Electron micrographs depicting short complete spindles at t = 12 min. Bar, 0.2 μm. (B) Spc98p staining and Spc110–226p staining of spc110–226 mutant cells reveal two dots of colocalized staining representing the poles of a short complete spindle 43 min after the midpoint of S phase. The cells were stained with affinity-purified anti-Spc110p antibodies (1:500) (Friedman et al., 1996) and mouse monoclonal anti-98 kDa antibodies (1:10; gift of J. Kilmartin). The secondary antibodies were a mixture of rhodamine-conjugated affinity-purified sheep IgG anti-mouse Ig (20 μg/ml) and fluorescein-conjugated goat anti-rabbit antibody (1:1000; Boehringer Mannheim Corp.). Bar, 2 μm. (C) Spc98p staining and Spc110–226p staining of spc110–226 mutant cells reveal three dots of colocalized staining and thus the presence of an aberrant structure 103 min after the midpoint of S phase in the synchronous shift experiment. Samples were processed for immunofluorescence as described in Figure 6B. Bar, 2 μm. (D) Timing of the appearance of three dots of Spc98p staining in the spc110–226 synchronous shift experiment. ▪, fraction of the cell population that has replicated at least one-half of its genome; •, fraction of spc110–226 cells with two dots of anti-98 kDa staining; ▴, fraction of spc110–226 cells with three or more dots of anti-98 kDa staining.
Cytological Techniques
Yeast cells were prepared for flow cytometry and analyzed for DNA content as described (Muller, 1991). Cells were prepared for tubulin immunofluorescence as described (Sundberg et al., 1996). Elutriated cells were prepared for SPB component immunolocalizations by a technique derived from a previous protocol (Rout and Kilmartin, 1990) and described previously (Sundberg et al., 1996). Asynchronous cells were prepared for calmodulin localization in an identical manner except that after being mounted on polylysine-coated slides, the cells were washed with phosphate-buffered saline instead of sorbitol phosphate buffer, resulting in more cell lysis and thus better visualization of calmodulin localization at the SPB. DNA was stained with 4′,6-diamidino-2-phenylindole at a concentration of 0.1 μg/ml. Stained cells were viewed with a Zeiss Axioplan fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY).
Electron Microscopy
Cells were prepared for thin-section electron microscopy as described (Sundberg et al., 1996). Serial thin sections were viewed under Philips EM300 and CM100 electron microscopes (Philips Electron Optics, Inc., Mahwah, NJ).
RESULTS
Temperature-sensitive Mutations in SPC110
To characterize the functions of Spc110p, we created temperature-sensitive mutations in SPC110. The PAIRCOIL (Berger et al., 1995) program predicts that the coiled-coil rod of Spc110p extends from amino acids 155–798. The COILER (Woolfson and Alber, 1995) program predicts a coiled-coil rod spanning amino acids 165–791. Both are in close agreement with earlier predictions [amino acids 164–791 (Mirzayan et al., 1992); amino acids 168–773 (Kilmartin et al., 1993)]. Deletion studies performed by Kilmartin et al. (1993) revealed that the coiled-coil rod of Spc110p functions as a nonessential spacer between the central and inner plaques. Thus, we targeted the mutagenesis to the amino terminus (amino acids 1–275) and the carboxy terminus (amino acids 761–944) of Spc110p (Figure 1). We isolated 2 amino-terminal alleles and 11 carboxy-terminal alleles (Table 3).
Figure 1.
Mutagenesis of SPC110. The mutagenized amino- and carboxy-terminal regions have been enlarged, and the locations of mutations are represented by vertical lines. The vertical lines topped with an x represent mutations that cause frameshifts resulting in truncations nearby. The vertical line topped with an o marks a mutation that creates a stop codon. Region I, amino acids 1–163; region II, amino acids 772–836; region III, amino acids 897–917.
Table 3.
spc110 alleles
| A. Amino-terminal
alleles
| ||||
|---|---|---|---|---|
| Allele | Mutations Region I | |||
| spc110-221 | M15T, T30M, D62E, S67P, S94P, K99N, K110E, L119S, N163Y | |||
| spc110-222 | E16G, E50G, I65V, K120N, R146S, I148L, M232K | |||
| B. Carboxy-terminal
alleles
| ||||
|---|---|---|---|---|
| Allele | Mutations Region II | Region III | ||
| Amino acids 772-836 | CaM | Truncations | Other | |
| spc110-223 | L836H | Frameshift D862M, E863N stop | ||
| spc110-224 | Y811D, H816L | Frameshift G850GAQSDHFTSSR stop | K849R | |
| spc110-228 | T776I, N782S, K796R, L804S, R820S, D830G | L899F | S923stop | |
| spc110-230 | C911R | |||
| spc110-220 | C911R | S853G | ||
| spc110-225 | I828L | V912E | R934K | |
| spc110-226 | L772M, R795L, D797V, N823S, L836P | |||
| spc110-227 | R784G, M802T, K812E, E818K, Y831N | |||
| spc110-229 | T776S, Q803E, L836H | E863D | ||
CaM, calmodulin-binding region (amino acids 897–917).
The two amino-terminal alleles, spc110–221 and spc110–222, contain nine and seven mutations, respectively (Table 3A). Of the 16 amino-terminal mutations, 15 map to the region between amino acids 1 and 163, although this region represents only 59% of the region mutagenized. Only 2 of the 16 mutations map to the predicted coiled coil consistent with the observation that the coiled coil is a nonessential spacer. The mutations present in each amino-terminal allele are widely distributed. A subset of mutations sufficient to confer temperature sensitivity was not identified. Neither an allele containing the first four mutations of spc110–221 (M15T, T30M, D62E, and S67P) nor an allele containing the last five mutations of spc110–221 (S94P, K99N, K110E, L119S, and N163Y) confers a temperature-sensitive phenotype. Similarly, an allele encoding either the first three mutations or the last four mutations of spc110–222 does not confer temperature sensitivity. Therefore, we define region I as the entire region between amino acids 1 and 163.
