Abstract
Ig class switch recombination (CSR) is initiated by activation-induced cytidine deaminase (AID) mediated deamination of the switch (S) regions; the resultant mismatch is processed to yield the DNA breaks required for recombination. Whereas many of the pathways involved in the mechanism of recombination have been identified, little is known about how CSR is regulated. AID action is known to require transcription of the Ig heavy-chain genes. However, it is not understood how AID is restricted to the Ig genes. Many aspects of gene expression are known to be regulated by modification of chromatin structure. In turn, chromatin is known to be regulated by several RNA-dependent activities. We have mapped the transcriptional and chromatin landscape of the human Ig heavy-chain locus to investigate the effect these activities have on CSR. We demonstrate that the Ig heavy-chain constant genes and 3′-regulatory regions are in an active chromatin conformation in unstimulated total human B cells: the locus undergoes both genic and intergenic transcription and possesses histone modifications associated with “active” chromatin (acetylated H3 and H4 and lysine 4 trimethylated H3). However, on cytokine stimulation, these modifications spread into the S regions, demonstrating a chromatin remodeling activity associated with switching. Surprisingly, after stimulation, the S regions also accumulate lysine 9 trimethylated H3, a modification previously associated with gene silencing. These data demonstrates that the Ig locus is maintained with a complex pattern of both positive and negative histone marks and suggest that some of these marks may have dual functions.
Keywords: human B cells, immunoglobulin class switching, chromatin
The vertebrate immune system generates an enormous diversity of antibody specificities and effector functions. To achieve this diversity, B cells undergo two somatic DNA recombination events, assembling functional Igs from an array of variable (V), diversity (D), joining (J), and constant (C) region gene segments. In the developing B cell V(D)J recombination assembles the variable regions of the Ig heavy-chain (IgH) and light-chain genes, determining antigen specificity. On activation, mature B cells can undergo CSR, linking the IgH variable regions with one of the downstream heavy-chain C (CH) genes, altering the effector function of the antibody (1).
All CH genes, except Cδ, are preceded by a short intervening (I) or germ-line exon, upstream of a 2–10 kb, GC rich, S region. Before recombination, the CH genes undergo a sterile transcription event (2), driven by promoter sequences located upstream of the I exons. The resultant germ-line transcripts (GLTs) are spliced, removing the S region sequences, to join the I exon to the CH exons (3). It is now widely accepted that the enzyme AID transforms cytidines to uridine in the transcribed S regions (4–6). The resultant mismatch is resolved by the base excision or mismatch repair pathways, generating the DNA breaks required for CSR. These DNA breaks are processed by the nonhomologous end-joining pathway (7), recombining the S regions of two CH genes, excising the intervening DNA (8), and linking the variable region to the new C region.
Mammalian S regions contain a high proportion of WRC motifs, the favored sites of AID action. However, AID is only able to deaminate single-stranded DNA (9). It has been known for many years that transcription of the S regions, occurring during the production of GLTs, is required for CSR (10). It now seems likely that “R-loops,” DNA/RNA hybrids between the template DNA strand and the GLT, form during germ-line gene transcription (11, 12), stabilizing a single-stranded substrate for AID on the nontemplate strand. This property makes the transcribed S regions ideal substrates for AID activity, and contributes toward its specificity for the Ig genes. However, AID can also deaminate single-stranded DNA found in “transcription bubbles” by its interaction with replication protein A (13), yet AID activity has only been detected at a few non-Ig genes, including BCL6 (14), Pax-5 (15), and B29 (16). Additional levels of control must restrict AID action primarily to the Ig genes.
Chromatin structure impacts on, and likely regulates, most aspects of gene expression. Several RNA-dependent mechanisms are now known to regulate the structure chromatin forms over a gene. Intergenic transcription has been detected at several multigene loci such as the β-globin locus (17, 18) and IL-4/IL-13 cytokine cluster (19, 20) and has recently been implicated in the regulation of VDJ recombination (21, 22). It is believed that these transcripts function to establish and maintain domains of modified histones over these loci. Also, recent studies in Schizosaccharomyces pombe have shown that transcripts capable of forming RNA duplexes may be connected with RNAi suppression mechanisms and heterochromatin formation (23). Chromatin structure also contributes to the regulation of CSR. After stimulation of germ-line transcription in mice, histones H3 and H4 have been shown to be acetylated at the I exon promoters and S regions (24–26). However, to our knowledge the involvement of specific histone methylation events and the distribution of these modifications in the human locus have not been investigated. We previously demonstrated that in humans, the CH genes are in an “accessible” chromatin conformation before CSR (27). We have now extended this analysis, examining the distribution of genic and intergenic transcripts and histone modifications across the C region and 3′-regulatory regions of IgH locus in human B cells stimulated to initiate CSR.
