Abstract
The rod and cone cells of the mammalian retina are the principal photoreceptors for image-forming vision. They transmit information by means of a chain of intermediate cells to the retinal ganglion cells, which in turn send signals from the retina to the brain. Loss of photoreceptor cells, as happens in a number of human diseases, leads to irreversible blindness. In a mouse model (rd/rd) of photoreceptor degeneration, we used a viral vector to express in a large number of retinal ganglion cells the light sensitive protein melanopsin, normally present in only a specialized subset of the cells. Whole-cell patch–clamp recording showed photoresponses in these cells even after degeneration of the photoreceptors and additional pharmacological or Cd2+ block of synaptic function. Interestingly, similar responses were observed across a wide variety of diverse types of ganglion cell of the retina. The newly melanopsin-expressing ganglion cells provided an enhancement of visual function in rd/rd mice: the pupillary light reflex (PLR) returned almost to normal; the mice showed behavioral avoidance of light in an open-field test, and they could discriminate a light stimulus from a dark one in a two-choice visual discrimination alley. Recovery of the PLR was stable for at least 11 months. It has recently been shown that ectopic retinal expression of a light sensitive bacterial protein, channelrhodopsin-2, can restore neuronal responsiveness and simple visual abilities in rd/rd mice. For therapy in human photodegenerations, channelrhodopsin-2 and melanopsin have different advantages and disadvantages; both proteins (or modifications of them) should be candidates.
Most retinal ganglion cells receive input from rods and cones and most of them project to the brain regions involved in image-forming vision. A small subset of ganglion cells are also intrinsically photosensitive (1–5). They express a light-sensitive protein, melanopsin, which may also function as a photoisomerase to regenerate its active retinal-based chromophore. Photoactivation of melanopsin leads, by a series of intermediate steps that are poorly understood, to opening of a cation channel in the ganglion cell membrane and generation of action potentials. A great majority of the melanopsin-expressing retinal ganglion cells project to the suprachiasmatic nucleus, a brain center regulating circadian rhythms. Accordingly, they have a major role in entraining the circadian clock to the ambient lighting conditions but have a negligible role in image-forming vision.
When expressed experimentally, melanopsin remains capable of initiating a response to light in various neuronal and nonneuronal cells (1, 3, 6–9). Here, we had three questions. First, would melanopsin mediate a response to light by retinal ganglion cells that do not normally express it? Melanopsin signals to the plasma membrane by intermediates. Such a signaling pathway is clearly present in the native melanopsin ganglion cells, but its presence in other types of retinal ganglion cells is not certain.
A related question was as follows. If the cells do posses a signaling system, so that ectopic expression of melanopsin causes ganglion cells to become intrinsically sensitive to light, does the normal synaptic physiology (i.e., the functional type) of a ganglion cell influence the responses initiated by melanopsin? Retinal ganglion cells have varied morphologies and distinct physiological responses; this diversity is accompanied by different assemblies of receptors and ion channels. The individual types of retinal ganglion cells have strikingly different responses to light (ON, OFF, sustained, transient, etc). How much of this distinctiveness is intrinsic to the individual cell, and how much depends on the way in which the cell is excited?
Last, can otherwise blind mice employ signals initiated by ectopically-expressed melanopsin for any useful form of vision? Channelrhodopsin-2 expressed in ON bipolar cells conferred responses to light on retinal ganglion cells and these responses led to an ability of the animal to respond to light behaviorally (10). However, it is not clear that channelrhodopsin-2 is the ideal molecule for therapy of photoreceptor degenerations: channelrhodopsin-2 requires stimulation with short-wavelength light at very high intensity, a potentially toxic combination (10, 11). In contrast, the intermediate signaling used by melanopsin could potentially amplify the light signal beyond the sensitivity imparted by the unamplified channelrhodopsin-2 molecule. Melanopsin also has limitations, notably those associated with its slow response to light. However, a positive result with melanopsin would extend the general principle of photoreceptor substitution, by using a light-sensitive molecule that operates by a quite different mechanism, and would provide an alternative candidate protein for possible therapeutic use. More generally, it would imply that various photosensitive molecules could serve this purpose, encouraging a search for specifically designed new molecules, or modification of existing ones.