Mutations present in the 11 temperature-sensitive carboxy-terminal alleles (Sundberg et al., 1996) were determined (Table 3B). Two alleles (spc110–223 and spc110–227) were represented twice. Interestingly, 22 of the 29 missense carboxy-terminal mutations are found between amino acids 772 to 836 (Table 3B). Thus, 76% of the mutations map to only 35% of the region mutagenized by PCR (amino acids 761–944). Furthermore, the density of missense mutations in this region is higher than in any other part of the carboxy terminus (Figure 1). This cluster of mutations identifies a new region of Spc110p, which we label region II (amino acids 772–836). Two alleles (spc110–226 and spc110–227) contain mutations only in region II, thus demonstrating that mutations in region II alone are sufficient to confer temperature sensitivity. The leucine at amino acid 836 is mutated in three of the carboxy-terminal alleles, yet an allele containing a mutation only at amino acid 836 (L836H) does not confer temperature sensitivity.
Two additional classes of carboxy-terminal mutations were isolated: 1) mutations that result in truncation of Spc110p; and 2) mutations in the calmodulin-binding region (amino acids 897–917) (Table 3B). We did not explore the role of truncations of Spc110p in conferring temperature sensitivity because previously we demonstrated that conversion of the codon for amino acid 856 to a nonsense codon was not lethal and did not confer a temperature-sensitive phenotype (Geiser et al., 1993). Only one missense mutation was isolated in the region between amino acids 856 and 898, thus further suggesting that this region is unimportant for Spc110p function. Although truncations that remove the calmodulin-binding region are not lethal (Geiser et al., 1993), we and others have shown that point mutations in the calmodulin-binding region confer profound defects in Spc110p function (Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996). In our carboxy-terminal screen, four alleles were identified that contained point mutations in the calmodulin-binding region. We define the third functional region (region III) of Spc110p as the calmodulin-binding region (Figure 1). Only 4 of the 29 missense mutations map outside of both region II and region III (Table 3B).
Six of the mutant spc110 alleles (spc110–220, spc110–221, spc110–222, spc110–224, spc110–225, and spc110–226) were integrated at the SPC110 locus as described in MATERIALS AND METHODS. A strain containing the integrated spc110–222 mutations was able to grow at 37°C; therefore, spc110–222 was not characterized further. Diploid strains homozygous for the carboxy-terminal spc110 alleles die at temperatures 2–4°C cooler than the temperatures at which the corresponding haploid strains die. Yet the amino-terminal spc110–221 diploid and haploid strains cease growth at the same temperature.
Intragenic Complementation of spc110 Alleles
Intragenic complementation can occur between different alleles of a gene encoding a homomultimeric protein (Fincham, 1966). If two alleles confer distinct defects, the hybrid multimer with subunits encoded by both alleles may function better than a homomultimer made up of either mutant protein alone. Given that Spc110p is likely to be a multimer (Kilmartin et al., 1993), we tested for intragenic complementation between the spc110 alleles. The viability of the heteroallelic diploid strains (for example, spc110–220/spc110–221) was compared with the viability of the homoallelic diploid strains (for example, spc110–220/spc110–220 and spc110–221/spc110–221). None of the homoallelic diploid strains grows at 37°C, but several of the heteroallelic diploids were able to grow at 37°C. The intragenic complementation results are summarized in Table 4. All five spc110 alleles are recessive.
Table 4.
Intragenic complementation analysis
| Growth
temperature
(°C)
|
||||||
|---|---|---|---|---|---|---|
| spc110-220 | spc110-225 | spc110-221 | spc110-224 | spc110-226 | SPC110 | |
| spc110-220 | 32 | 34 | 37 | 34 | 34 | 37 |
| spc110-225 | 30 | 37 | 30 | 32 | 37 | |
| spc110-221 | 34 | 34 | 37 | 37 | ||
| spc110-224 | 25 | 30 | 37 | |||
| spc110-226 | 32 | 37 | ||||
| SPC110 | 37 | |||||
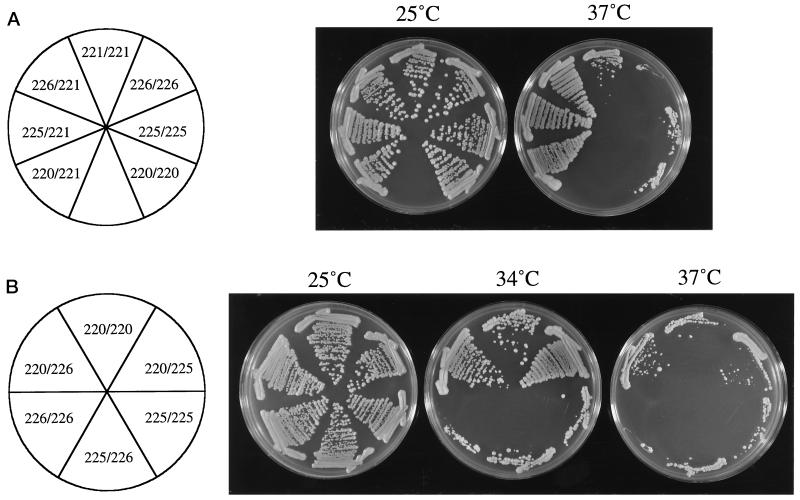
The amino-terminal allele spc110–221 (region I) fully complements the carboxy-terminal alleles, spc110–220 (region III), spc110–225 (regions II and III), and spc110–226 (region II) (Figure 2A), demonstrating that the defect conferred by the spc110–221 allele is different from the defects conferred by the carboxy-terminal alleles. The hybrid multimers made by the heteroallelic diploids function well enough to allow normal growth at 37°C. A haploid yeast strain containing a single gene encoding the mutations of spc110–220 along with the mutations of spc110–221 as the only source of SPC110 is viable at 21°C but dead at 30°C. Therefore, it is the cooperation of Spc110–220p and Spc110–221p molecules to contribute distinct functional domains that confers viability to the heteroallelic diploid.