Results
The Ig Heavy-Chain Locus Is Constitutively Transcribed.
Transcription across the C region and 3′-regulatory regions of the human IgH locus was quantified by analyzing steady state transcript levels by qRT-PCR (Fig. 1A). Primers were designed to detect transcripts at specific regions across the locus [Fig. 1C and supporting information (SI) Table S1]. However, the locus contains multiple sequence homologies surrounding the IgG genes and 3′Cα1 and 3′Cα2 enhancers, preventing the design of specific primers in these regions: primer set I was 100% homologous for both the Cα1 and Cα2 enhancers, preventing differentiation between these two sequences. Similarly, primer sets D, E, F, G, and H amplified sequences surrounding the IgG genes with very few mismatches and, thus, could not distinguish among the four IgG isotypes. Transcription was analyzed in total B cells assayed immediately after isolation and after 48-h culture with IL-4/anti-CD40 stimulation, previously shown to induce CSR to IgG and IgE (27), and compared with fibroblasts. Under these conditions, circle transcripts and DNA breaks are first detected at this time, demonstrating the initiation of recombination to IgG and IgE (D.J.F., unpublished result).
Fig. 1.
Analysis of transcript levels (A) and direction of transcript production (B) across the Ig-heavy chain locus. (A) Analysis of transcription across the Ig heavy-chain locus. Transcription across the Ig locus was quantified by measuring steady-state transcript levels by qRT-PCR. The location of the Taqman primer sets used for this analysis are depicted on the diagram of the locus below the graph (C, not to scale). Note that primers D, E, H, and I hybridize to multiple regions across the locus. All qRT-PCR assays were quantified against a standard sample of genomic DNA and normalized to the expression of β2-microglobulin; β2-microglobulin expression routinely had a Ct value four cycles less than primer set A, i.e., it was ≈16 times more abundant. Transcript expression level is shown on an arbitrary logarithmic scale, based on the genomic DNA standard used throughout. The mean results from total B cells isolated from the tonsils of four donors are shown; error bars show standard deviation. B cells were analyzed immediately after isolation (0 h) or after 48-h culture with IL-4/anti-CD40 (+4/40). The signals from the −RT controls is superimposed onto the respective bars to demonstrate the amount of the signal originating from gDNA contamination. (B) Sense and antisense transcription across the Ig heavy-chain locus. The direction of transcription across the locus was determined by employing strand-specific priming of the RT reaction, followed by qRT-PCR analysis. The location of the Taqman primer sets used for this analysis, and orientation of sense/antisense transcription, are depicted on the diagram of the locus below the graph (C, not to scale). Note that primers D, E, H, and I hybridize to multiple regions across the locus. The proportion of transcription originating from the sense (S) and antisense (AS) strands is shown compared with self-priming in the absence of RT primers (NoP, white bars). Mean data are shown from B cells isolated from three donors analyzed immediately after isolation (−) or after 48-h culture with IL-4/anti-CD40 (+). Error bars show SEM.