Results
We used adeno-associated virus (AAV) to ectopically express mouse melanopsin in the retina of rd mice homozygous for the Pde6brd1 mutation (12, 13). This gene codes for a specific cGMP phosphodiesterase present exclusively in rod photoreceptors. These cells in the retina of rd/rd mice begin to degenerate soon after their terminal differentiation and are essentially absent by postnatal day 30 (P30). Cone photoreceptors subsequently degenerate, and all but a very small residual subset in the peripheral retina are lost by P90 (14–16). At ≈P80, we injected intravitreally one of three viral constructs: AAV-Opn4, coding for the melanopsin protein; AAV-Opn4-IRES-EGFP, coding for melanopsin protein and EGFP; and AAV-GFP, coding for the green fluorescent protein alone. Because we planned whole-animal behavioral testing of the animals, both eyes of a mouse were injected with the same construct.
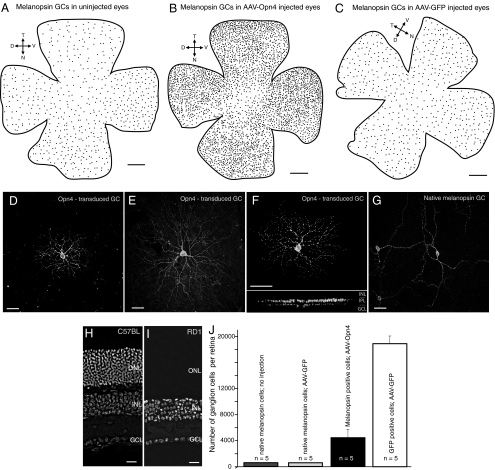
Four weeks later, we studied the retinas morphologically and electrophysiologically and evaluated the visual behavior of the mice. The testing occupied approximately two weeks. All of the constructs were expressed in retinal neurons (Fig. 1). The most effective, as judged by the number of cells transduced, brightness of GFP, and/or intensity of melanopsin immunostaining, was AAV-GFP (18,906 ± 1,184 GFP-expressing cells per retina), followed by AAV-Opn4 (4,437 ± 1,222 Opn4-expressing cells per retina) (Fig. 1J). Retinas injected with AAV-Opn4-IRES-EGFP were used primarily to identify the transduced cells for recording; the number of cells was not counted. Most of the transduced cells were retinal ganglion cells, although a scattering of amacrine and bipolar cells were also transduced. This selectivity may occur in part because ganglion cells are the first cells encountered by the virus particles after intravitreal injection, but there is a viral tropism as well (17). In untreated or sham-injected (AAV-GFP) retinas, the Opn4-expressing ganglion cells made up 572 ± 10.7 and 577 ± 23 cells per retina. Thus, the total number of Opn4-expressing ganglion cells increased by almost an order of magnitude in the treated animals.
Fig. 1.
Ectopic expression of melanopsin protein in retinal ganglion cells of different morphological types in the rd/rd mouse. (A–C) Spatial distributions of native melanopsin-expressing ganglion cells in uninjected (A) and sham-injected (C) rd/rd mouse retinas, and of total melanopsin-expressing ganglion cells in an AAV-Opn4 injected rd/rd mouse retina (B). Orientation of the retina: T, temporal; N, nasal, D, dorsal; and V, ventral. (D–G) Different types of ganglion cells were targeted in the melanopsin-treated mouse retina. Two monostratified ganglion cells (D and E) and one bistratified ganglion cell (F) are indicated here. (I) Their dendritic arbors are denser and smaller than those of the native melanopsin cell (G). (H and I) Sections of mouse retina stained with DAPI. In the rd/rd mice there appeared to be total loss of rod photoreceptors. Rare cones, which lacked inner and outer segments, were present but limited to the retinal periphery. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (Scale bars, 500 μm in A, B, and C; 100 μm in D–G; and 20 μm in H and I.) (J) Quantitation of melanopsin-expressing ganglion cells in rd/rd mice. For comparison, numbers of GFP-expressing ganglion cells in retinas injected with AAV-GFP are also indicated. Values are mean ± SD, with n indicating the number of retinas counted.