Figure 2.
Growth of strains demonstrating intragenic complementation. (A) The amino-terminal allele fully complements the carboxy-terminal alleles. Strains HSY21 (spc110–221/spc110–221), HSY79 (spc110–226/spc110–221), HSY83 (spc110–226/spc110–226), HSY42 (spc110–225/spc110–221), HSY48 (spc110–225/spc110–225), HSY31 (spc110–220/spc110–221), and HSY20 (spc110–220/spc110–220) were plated onto YPD and incubated at the indicated temperatures for 3 days. (B) Partial complementation exists between the spc110–226 (region II) and spc110–220 (region III) alleles. Strains HSY20 (spc110–220/spc110–220), HSY39 (spc110–220/spc110–225), HSY48 (spc110–225/spc110–225), HSY82 (spc110–225/spc110–226), HSY83 (spc110–226/spc110–226), and HSY78 (spc110–220/spc110–226) were plated onto YPD and incubated at the indicated temperatures for 3 days.
None of the carboxy-terminal alleles fully complements any of the other carboxy-terminal alleles. However, the spc110–220/spc110–226 and the spc110–220/spc110–225 heteroallelic diploids are able to grow substantially better than the homoallelic diploids (Figure 2B), indicating that spc110–220 (region III) confers a defect different from that of the other carboxy-terminal missense alleles. The spc110–225 allele and the spc110–226 allele do not complement each other; therefore, they confer a common defect.
The spc110–224 allele only shows complementation, albeit slight, with the spc110–220 allele (Table 4). Western blot analysis reveals that Spc110–224p is present at roughly 10% of the level of wild-type Spc110p, even at the permissive temperature of 22°C. Perhaps the slight intragenic complementation seen between spc110–220 and spc110–224 is the result of enhancement of the solubility of Spc110–220p by Spc110–224p and prevention of its aggregation with other pole components. Presumably, the low levels of Spc110–224p result in the null behavior of the spc110–224 allele with regard to the other alleles. The other mutant Spc110p proteins are present at levels within twofold the levels of wild-type Spc110p. Furthermore, the other spc110 alleles are capable of complementation, thus confirming that they are not behaving as nulls at the nonpermissive temperature.
Calmodulin Localization
Calmodulin localization at the SPB is dependent on the calmodulin-binding site of Spc110p (Spang et al., 1996b; Sundberg and Davis, unpublished results). Previously, we demonstrated by immunoelectron microscopy that calmodulin levels at the SPB decrease at the nonpermissive temperature in a diploid strain containing Spc110–220p, which has a point mutation in the calmodulin-binding region. At the time that work was published, we were unable to detect calmodulin at the SPB in the spc110–220 mutant cells by indirect immunofluorescence, thus necessitating our use of immunoelectron microscopy (Sundberg et al., 1996). We have modified the methanol/acetone fixation technique (see MATERIALS AND METHODS) to enable us to examine calmodulin localization at the SPB in diploid strains containing spc110 alleles by indirect immunofluorescence. Calmodulin localization at the SPB is perturbed at the nonpermissive temperature in strains in which Spc110p has a point mutation in the calmodulin-binding site but normal in strains with missense mutations elsewhere in Spc110p. After incubation for 1 h at 37°C, calmodulin colocalizes with Spc98p in 99% of the wild-type cells compared with only 48% of the spc110–220 mutant cells and with 87% of the spc110–225 mutant cells. Thus, calmodulin localization to the SPB appears to be only slightly defective in the spc110–225 strain as compared with the spc110–220 strain. In the spc110–220/spc110–225 heteroallelic diploid, calmodulin colocalizes with Spc98p in 61% of the mutant cells, suggesting that the hybrid multimer formed by Spc110–220p and Spc110–225p remains defective in binding calmodulin. Calmodulin localization at the SPB in the spc110–221 and spc110–226 strains appears the same as in a wild-type strain after 1 h of incubation at 37°C.
Dosage-dependent Suppression of spc110 Alleles
Because calmodulin binding was defective in two of the mutants, we determined whether the temperature sensitivity of strains containing spc110 alleles could be suppressed by overproduction of calmodulin. A high-copy-number plasmid encoding a 17-fold excess of calmodulin allows an spc110–220 homozygous diploid to grow at 37°C (Sundberg et al., 1996). Similar overproduction of calmodulin allows partial suppression of the temperature sensitivity conferred by spc110–225, indicating that Spc110–225p is impaired not only in calmodulin binding but also in an additional function unrelated to calmodulin binding (Table 5). In support of this hypothesis, the V912E mutation of spc110–225 alone does not produce a temperature-sensitive phenotype, whereas the C911R mutation of spc110–220 alone is temperature sensitive. Overexpression of CMD1 (the gene encoding calmodulin) from a high-copy-number plasmid allows growth of the spc110–220/spc110–225 and the spc110–220/spc110–226 heteroallelic diploid strains at 37°C (Table 5), indicating that calmodulin binding is the only defect remaining in these strains. Overproduction of calmodulin does not suppress the temperature sensitivity conferred by spc110–221 or spc110–226, which do not encode mutations in the calmodulin-binding site or alter calmodulin localization at the SPB (Table 5). Spc110–224p is truncated to remove the calmodulin-binding site and thus calmodulin is not found at the SPB in the spc110–224 strain. As expected, overproduction of calmodulin does not suppress the spc110–224 mutant strain (Table 5).
Table 5.