Fig. 1A shows the mean results from four donors. In both freshly isolated and stimulated B cells transcript levels over the μ enhancer (primer set A) and a region 1.2 kb 3′ of Cδ (set B) were ≈100-fold higher than across the rest of the locus (P > 0.001). Transcript expression was remarkably uniform across the remaining regions, irrespective of their genic or intergenic origin; however, transcripts significantly decreased 5′ of the ELK2ψ2 pseudo gene (set P) (P = 0.02). Transcript levels were not significantly affected by IL-4/anti-CD40 stimulation, except at sequences surrounding the ε gene (set K and L): transcripts over the Iε promoter (set K) increased 5-fold (P = 0.03), whereas transcripts over the Iε exon (set L) increased 150-fold after stimulation (P = 0.03). Conversely, transcripts over the intergenic region between IgD and IgG3 (set C) decreased ≈4-fold after stimulation. Transcript expression was detectable in fibroblasts at some regions across the locus. However, the level of expression was highly variable (often not being detectable) and generally >10-fold below the level of expression seen in B cells. The presence of high levels of transcription across the locus in unstimulated B cells is evidence that, in humans, the region containing the IgH constant genes (spanning from the μ enhancer to the 3′α2 enhancer) is maintained in an accessible chromatin conformation.
Sense and Antisense Transcription Is Detected Across the Ig Heavy-Chain Locus.
The direction of transcription across the C region and 3′-regulatory regions was determined by employing strand-specific priming of a reverse transcription (RT) reaction. Oligonucleotides that annealed to RNA produced in either a sense or antisense direction were used to synthesize strand-specific cDNA (Fig. 1B). The amount of cDNA produced in the sense and antisense reactions was determined by qRT-PCR and compared with cDNA produced in the absence of primers, a measure of the amount of self-priming of the RT reaction by RNA fragments. Fig. 1B shows the proportion of transcription originating from the sense or antisense strands, compared with self-priming, from B cells isolated from three donors and cultured as described above. It should be noted that the efficiency of the sense and antisense primed RT reactions may differ, therefore these results are only indicative of the degree of sense and antisense transcription across the locus. Similarly, a comparative study between the different primer sets cannot be made, because the efficiency of the differently primed RT reactions could not be assessed by using this method.
In Fig. 1B, transcription over the μ enhancer (set A), IgD-IgG3 intergenic region (set C), and over the Iγ (set D) and Iε (set K) germ-line promoters was predominantly in the sense direction. However, transcription could be readily detected on both strands over the Iε exon (set L): the direction of transcription occurring over the Iγ exons and S regions could not be determined due to the high level of self-priming in these regions. Although considerable variation in the proportion of sense or antisense transcription over Iε (set L) was seen between the samples, robust bidirectional transcription was seen in all samples measured. In contrast, transcription from the intergenic regions between the IgG genes (set H), between IgE and IgA2 (set O), and surrounding the Cα enhancers (sets I and J) was predominantly antisense. Despite the distinct directional bias at most locations, bidirectional transcription was detected at most regions and was particularly evident over Iε.
Both Repressive and Stimulatory Histone Modifications Exist at the Ig Heavy-Chain Locus.
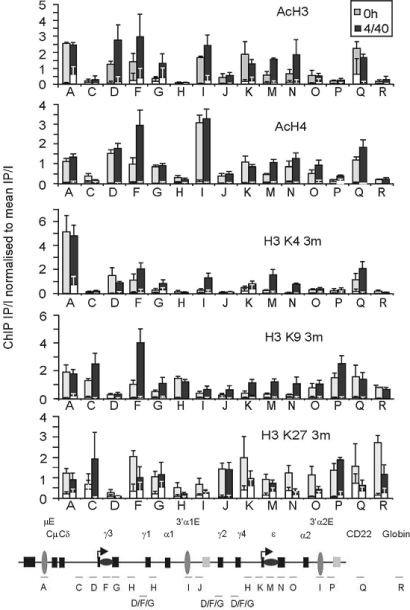
A detailed analysis of histone modification patterns across the C region and 3′-regulatory regions of IgH was carried out by ChIP of native mononucleosomes and dinucleosomes isolated from total B cells, cultured as described above. The distribution of each histone modification was analyzed by qPCR, compared with the input DNA, and normalized to the mean efficiency of the immunoprecipitation (see Materials and Methods). Fig. 2 shows the distribution of each modification across the locus compared with CD22 (set Q), a constitutively expressed B cell gene (28), and the ε-globin gene (set R), silenced in B cells. Mean data from four donors are shown.
Fig. 2.