The transduced cells had various different dendritic morphologies, stratifications, and sizes (Fig. 1 D–F; see also Fig. S1). They were distinctly different from the unique and stereotyped native melanopsin cells (1, 18), which have sparse, crooked dendrites that spread far across the retinal surface (Fig. 1J). For six retinas, we mapped the position of every melanopsin-expressing ganglion cell in the transduced retinas (Fig. 1 A–C). They were distributed across entire surface of the retina, with occasional concentrations that may have been near the injection sites. We cannot be certain that representatives of all ≈12 types of ganglion cells in the mouse (19) were transduced, but it is clear that many of them were.
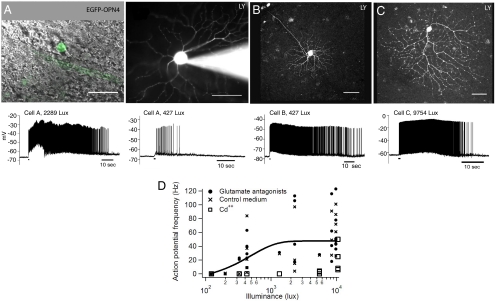
The responses of the ganglion cells to light were studied by whole-cell patch–clamp recording (Fig. 2). Cells were targeted for recording by their expression of GFP in eyes injected with AAV-Opn4-IRES-EGFP. GFP was identified in a ganglion cell soma during brief fluorescence illumination, after which the electrode was advanced while visualized by infrared differential interference contrast (DIC) microscopy optics. The recording pipettes were filled with Lucifer yellow CH, allowing visualization of the dendritic arbors. Although there was considerable variability from cell to cell (presumably due to varying amounts of melanopsin expression and/or bleaching during exploration of the retina) all of the cells that expressed Opn4 and GFP (n = 18) responded to light. The responses had the characteristics expected for melanopsin: long latency of onset (several hundreds of milliseconds to several seconds), and persistence for seconds after termination of the stimulus (1, 3, 18, 20, 21).
Fig. 2.
Long-lasting responses from retinal ganglion cells expressing Opn4 and EGFP. (A–C) Examples of EGFP- and Opn4-expressing retinal ganglion cells (Scale bars: 50 μm). (A Left) Combined DIC and fluorescence image of a cell that expresses EGFP (green) and Opn4. (A Right) After patch–clamp recording, the cell was filled with Lucifer yellow (0.1%) to visualize the morphology. Indicated in the traces below, the cell responded to light (480 nm peak; 2,289 lux; 1 s) with long-lasting trains of action potentials. A lower frequency and shorter duration train of action potentials were observed with a lower intensity of light (427 lux). (B and C) Heterogeneous types of ganglion cells (here, one very small and one large cell) expressed GFP and melanopsin, but they responded similarly to light. (D) Responses to light of the Opn4-transduced cells under normal or pharmacological conditions. The solid line is the average of all of the data in glutamate antagonists and control medium.