Multicopy suppression of spc110 alleles
| Growth temperature
(°C)
|
||||
|---|---|---|---|---|
| 2 μm vector | 2 μm CMD1 | 2 μm SPC98 | 2 μm SPC110 | |
| spc110-220/spc110-220 | 32 | 37 | 32 | 37 |
| spc110-225/spc110-225 | 30 | 34 | 30 | 37 |
| spc110-226/spc110-226 | 32 | 32 | 32 | 37 |
| spc110-220/spc110-225 | 34 | 37 | 34 | 37 |
| spc110-220/spc110-226 | 34 | 37 | ND | 37 |
| spc110-225/spc110-226 | 32 | 34 | ND | 37 |
| spc110-221/spc110-221 | 34 | 34 | 37 | 37 |
| spc110-224/spc110-224 | 25 | 25 | 25 | 37 |
ND, not determined.
Next we examined whether the temperature-sensitive phenotypes conferred by the spc110 alleles could be suppressed by overexpression of SPC98, SPC97, or TUB4, genes encoding known inner plaque components. The temperature sensitivity of spc110–221 is suppressed by overexpression of SPC98 (Table 5) but not by overexpression of TUB4 or SPC97. Overexpression of SPC98 does not affect the temperature sensitivity conferred by any of the other alleles.
Cell Cycle-specific Defects of the Temperature-sensitive Mutants
The previous results argue that missense mutations in each region of Spc110p confer distinct defects. The nature of the defects conferred by each allele was analyzed in detail. Homozygous diploid strains were synchronized in early G1 by centrifugal elutriation and released at the nonpermissive temperature of 37°C. Analysis by electron microscopy revealed that SPB duplication occurs in all four strains examined (spc110–220, spc110–225, spc110–226, and spc110–221). All four mutant strains progressed through S phase, completed bud emergence, and completed bud growth at rates similar to those of a wild-type strain. After 2 h of incubation at 37°C, all mutant strains had accumulated large buds and a G2 content of DNA and remained in this state after 4 h of incubation at 37°C. 4′,6-Diamidino-2-phenylindole staining revealed that in the majority of the mutant cells the DNA had not segregated as expected for a classic arrest at G2-M. However, tubulin immunofluorescence and/or electron microscopy at late times revealed that although cells containing the amino-terminal allele spc110–221 exhibited the short spindle of a G2-M arrest, cells containing the carboxy-terminal alleles spc110–226, spc110–225, or spc110–220 frequently contained broken spindles. The viability of the synchronous mutant cultures was determined by their ability to form colonies when returned to the permissive temperature. Consistent with a classic G2-M arrest, the spc110–221 diploid strain shows little loss of viability at the nonpermissive temperature. In contrast, strains containing the carboxy-terminal mutant alleles suffer irreversible damage (Figure 3).
We monitored spindle formation in the synchronous shift experiments using immunofluorescent staining with antibodies directed against Spc98p (gift of J. Kilmartin). An unbudded wild-type cell has a single SPB and thus only one dot of anti-98 kDa staining. Duplicated, paired side-by-side SPBs also appear as a single dot of anti-98 kDa staining. However, when a short spindle is formed (at about the time when the bud diameter is one-third the diameter of the mother), the anti-98 kDa staining resolves the two SPBs as two dots of staining. Spindle formation as measured by two dots of anti-98 kDa staining in the diploid spc110–221, spc110–225, and spc110–226 strains occurs 10–15 min after spindle formation in a wild-type strain (Figure 4). In the spc110–220 mutant strain, the IMO, which contains Spc98p, Spc110–220p, and Tub4p (our unpublished observations), forms during SPB duplication, resulting in the appearance of a second dot of Spc98p staining before spindle formation as detected by electron microscopy (Sundberg et al., 1996). Strains containing the amino-terminal allele spc110–221 and the carboxy-terminal alleles spc110–225 and spc110–226 do not exhibit extra dots of anti-98 kDa staining early and thus do not form IMOs. Therefore, the spc110–221, spc110–226, and spc110–225 mutant strains behave similarly during SPB duplication and spindle formation, but further examination of the mutant strains revealed differences that occur later in the cell cycle.
Analysis of spc110–221: Mutations in Region I
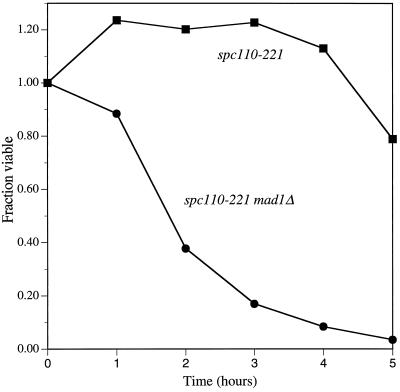
No cytological defect in an spc110–221 strain was detected when cells were synchronously shifted to the nonpermissive temperature. Examination by electron microscopy of spc110–221 cells (n = 24) at early times after the shift to 37°C revealed normal-appearing short spindles and SPBs. Immunofluorescent staining revealed that Spc110–221p and calmodulin colocalized with Spc98p. No extra immunofluorescent dots of spindle pole component staining were seen before spindle formation, and no aberrant structures were detected after spindle formation. However, the spc110–221 cells do arrest, failing to enter another cell cycle. We tested whether the arrest of an spc110–221 strain was dependent on the spindle assembly checkpoint gene MAD1 (Li and Murray, 1991; Hardwick and Murray, 1995). An spc110–221 mad1Δ strain loses viability rapidly at the nonpermissive temperature compared with an spc110–221 strain (Figure 5). Therefore, Mad1p is required to check the defect conferred by spc110–221 at the nonpermissive temperature.
Figure 5.
spc110–221 defect is checked by MAD1. Asynchronous cultures of an spc110–221 strain (▪) and an spc110–221 mad1Δ strain (•) were shifted to the nonpermissive temperature of 37°C at t = 0 h. At regular intervals, aliquots were removed, sonicated, and titered for colony-forming units at 21°C on YPD. A mad1Δ strain behaved like the wild-type control strain (our unpublished observations).