Analysis of histone modifications across the Ig heavy-chain locus. Histone modification patterns across the locus were analyzed by performing ChIP on mononucleosomes and dinucleosomes, recovered from micrococcal nuclease treated, native chromatin isolated from B cells immediately after purification (0 h) or after 48-h culture with IL-4/anti-CD40 (+4/40). The distribution of each histone modification was determined by qPCR and normalized to the amount of “input” DNA and mean efficiency of the immuno-precipitation. Mean data are shown from total B cells isolated from four donors, errors bars show SEM. IP signal obtained from the IgG controls are shown in white. The location of the Taqman primer sets used for this analysis are depicted on the diagram of the locus below the graph (not to scale). Note that primers D, E, F, G, H, and I hybridize to multiple regions across the locus. Levels of H3 and H4 acetylation (AcH3 and AcH4), H3 trimethylated at lysine 4 (H3 K4 3m), histone H3 trimethylated at lysine 9 (H3 K9 3m), and lysine 27 (H3 K27 3m) are shown.
Histones H3 and H4 are acetylated at regions of activated chromatin, where genes are transcribed (29) or are undergoing DNA repair (30). In Fig. 2, levels of H3 and H4 acetylation (AcH3 and AcH4) were constitutively high at genic (sets F, G, M, and N) and regulatory regions (sets A, D, I, and K) across the Ig locus but less abundant at intergenic regions (sets C, H, and O) and at the ELK2 pseudogenes (sets J and P). Histones H3 and H4 were acetylated at a high level over the Iγ (set D) and Iε (set K) germ-line promoters but were not significantly affected by IL-4/anti-CD40 stimulation; the increase in H3 acetylation seen at D was within the limits of the experimental error. In contrast, H3 and H4 acetylation increased over the S regions of the ε and γ genes (sets F and M) after stimulation: The increase in H3 acetylation at Sγ was within the limits of the errors; however, acetylation increased in three out of four individual experiments.
The regulatory regions of activated genes are also enriched for lysine 4 trimethylated histone H3 (H3 K4 3m) (31). In Fig. 2, this modification was very abundant over the μ enhancer (set A), and to a lesser extent over the 3′Cα enhancer (set I) after stimulation. The Iγ germ-line promoters (set D) and S regions (set F) were also found to be constitutively enriched for H3 K4 3m. Conversely, although Sε (set M) was enriched for this modification after stimulation, H3 K4 3m was poorly enriched at the Iε promoter (set K) and not significantly affected by IL-4/anti-CD40 stimulation. Together, these data support the conclusion that both the γ and ε genes are in a constitutively activated chromatin state but that further modification is associated with the addition of IL-4/anti-CD40.
Trimethylation of histone H3 at lysine 9 (H3 K9 3m) and lysine 27 (H3 K27 3m) has previously been associated with “silenced” genes and the formation of heterochromatin (31, 32). In Fig. 2, constitutive H3 K9 3m was seen at the intergenic regions (sets C, H, and O), ELK2ψ2 pseudogene (set P) and over the μ enhancer (set A). However, this mark was largely absent from the Iγ germ-line promoter (set D), although it was present at the Iε promoter (set K) after stimulation. Surprisingly, after IL-4/anti-CD40 stimulation, levels of H3 K9 trimethylation rose over the γ and ε S regions (sets F and M) and C exons (sets G and N). Levels of H3 K27 3m across the locus were far lower, only 2- to 3-fold above the IgG controls at most locations. However, H3 K27 3m was enriched at the ELK2 pseudogenes (sets J and P), and showed a decrease from the Ig genes after stimulation. These data demonstrate that the Ig locus is maintained with a complex pattern of both positive and negative histone marks and suggests that some of these marks may have dual, antagonistic, functions.
Discussion
Within the last 10 years, much effort has been focused on elucidating the mechanisms of CSR, greatly expanding our understanding of this complex process. Despite this effort, to our knowledge it is still unclear how CSR is regulated and particularly how it is targeted to the Ig genes. For CSR to take place the Ig genes are subjected to transcriptional activation, RNA splicing, AID-dependent cytidine deamination, DNA strand breakage, recombination, and repair. Each of these events is likely to be accompanied by, and possibly regulated by, specific changes in chromatin structure. We have examined the transcriptional and epigenetic landscape of the C region and 3′-regulatory regions of the IgH locus in human B cells before and after stimulation of the early stages of CSR to investigate how these events may affect class switching.