The retinas of the rd/rd mice appeared to lack rod photoreceptors (Fig. 1B), and the few residual cones were truncated (they lacked inner and outer segments) and restricted to the retinal periphery. To eliminate any possibility that the responses were driven by these cones (14–16, 22), the experiment was repeated in a series of rd/rd retinas (nine retinas, 15 cells) incubated in a mixture of NMDA-receptor antagonist [2-amino-5-phosphonovaleric acid (APV)], AMPA-receptor antagonist [6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)], and metabotropic glutamate receptor agonist 2-amino-4-phosphonobutyrate (APB), to block the glutamergic synapses that would transmit potential signals from these photoreceptors to the ganglion cells. Responses to light persisted under these conditions, indicating that the ganglion cells had become intrinsically photosensitive. In four cases, Cd2+ at 200 μM was used to block synaptic transmission instead of the glutamate receptor drugs. The cells continued to respond to light, albeit at a higher intensity than in untreated retinas or retinas treated with glutamate antagonists. The higher threshold is likely due to the direct effect of Cd2+ on the ganglion cells themselves, because Ca2+ ions permeate the channel opened by melanopsin in response to light (23, 24). The dendritic arbors of the cells expressing melanopsin and GFP had many morphologies (Fig. 2), corresponding to many types of ganglion cells. There was no noticeable correlation between the morphology of the cell and the characteristics of the light-induced response. We conclude that ectopic expression of the melanopsin protein rendered many types of retinal ganglion cells intrinsically sensitive to light.
Could the visual information transmitted to the brain by the transduced ganglion cells restore any of the visual function that is lost when the photoreceptor cells degenerate in these mice? We performed several tests of visual function. The first was the PLR (25–27). A high-threshold PLR is retained in rd/rd mice, mediated by the small complement of native melanopsin cells (4, 26). Our results in the sham-injected (transduced with GFP alone) mice confirm this finding (Fig. 3C). In these animals, as in untreated mice with photoreceptor degeneration, the PLR was ≈3 log units less sensitive than the PLR in wild-type C57BL mice and had a long latency. In rd/rd mice with ectopic expresssion of melanopsin, the PLR was returned to almost the sensitivity observed in mice that had not suffered photoreceptor degeneration (Fig. 3C). The responses to light did have somewhat longer latencies than normal (Fig. 3 D and E). This delay is in accord with the long latency of response recorded electrophysiologically in the transduced ganglion cells (Fig. 2).
Fig. 3.
Restoration of light sensitivity in the eye of the AAV-Opn4 treated rd/rd mouse. (A and B) Representative infrared images of pupil area taken in dark (A) and light (B). White dots indicate the pupil areas. For a time series of images, see Fig. S2. Pupil area was measured from such images by using ImageJ. (C) Intensity-response curves for pupillary constriction. The stimulus was exposure to 20 s of white light. The threshold for response is dramatically reduced in melanopsin treated eyes (red curve) compared with sham-injected eyes (blue curve). Data from uninjected C57BL mice are shown for comparison. The data are fitted with a sigmoidal function. (D and E) Time course of pupil constriction over the first few seconds of dim (D) and bright (E) light exposure are shown for melanopsin-treated (red) and sham-injected (blue) rd/rd mice, and uninjected C57BL mice (black). The area of the pupil is depicted as a percentage of its size immediately preceding the onset of light. Values are mean ± SEM, with n indicating the number of eyes examined.
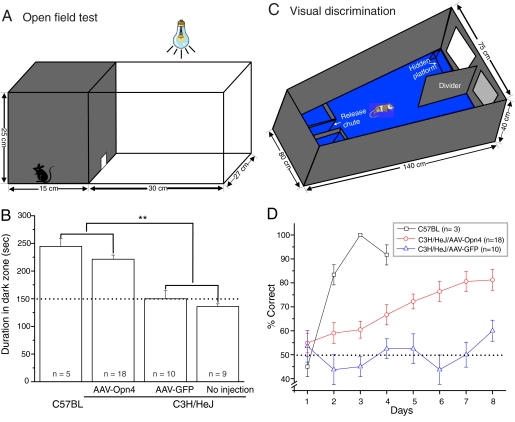
The ectopic melanopsin cells could also guide more complex behaviors. Normal mice avoid open, brightly lit spaces, and this innate tendency is the basis of a simple test of their ability to see. The test is to place mice in an illuminated open field that also contains a dark refuge. The fraction of time spent by the mice in the open space is measured. Mice were placed in the apparatus shown in Fig. 4A for a total of 300 s. The distorted cones persisting in the dorsal retinas of rd/rd mice could conceivably mediate a preference of mice for nesting in the dark under chronic living conditions (16). However, these cones were not sufficient to mediate light avoidance by rd/rd mice under our conditions: mice with normal retinas spent 244 ± 14.2 s (mean ± SEM, n = 5) in the dark field, whereas untreated rd/rd mice and mice injected with AAV-GFP spent 136 ± 4.8 s (n = 10) and 150 ± 14.5 s (n = 9) in the dark area, respectively. Injection of AAV-Opn4 returned the mice lacking rods and cones almost to normal behavior (221 ± 7.7 s, n = 18). The difference between treated and untreated or AAV-transduced rd/rd animals was statistically significant at P < 0.01 (Fig. 4B).