Analysis of spc110–226: Mutations in Region II
All of the mutations of spc110–226 are in region II. The complementation analysis revealed that the spc110–226/spc110–220 heteroallelic diploid can live at a higher temperature than either homoallelic diploid, suggesting that the mutations in region II confer a different defect than do mutations in the calmodulin-binding site. Consistent with this idea, analysis of the phenotype conferred by spc110–226 revealed several differences from the phenotype conferred by spc110–220. A synchronous population of spc110–226 mutant cells shifted to the nonpermissive temperature suffers the worst lethality of all the temperature-sensitive alleles (Figure 3). Cell death begins later in an spc110–226 strain than in an spc110–220 strain (Figure 3C, t = 0–45 min). Electron microscopy at t = 12 min revealed that the spc110–226/spc110–226 mutant cells do not contain IMOs. Six of eight cells at this time contained normal short complete spindles (Figure 6A), and the other two cells contained duplicated SPBs, paired in the side-by-side configuration. Although spindle formation appeared normal early in the cell cycle (Figure 6B), an aberrant third structure that stains with anti-98 kDa antibodies is seen at late times after spindle formation (Figure 6C). At t = 58 min, 9% of the cells contain a third dot of 98-kDa staining. At t = 103 min, 52% of the cells contain three or more dots of 98-kDa staining (Figure 6D). In addition to containing Spc98p, the majority of the aberrant structures also contain Spc110–226p (Figure 6C) and calmodulin (our unpublished observations) as determined by indirect immunofluorescence. The aberrant structures that did not contain Spc110–226p or calmodulin were often seen in the cytoplasm, suggestive of an outer plaque that has become disassociated from the remainder of the SPB.
Examination of the spc110–226 mutant cells by electron microscopy at t = 42 min revealed that four of six nuclei had short complete spindles and two nuclei had monopolar spindles. One of the nuclei with a monopolar spindle had what appeared to be a portion of an SPB nucleating microtubules from within the nucleoplasm, not from the nuclear envelope. At t = 102 min, 9 of 11 nuclei examined contained only a single SPB and 2 nuclei had broken spindles, the microtubules of which were organized by SPBs that appeared structurally compromised. Two of the nine nuclei with monopolar spindles again had what appeared to be a portion of an SPB nucleating microtubules from within the nucleoplasm. Structures corresponding to the extra dots of Spc98p staining seen so strikingly by immunofluorescence were not visible by electron microscopy. These results suggest that one of the SPBs disintegrates after spindle formation, resulting in the appearance of a monopolar spindle by electron microscopy. The spindle components Spc110–226p, calmodulin, and Spc98p may continue to associate leading to the extra dots of immunofluorescent staining, although the second SPB proper no longer exists.
Analysis of spc110–225: Mutations in Regions II and III
The spc110–225 allele has mutations in both region II and region III and causes defects associated with both regions. Calmodulin localization at the SPB at the nonpermissive temperature in an spc110–225 diploid strain is slightly perturbed compared with a wild-type strain. Furthermore, overexpression of CMD1 partially suppresses the temperature-sensitive phenotype of spc110–225. The spc110–226/spc110–225 heteroallelic diploid cannot live at a higher temperature than either homoallelic diploid, suggesting that the spc110–225 allele is also defective in the function of Spc110p associated with region II. The spc110–225 cells do not exhibit the additional dots of anti-98 kDa staining indicative of the presence of an IMO before the time of spindle formation. SPBs and spindle morphologies were analyzed by electron microscopy. At t = 21 min, short complete spindles were present in six nuclei, and a monopolar spindle was observed in one nucleus. If the defect caused by Spc110–225p were similar to the defect of Spc110–220p, IMOs would have been detectable in this time point. No IMOs were observed although three of the seven nuclei contained a single electron-dense structure that did not nucleate microtubules. No extra dots of anti-98 kDa staining (analogous to the extra dots of staining observed in the spc110–226 strain) were detected after spindle formation in the spc110–225 mutant strain. However, one cell with a monopolar spindle similar to those produced by the spc110–226 strain was observed by electron microscopy. Additionally, tubulin immunofluorescence of an asynchronous spc110–225 mutant culture at late times after the shift to 37°C revealed broken spindles similar to those observed in spc110–220 and spc110–226 mutant strains.
spc110–225 has three mutations: isoleucine 828 to leucine (I828L), valine 912 to glutamic acid (V912E), and arginine 934 to lysine (R934K). The V912E mutation resides in the calmodulin-binding site yet alone does not produce a temperature-sensitive phenotype. The V912E mutation in combination with the R934K mutation also does not confer temperature sensitivity. The I828L mutation alone also does not have a temperature-sensitive phenotype. Thus, the spc110–225 allele requires at least I828L and one, if not both, of the other mutations for temperature sensitivity. Our results suggest that the mutations of spc110–225 cause both mild calmodulin-binding defects similar to the defects conferred by spc110–220 and mild spindle integrity defects similar to the defects conferred by spc110–226.