The high level of constitutive transcript expression seen (Fig. 1A) over the μ enhancer (set A) and a region 1.2 kb 3′ of IgD (set B) correlates with the expression of the primary IgM-IgD transcript. However, although lower transcript levels are seen 3′ of this region, expression is readily detected from both genic and intergenic sequences. Genome wide “transcriptome” analyses have previously demonstrated that the majority of the mammalian genome is transcribed to some extent (33, 34). Whereas it is undoubtedly possible to detect this “transcriptional noise,” the level of expression seen from the Ig locus in B cells is significantly higher than in fibroblasts (Fig. 1A), demonstrating that it is a B cell specific phenomenon and not the result of “leaky” transcriptional repression. We previously showed that in the majority of human B cells the CH genes undergo constitutive germ-line gene transcription (27). These data build on our previous results, demonstrating that the C region and 3′-regulatory regions of IgH are constitutively accessible, at least to the transcriptional machinery, before cytokine stimulation. This finding is in contrast to data obtained from murine B cells where S region transcription is only detected after cytokine stimulation (35). Although Perlot et al. (35) did not investigate intergenic transcription, it would appear that significant differences exist between the level of accessibility, and therefore regulation, of the locus in man versus mouse.
Transcription over the genic regions occurred predominantly on the sense strand, or was bidirectional in the case of Iε (Fig. 1B). In contrast, transcription over the intergenic regions (sets H and O), 3′Cα enhancers (set I) and ELK2 pseudogenes (set J) occurred predominantly in an antisense direction. Intergenic transcription, emanating from locus control regions (LCRs), is thought to regulate both the β-globin and human growth hormone loci (18, 36). It is thought that the 3′Cα enhancers may have LCR activity (37, 38). Thus, the discovery of abundant antisense transcription in the vicinity of these enhancers may suggest that they influence locus wide chromatin structure by delivering RNA polymerase associated chromatin modifiers to the distal genes (39).
In contrast to the rest of the locus, transcript levels decrease significantly over the ELK2ψ2 pseudogene (set P), approaching levels seen in fibroblasts (Fig. 1A). This region displays a markedly different pattern of histone modifications compared with the rest of the locus, showing elevated K27 and K9 trimethylation of histone H3 and reduced acetylation of H3 and H4 (Fig. 2). This dramatic change suggests that this region represents the 3′ end of the functional Ig locus chromatin domain, and raises the possibility that a boundary element may exist at this location, as has been suggested in the mouse (40). Interestingly, the ELK2ψ1 pseudogene, 3′ of the Cα1 gene, also displays elevated levels of H3 K27 trimethylation and reduced histone acetylation. This element may separate, and allow differential regulation of, the two duplicated halves of the human IgH locus.
Both sense and antisense transcripts are readily detected over the Iε exon (set L) (Fig. 1B). It is likely that the amount of sense transcript is underestimated by this analysis due to it being spliced; therefore, the primary transcript will probably not accumulate in the cell. However, the detection of antisense transcripts may have important implications for the regulation of CSR. Single-stranded DNA, the substrate for AID, is thought to be exposed on the nontemplate strand by transcription, leading to the formation of R-loops (11, 12). Antisense transcription would provide a means to produce single-stranded DNA, and subsequently DNA breaks, on the template strand. Although antisense transcription of the S regions does not form R-loops (41, 42), it would expose short regions of single-stranded DNA in the transcription bubble, sufficient for AID action (9). Antisense transcripts have recently been detected over murine S regions (35) and chromosomal translocations (43–45), strongly supporting their possible role in facilitating class switching.
To further investigate the chromatin environment across these regions of the IgH locus, ChIPs were performed to investigate the distribution of various histone modifications associated with either activated or silenced chromatin (Fig. 2). The μ and 3′Cα enhancers were found to contain high levels of acetylated H3 and H4 and H3 trimethylated at lysine 4 (H3 K4 3m), modifications also associated with the germ-line promoters; however, these modifications were depleted at the intergenic regions. The distribution of these modifications correlated with their reported genomic distributions (31, 46, 47). However, their abundance in the unstimulated cells, particularly at the IL-4/anti-CD40 responsive Iε promoter, is further evidence that in these cells the locus is in a constitutively accessible, and at least partially activated, conformation. The absence of H3 K4 3m at the Iε but not Iγ promoter in the unstimulated cells could reflect the requirement of IL-4/anti-CD40 stimulation to fully activate the ε gene.