Fig. 4.
Enhancement of visual function in the AAV-Opn4 treated rd/rd mice. (A) The open-field test box consisted of a dark compartment (one third of the floor area) and a larger illuminated compartment (two thirds). A small opening located at floor level in the center of the dividing wall allowed mice to freely move between the lit and dark chambers. (B) Time spent in dark area by four groups of mice. The AAV-Opn4 treated rd/rd mice showed behavioral aversion to light: they spent significantly longer time in the dark chamber than their counterparts of either sham-injected or uninjected rd/rd mice (P < 0.01, t test). The exploratory behavior of uninjected C57BL mice is shown as comparison. Dotted line shows the behavior to be expected by chance. (C) Visual discrimination alley. Mice swam down a water-filled alley toward an illuminated or a dark target. The rewarded stimulus indicated the location of a submerged platform. (D) Melanopsin-treated (open circles) mice outperformed sham-injected (triangles) rd/rd mice in visual detection task over an 8-day trial (P < 0.001, two-way ANOVA test). Values represent mean ± SEM; n = number of mice.
Last, we sought a test that had a cognitive component (i.e., one in which the mice were required to make a decision based on visual information) (28). For this purpose, we used a two-choice visual discrimination. Mice were taught that a bright target represented safety in the form of a submerged platform. The mice swam down an alley and had to choose between a bright target (safe platform) or a dim target (no platform) (Fig. 4C). For normal mice, this task is easy: they learned it to >90% accuracy in only a few days (Fig. 4D, open squares). Rd/rd mice injected with the AAV-GFP construct did not reach above-chance performance after 8 days of training. Rd/rd mice injected with the AAV-Opn4 construct showed a steady improvement, reaching a level of ≈80% correct after the 8-day sequence (Fig. 4D). The difference between the latter two groups was significant at P < 0.001 (two-way ANOVA).
The PLR was retested in one series of mice 11 months after injection of AAV vectors. The sensitivity of the PLR in the melanopsin-treated rd/rd animals remained near the sensitivity of the PLR in the wild-type animals (Fig. S3).
Discussion
These results show that the signaling system that couples melanopsin to membrane depolarization is ubiquitous, or at least very widespread, in retinal ganglion cells; it is clearly not restricted to the native melanopsin-expressing cells. Although there is no certainty that the signaling pathway is identical in every ganglion cell, a functional pathway that can couple activation of melanopsin to a membrane cation channel appears to be present in most types of retinal ganglion cell, as well as in many other neural and nonneuronal cells (1, 3, 6–9).
In the absence of light-driven inputs, the responses of the different morphological types of ganglion cells were similar to each other. This finding suggests that ganglion cells, which normally send diverse kinds of functional signals to the brain (ON responses, OFF responses, sustained, transient, etc.), become electrophysiologically more uniform when driven by melanopsin. Presumably, the melanopsin system bypasses the normal interplay of excitatory and inhibitory inputs to the cells. Subtle differences, for example, those due to differing expression of ion channel proteins or amounts of melanopsin, may well exist (20), because their effects could have been obscured by the large and long-lasting depolarization initiated by the photoactivation of melanopsin.