Execution of Essential Spc110p Carboxy-terminal Functions Occurs after Hydroxyurea Block
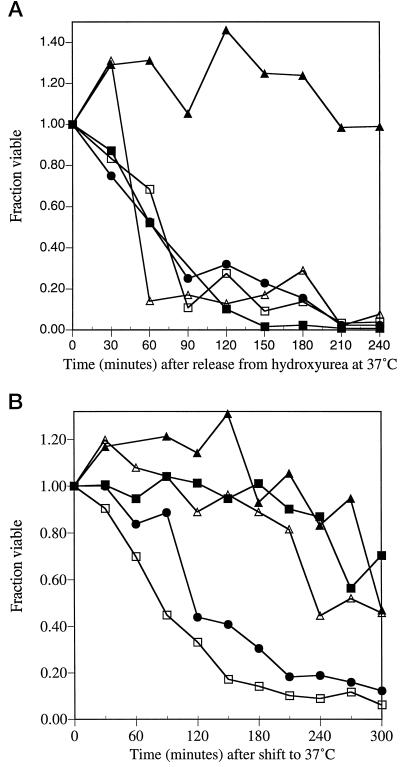
Analysis of the spc110 mutant strains suggested functions for Spc110p during mitosis. If the mitotic functions of Spc110p are the essential functions of Spc110p, mutant cultures that are blocked before mitosis at the nonpermissive temperature should remain viable. Haploid cultures of the five spc110 mutant strains (spc110–221, spc110–220, spc110–225, spc110–226, and spc110–224) were synchronized by elutriation and released into hydroxyurea, thus blocking DNA replication and preventing mitosis. All of the spc110 mutant strains remained viable in hydroxyurea at 37°C as well as at the permissive temperature of 22°C, indicating that the execution point of the carboxy-terminal Spc110p functions occurs after the hydroxyurea block. Confirmation that the execution point is after the hydroxyurea block came from the reciprocal experiment. When asynchronous cultures of the five spc110 mutant strains were blocked in hydroxyurea at the permissive temperature and then released at the nonpermissive temperature, the strains with carboxy-terminal mutant alleles died immediately, not entering another cell cycle (Figure 7A). The strain with the amino-terminal mutant allele spc110–221 also did not enter another cell cycle and maintained the large budded arrest (Figure 7A).
Figure 7.
Execution of essential Spc110p carboxy-terminal functions occurs after hydroxyurea block. (A) Carboxy-terminal spc110 mutant strains die in the first cell cycle when released from hydroxyurea at the nonpermissive temperature. The amino-terminal spc110–221 strain remains viable. Asynchronous cultures of the five spc110 mutant strains were blocked in hydroxyurea (0.05 M) at the permissive temperature and then released at the nonpermissive temperature at t = 0 min. ▴, fraction of spc110–221 cells viable; □, fraction of spc110–220 cells viable; •, fraction of spc110–225 cells viable; ▪, fraction of spc110–226 cells viable; ▵, fraction of spc110–224 cells viable. (B) Elutriated spc110–220 and spc110–225 mutant cultures die when released into α-factor at 37°C. Synchronous populations of haploid spc110–221, spc110–220, spc110–225, spc110–226, and spc110–224 mutant cultures (from strains HSY14, HSY10, HSY9, HSY91, and HSY8, respectively) were obtained by centrifugal elutriation as described in MATERIALS AND METHODS and shifted to 37°C in the presence of 10 μg/ml α-factor at t = 0 min. At regular intervals, aliquots were removed, sonicated, and titered for colony-forming units at 21°C on YPD. ▴, fraction of spc110–221 cells viable; □, fraction of spc110–220 cells viable; •, fraction of spc110–225 cells viable; ▪, fraction of spc110–226 cells viable; ▵, fraction of spc110–224 cells viable.
The five elutriated haploid spc110 mutant cultures were also released into α-factor at 37°C and 22°C. All of the mutant cultures remained viable in α-factor at the permissive temperature of 22°C. In agreement with previous results (Kilmartin and Goh, 1996), alleles with point mutations in the calmodulin-binding site (region III), cause rapid loss of viability in α-factor at 37°C. The other mutant strains remained viable in α-factor at 37°C (Figure 7B).
DISCUSSION
Analysis of the temperature-sensitive spc110 alleles defined two new functional regions of Spc110p. Region I is located at the amino terminus of Spc110p. Region II (amino acids 772–836) extends beyond the carboxy-terminal end of the predicted coiled coil. Mutations in either region confer distinct defects that differ from those conferred by mutations in the calmodulin-binding region (amino acids 897–917), which we refer to as region III. Our results strongly support the hypothesis that Spc110p links the γ-tubulin complex to the central plaque of the SPB.
The amino-terminal allele (spc110–221) fully complements all of the carboxy-terminal alleles. Intragenic complementation between two alleles can occur if the alleles affect different functions of the protein. Alternatively, complementing alleles can cause different defects in a single function such that together the alleles reconstitute the single function. Although the amino and carboxy termini of Spc110p function together to provide an essential structure of the SPB, we favor the idea that the amino- and carboxy-terminal regions are responsible for different aspects of this common function for two reasons. First, the phenotype conferred by the amino-terminal allele is entirely different from the phenotypes conferred by the carboxy-terminal alleles. The amino-terminal allele causes a fully reversible cell cycle arrest with spindles that appear normal, whereas strains carrying the carboxy-terminal alleles suffer irreversible spindle damage. Second, the amino terminus is localized in the inner plaque, 700 Å away from the carboxy terminus in the central plaque of the SPB (Bullitt et al., 1997). Clues about the function of the amino terminus come from the dosage-dependent suppression analysis. Overexpression of SPC98 suppresses the temperature-sensitive phenotype conferred by spc110–221, but not the phenotype conferred by any of the other alleles. Thus, the amino terminus of Spc110p appears to be involved in an interaction with the γ-tubulin complex composed of Spc98p, Spc97p, and Tub4p.
Random mutagenesis of the carboxy terminus of Spc110p revealed that temperature-sensitive mutations cluster in two regions, designated region II and region III. Previous deletion studies demonstrated that a region at the carboxy-terminal end of the coiled coil is important for viability (Geiser et al., 1993; Kilmartin et al., 1993). Kilmartin et al. (1993) created a series of deletions of the central-rod region of Spc110p. A deletion of amino acids 216–710 of Spc110p allows growth, albeit slow, showing that most of the coiled-coil rod region is not essential. A deletion of amino acids 489–809 produces a severe growth phenotype, and a deletion of amino acids 489–824 is lethal (Kilmartin et al., 1993). Truncation of Spc110p at amino acid 828 is lethal whereas truncation of Spc110p at amino acid 856 supports growth of an spc110Δ strain (Geiser et al., 1993). Combining the two sets of results argues that a region between amino acids 711 and 856 is important for viability. Clustering of carboxy-terminal temperature-sensitive mutations in region II between amino acids 772 to 836 further delineates the region important for Spc110p function.