Three different behavioral measures indicated that visual function, at least of a simple sort, was restored in nominally blind rd/rd mice by ectopic expression of melanopsin. The PLR is the simplest of the three, because it is a subcortically mediated visual reflex. At the other extreme, the light–dark discrimination task would ordinarily be classified as a learned visual discrimination. However, none of the three tasks studied here require great temporal or spatial resolution; they can evidently be performed by using the slow- and long-lasting responses mediated by melanopsin. The results represent proof of principle that the visually driven information available from the ectopic expression of melanopsin in a few thousand ganglion cells can be used to guide behavior, but do not define the limits of the visual abilities provided. An obvious next step will be to see whether these mice can carry out visual discriminations more demanding than those tested so far.
Could ectopic expression of melanopsin aid vision in humans suffering from photoreceptor cell degenerations (7)? These results suggest that the AAV vector could transduce enough cells in rodents for a crude but useful visual resolution, and that the sensitivity of the cells to light would be within a functional range. However, responses mediated by melanopsin can last for many seconds after the visual stimulus is off, and this persistence would limit the temporal resolution of vision. Modifications of the system to improve temporal resolution [coexpression of arrestin (8) and/or genetic modification of the signal pathway] might reduce this problem.
AAV-mediated expression of channelrhodopsin-2, a light-sensitive microbial protein that contains an intrinsic ion channel, renders ganglion and bipolar cells electrophysiologically responsive to light (11). When expressed in ON bipolar cells of rd/rd mice, it also permitted recovery of certain visual reflexes to high-intensity light: the PLR, an activity test, and the optokinetic response (10). Channelrhodopsin-2 is attractive because it yields responses to light on a millisecond time scale close to that of neurons in normal retinas. However, channelrhodopsin-2 is not a native mammalian protein, raising the possibility of an adverse immune response on long-term expression; this risk would be a significant factor when contemplating its use in humans. Also, it requires stimulation with light at exceedingly high intensities, potentially damaging to the retina when applied chronically. After transduction of ganglion cells with melanopsin, in contrast, the PLR of rd/rd mice returned virtually to its normal sensitivity; and our other behavioral tests were carried out by using stimulus intensities that fall in the range of ordinary indoor lighting. In the future, it might be possible to engineer a fast-acting melanopsin, or a more sensitive channelrhodopsin-2.
Materials and Methods
These experiments were carried out, with the same fundamental results, in two parallel sets of mice, one in San Diego and one in Boston. Because there were many small differences in the protocols, for simplicity this report describes only the Boston experiments, which were the larger series of the two. All methods are described in more detail in SI Methods.
Constructs and Viral Vectors.
Three constructs were used: AAV-GFP, AAV-Opn4, and AAV-Opn4-GFP. ORFs coding for GFP, full-length mouse melanopsin (GenBank accession no. 6693702), or Opn4-IRES-GFP were cloned into pAAV-MCS8 vector under the transcriptional control of CMV promoter. These three constructs were packaged into AAV2 serotype virus at the Harvard virus production core. The packaged viruses were concentrated and purified in PBS at titers as follows: AAV-Opn4, 2.1 × 1012; AAV-GFP, 7.8 × 1012; and AAV-Opn4-GFP, 2.9 × 1012 genome copies per milliliter.
Immunocytochemistry and Electrophysiology.
The retina was fixed in 4% paraformaldehyde (PFA) for 1 h. Antimelanopsin antibody was applied to reveal melanopsin. The primary antibody was antimelanopsin (1:200; Fisher Scientific), which was diluted in 5% NGS/1% BSA/0.5% Triton X-100 in PBS and applied overnight. After washes in PBS, secondary antibody conjugated either to Alexa TM 488 (1:500; Molecular Probes) or Alexa TM 594 (1:500; Molecular Probes) were applied for 2 h. Confocal micrographs of fluorescent specimens were taken from retinal flat-mounted preparations with a Bio-Rad Radiance confocal microscope. Images were adjusted in brightness and contrast by using Photoshop 8 (Adobe Systems).
Whole-cell patch–clamp recording was carried out by conventional techniques (24) on intact retinas as whole mounts by using retinas injected with AAV-Opn4-GFP. The expression of GFP was relatively weak in these retinas (Fig. 2) and allowed visualization of only the soma. To prevent photodamage to the retina, after a GFP-expressing soma had been identified by fluorescence microscopy, further manipulation and electrode approach to the cell were carried our under infrared DIC. Photic stimuli were generated by the mercury lamp of the microscope (peak wavelength, 480 nm) attenuated by neutral density filters and were delivered through the epifluorescence pathway of the microscope optics. Note that the process of searching for a transduced cell requires strong short-wavelength illumination, which inevitably isomerizes photopigment and causes an unknown degree of light adaptation (20). For that reason, the unbleached sensitivities cannot be estimated from these electrophysiological experiments. From the results of the behavioral experiments they appear to be near the normal sensitivity of melanopsin, as judged from the sensitivity of the PLR.
Behavioral Tests.
The PLR and open-field avoidance tests were carried out by standard techniques. The two-choice visual discrimination closely followed procedures systematically evaluated for various mouse strains by Wong and Brown (28). The apparatus (shown in Fig. 4B) and the testing protocol were close replicas of theirs. It is essentially a classic two-choice alley, with visual stimuli at the end of the alley. The alley was filled to a depth of 15 cm with water, and the reward was access to a safe platform located 1 cm beneath the surface of the water under the positive stimulus.
Supplementary Material
Acknowledgments.
We thank Aimee Wong and Richard Brown for advice on the testing of mouse visual discriminations and Jeng-Shin Lee and the Harvard vector core for providing the AAV constructs. This work was supported by National Institutes of Health Grants EY 017169 (to R.H.M.) and EY016807 (to S.P.). S.P. was supported by a Pew Scholars award. R.H.M. is a Senior Investigator of Research to Prevent Blindness.
Footnotes
Conflict of interest statement: A patent application (U.S. no. 60/397,088; July 18, 2002) has been filed by R.H.M. and assigned to the Massachusetts General Hospital.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806114105/DCSupplemental.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Fu Y, et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci USA. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 5.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 8.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 9.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 10.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 11.Bi A, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowes C, et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 13.Farber DB, Lolley RN. Cyclic guanosine monophosphate: Elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974;186:449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- 14.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 15.García-Fernández JM, Jimenez AJ, Foster RG. The persistence of cone photoreceptors within the dorsal retina of aged retinally degenerate mice (rd/rd): Implications for circadian organization. Neurosci Lett. 1995;187:33–36. doi: 10.1016/0304-3940(95)11330-y. [DOI] [PubMed] [Google Scholar]
- 16.Mrosovsky N, Hampton RR. Spatial responses to light in mice with severe retinal degeneration. Neurosci Lett. 1997;222:204–206. doi: 10.1016/s0304-3940(97)13374-2. [DOI] [PubMed] [Google Scholar]
- 17.Harvey AR, et al. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: A comparison with lentiviral vectors. Mol Cell Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- 18.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 19.Kong JH, Fish DR, Rockhill RL, Masland RH. The Diversity of Ganglion Cells in the Mouse Retina: Unsupervised Morphological Classification and its Limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- 20.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Mrosovsky N, Salmon PA, Foster RG, McCall MA. Responses to light after retinal degeneration. Vision Res. 2000;40:575–578. doi: 10.1016/s0042-6989(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 23.Hartwick AT, et al. Light-Evoked Calcium Responses of Isolated Melanopsin-Expressing Retinal Ganglion Cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koizumi A, Jakobs TC, Masland RH. Inward rectifying currents stabilize the membrane potential in dendrites of mouse amacrine cells: Patch–clamp recordings and single-cell RT-PCR. Mol Vis. 2004;10:328–340. [PubMed] [Google Scholar]
- 25.Gamlin PD, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]
- 28.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.