The intragenic complementation analysis indicates that mutations in region II cause a defect that is different from the defect caused by the point mutation in the calmodulin-binding site (region III) of Spc110–220p. These different defects result in distinct phenotypes. First, an spc110–220 strain (region III) suffers considerable lethality when released into α-factor at the nonpermissive temperature, whereas an spc110–226 strain (region II) remains viable. Second, overproduction of calmodulin does not suppress the temperature-sensitive phenotype of the spc110–226 mutant strain. Third, calmodulin and Spc110–226p assemble correctly at the new SPB, whereas in the spc110–220 mutant strain, spindle pole components misassemble into the IMO as the nascent SPB is forming. Finally, after spindle formation has been completed in the spc110–226 mutant (region II), one of the SPBs appears to disintegrate, resulting in a monopolar spindle as determined by electron microscopy. The fragmented SPBs as visualized by immunofluorescence contain Spc110–226p and calmodulin in addition to Spc98p, suggesting that delamination from the central plaque of the SPB is occurring at the carboxy terminus of Spc110–226p. Thus, the region between amino acids 772–836 is required to maintain SPB integrity after the spindle has formed, possibly when the spindle is attempting to elongate. Perhaps the connection of Spc110–226p to another pole component required for mitosis is not properly made at the nonpermissive temperature. The fact that only one of the SPBs disintegrates at the nonpermissive temperature could be explained if the mother SPB, which was assembled under permissive conditions, is more stable than the new pole, which was assembled under nonpermissive conditions.
Calmodulin binding to region III in the carboxy terminus of Spc110p is essential for function (Geiser et al., 1993; Stirling et al., 1994; Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996). Detailed characterizations of the phenotypes conferred by five alleles with at least one mutation in the calmodulin-binding site have been presented (Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996; and this article). Each allele causes lethality during mitosis. We and Kilmartin and Goh (1996) demonstrated that the execution point of Spc110p was after the formation of the short spindle that appears in cells arrested by treatment with hydroxyurea. Furthermore, we showed that even if the SPB is assembled at the permissive temperature, a release from hydroxyurea at the nonpermissive temperature results in death in the first cell cycle. Four of the five region III alleles result in the appearance of broken spindles. Kilmartin and Goh (1996) observed a reduced number of microtubules associated with the SPBs, suggesting that mutations in region III interfere with attachment of Spc110p to the pole. The disruption of the calmodulin-Spc110p interaction by our spc110–220 allele also leads to the misassembly of spindle components into the IMO. One way to explain these observations is to propose that the assembly of Spc110p requires the self-association of Spc110p as well as the attachment of Spc110p to the SPB. By this line of reasoning, the IMO forms because mutations in the calmodulin-binding site interfere with attachment of Spc110p to the SPB but do not prevent the self-association of molecules of Spc110p, which therefore assemble in the nucleoplasm.
Interestingly, although point mutations in the calmodulin-binding site (region III) lead to lethal phenotypes (Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996), truncations of Spc110p that remove the calmodulin-binding site are able to support growth of an spc110Δ strain (Geiser et al., 1993). Therefore, deletion of the calmodulin-binding site in Spc110p relieves a requirement for calmodulin. This is reminiscent of the majority of calmodulin-activated enzymes in which the calmodulin-binding site overlaps an inhibitory region. When calmodulin is not bound, the inhibitory region binds at or near the active site and inhibits the enzyme. When calmodulin binds, the inhibitory region is removed from the active site and the enzyme is active. Truncations of the enzyme that remove the calmodulin-binding site also remove the inhibitory region, thereby constitutively activating the enzyme. For example, truncation of phosphorylase b kinase before the two calmodulin-binding sites yields a highly active, calmodulin-independent enzyme (Harris et al., 1990). Furthermore, the phosphorylase b kinase calmodulin-binding sites also include the inhibitory region because synthetic peptides corresponding to the calmodulin-binding sites bind to the enzyme and inhibit its activity (Dasgupta and Blumenthal, 1995).
By analogy to calmodulin-activated enzymes, one hypothesis proposes that calmodulin binding to Spc110p is required to reveal region II of Spc110p essential for SPB integrity. If this model were true, mutations in the calmodulin-binding site of Spc110p (region III) that perturb calmodulin binding would cause region II to remain blocked from performing its essential functions relating to SPB integrity. This hypothesis predicts that mutations in region III should lead to defects in SPB integrity. We have shown that both region II and region III alleles have a similar execution point after formation of the short spindle. However, none of the region III alleles leads to the disintegration of an SPB exhibited by the region II allele spc110–226. This could simply represent a difference in the severity of the alleles or region II may not be the region revealed by calmodulin binding to Spc110p.
Recent analysis of the structure of the SPB by cryoelectron microscopy shows a conserved multilayered vertical architecture (Bullitt et al., 1997). The coiled coil of Spc110p forms a fibrous layer on the nucleoplasmic side of the central plaque with the carboxy terminus of Spc110p anchored in the central plaque (Kilmartin and Goh, 1996; Sundberg et al., 1996). Our results support the hypothesis that Spc110p links the γ-tubulin complex in the inner plaque to the central plaque of the SPB. Moreover, analysis of the phenotypes conferred by mutations in different regions of Spc110p suggest four steps in the assembly of Spc110p: 1) initial attachment of Spc110p to the central plaque, 2) self-association of Spc110p, 3) stabilization of the attachment of Spc110p to the central plaque, and 4) attachment of the γ-tubulin complex to the amino terminus of Spc110p. Future studies will aim at dissecting the order and timing of these four steps in Spc110p assembly in wild-type cells. Moreover, calmodulin is a component of the mammalian centrosome (Willingham et al., 1983), so it should be of interest to determine whether a mammalian homologue of Spc110p exists.
ACKNOWLEDGMENTS
We thank Susan Francis for critical reading of the manuscript. We are indebted to John Kilmartin for anti-98 kDa antibodies and to Tim Stearns for plasmid pTS477 encoding TUB4 and for anti-Tub4p antibodies. We gratefully acknowledge Tom Alber for performing the PAIRCOIL and COILER analysis of Spc110p. We thank Loretta Goetsch for advice regarding electron microscopy. We thank Breck Byers and Robin Wright for helpful discussions. This work was supported by National Institutes of Health grant GM40506 (T.N.D.). H.A.S. was supported by Public Health Service National Research Service Award T32 GM07270, National Institute of General Medical Sciences.
Footnotes
Abbreviations used: SPB, spindle pole body; IMO, intranuclear microtubule organizer; kb, kilobase.
REFERENCES
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Bullitt E, Rout MP, Kilmartin JV, Akey CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–1086. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Byers B. Cytology of the yeast life cycle. In: Strathern JN, Jones EW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1981. pp. 59–96. [Google Scholar]
- Dasgupta M, Blumenthal DK. Characterization of the regulatory domain of the gamma-subunit of phosphorylase kinase. The two noncontiguous calmodulin-binding subdomains are also autoinhibitory. J Biol Chem. 1995;270:22283–22289. doi: 10.1074/jbc.270.38.22283. [DOI] [PubMed] [Google Scholar]
- Davis T. Genetic analysis of calcium-binding proteins in yeast. In: Smith VL, Dedman JR, editors. Stimulus Response Coupling: The Role of Intracellular Calcium-binding Proteins. Boston: CRC Press; 1990. pp. 237–249. [Google Scholar]
- Davis TN. A temperature-sensitive calmodulin mutant loses viability during mitosis. J Cell Biol. 1992;118:607–617. doi: 10.1083/jcb.118.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham JRS. Genetic Complementation. New York: W.A. Benjamin, Inc.; 1966. [Google Scholar]
- Friedman DB, Sundberg HA, Huang E, Davis TN. The 110-kD spindle pole body component of Saccharomyces cerevisiae is a phosphoprotein that is modified in a cell cycle-dependent manner. J Cell Biol. 1996;132:903–914. doi: 10.1083/jcb.132.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, Sundberg HA, Chang BH, Muller EGD, Davis TN. The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7913–7924. doi: 10.1128/mcb.13.12.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, van-Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Murray AW. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WR, Malencik DA, Johnson CM, Carr SA, Roberts GD, Byles CA, Anderson SR, Heilmeyer LM, Jr, Fischer EH, Crabb JW. Purification and characterization of catalytic fragments of phosphorylase kinase gamma subunit missing a calmodulin-binding domain. J Biol Chem. 1990;265:11740–11745. [PubMed] [Google Scholar]
- Kilmartin JV, Dyos SL, Kershaw D, Finch JT. A spacer protein in the Saccharomyces cerevisiae spindle pole body whose transcript is cell cycle-regulated. J Cell Biol. 1993;123:1175–1184. doi: 10.1083/jcb.123.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Goh P-Y. Spc110p: assembly properties and role in the connection of nuclear microtubules to the yeast spindle pole body. EMBO J. 1996;15:4592–4602. [PMC free article] [PubMed] [Google Scholar]
- Knop M, Pereira G, Geissler S, Grein K, Schiebel E. The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 1997;16:1550–1564. doi: 10.1093/emboj/16.7.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Marschall LG, Jeng RL, Mulholland J, Stearns T. Analysis of Tub4p, a yeast γ-tubulin-like protein: implications for microtubule-organizing center function. J Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayan C, Copeland CS, Snyder M. The NUF1 gene encodes an essential coiled-coil related protein that is a potential component of the yeast nucleoskeleton. J Cell Biol. 1992;116:1319–1332. doi: 10.1083/jcb.116.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Muller EGD. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991;266:9194–9202. [PubMed] [Google Scholar]
- Rout MP, Kilmartin JV. Components of the yeast spindle and spindle pole body. J Cell Biol. 1990;111:1913–1927. doi: 10.1083/jcb.111.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel SG, Snyder M. A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol. 1995;131:1775–1788. doi: 10.1083/jcb.131.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E. γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J Cell Biol. 1996a;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Grein K, Schiebel E. The spacer protein Spc110p targets calmodulin to the central plaque of the yeast spindle pole body. J Cell Sci. 1996b;109:2229–2237. doi: 10.1242/jcs.109.9.2229. [DOI] [PubMed] [Google Scholar]
- Stirling DA, Rayner TF, Prescott AR, Stark MJR. Mutations which block the binding of calmodulin to Spc110p cause multiple mitotic defects. J Cell Sci. 1996;109:1297–1310. doi: 10.1242/jcs.109.6.1297. [DOI] [PubMed] [Google Scholar]
- Stirling DA, Welch KA, Stark MJR. Interaction with calmodulin is required for the function of Spc110p, an essential component of the yeast spindle pole body. EMBO J. 1994;13:4329–4342. doi: 10.1002/j.1460-2075.1994.tb06753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg HA, Goetsch L, Byers B, Davis TN. Role of calmodulin and Spc110p interaction in the proper assembly of spindle pole body components. J Cell Biol. 1996;133:111–124. doi: 10.1083/jcb.133.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham MC, Wehland J, Klee CB, Richert ND, Rutherford AV, Pastan IH. Ultrastructural immunocytochemical localization of calmodulin in cultured cells. J Histochem Cytochem. 1983;31:445–461. doi: 10.1177/31.4.6338107. [DOI] [PubMed] [Google Scholar]
- Woolfson DN, Alber T. Predicting oligomerization states of coiled coils. Protein Sci. 1995;4:1596–1607. doi: 10.1002/pro.5560040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Muller EGD, Amacher SL, Northrop JL, Davis TN. A dosage-dependent suppressor of a temperature-sensitive calmodulin mutant encodes a protein related to the fork head family of DNA-binding proteins. Mol Cell Biol. 1993;13:1779–1787. doi: 10.1128/mcb.13.3.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]