After stimulation by IL-4/anti-CD40, acetylation of histones H3 and H4 and H3 K4 trimethylation spreads into the S regions. These results confirm previous studies in the mouse which showed the accumulation of acetylated H3 and H4 at S regions stimulated to undergo CSR (24, 25, 48). However, to our knowledge, this is the first such report in primary human B cells linking H3 and H4 acetylation and H3 K4 trimethylation to such stimulation. The spreading of these modifications into Sγ required IL-4/anti-CD40 stimulation, despite stimulation not greatly affecting γ germ-line gene transcription (27). This result suggests that cytokine stimulation may have additional functions in chromatin remodeling, independent of its effect on transcription.
Trimethylation of H3 at lysine 9 (H3 K9 3m) has been viewed as a modification associated with silenced genes and heterochromatin formation (31, 32, 49). When this modification was investigated across the locus (Fig. 2), it was found to be enriched at the intergenic sequences (sets C, H, and O) and at the ELK2ψ2 pseudogene (set P). These sites were also enriched for H3 K27 3m; thus, it is conceivable that these modifications facilitate silencing at these sites. However, surprisingly H3 K9 3m was enriched at both γ and ε S regions (sets F and M) and CH exons (sets G and N) after IL-4/anti-CD40 stimulation. H3 K9 3m has recently been shown to be associated with actively transcribed genes as well as silenced regions (31, 50–52). Thus, it would appear that H3 K9 3m can have dual roles within the cell, possibly dependent on its association with other marks (53). The IgH locus is “biallelically expressed,” one allele having undergone a productive VDJ recombination event, whereas the other is unrearranged or nonproductively rearranged. It is known that the nonproductive allele is silenced by its nuclear location (54), and possibly by a modified chromatin structure (55). Because of the dichotomous nature of the locus, we cannot exclude the possibility that the detected H3 K9 3m may only associate with the silenced allele. However, if this were the case, it might be expected to be distributed more uniformly across the locus, and would be unlikely to respond to stimulation. The strong association of H3 K9 3m with the S regions after stimulation suggests that it may have a role in the regulation of CSR. This proposition is supported by recent reports linking two H3 K9 methyltransferase enzymes with switching to specific isotypes or Ig serum levels and human disease: Suv39h1 is linked to switching to IgA (56), whereas SETDB2 is linked to serum IgE levels and atopic dermatitis (57, 58).
The modifications described here suggest that a level of control of CSR exists in addition to, and occurring independently from, transcription. Woo et al. (59) observed a similar phenomenon; they found that VH regions were constitutively transcribed and hypoacetylated, but that induction of somatic hypermutation (SHM) led to VH gene hyperacetylation in the absence of additional transcription. In addition to the changes we have described occurring at the Ig locus, AID expression is stimulated by IL-4/anti-CD40 (60) and must translocate into the nucleus to perform its function (61). Therefore, it is evident that, as would be expected for such a potent and potentially harmful mutagenic activity, CSR is subject to multiple levels of control affecting both the proteins that carry out the activity and the DNA on which they act.
Materials and Methods
Cell Purification.
B cells were isolated from tonsils obtained during routine tonsillectomies (ethical approval from GKT Hospital Trust). All patients gave informed consent and were uncharacterized with respect to atopy. Total B cells were isolated from the tonsil as previously described (62) and purity assessed by flow cytometry. B cell populations were routinely >95% CD19+, with <5% contaminating CD3+ T cells; ≈60% of these cells expressed IgM, with <2% IgG or IgE expressing cells (data not shown).
Cell Culture.
B cells were cultured at 0.5 × 106 cells per ml in RPMI medium (Invitrogen), supplemented with Transferrin (35 μg/ml, Sigma–Aldrich), Insulin (5 μg/ml, Sigma–Aldrich), penicillin and streptomycin antibiotics (Invitrogen) and 10% FBS (HyClone, Perbio Biosciences). Where indicated, media were supplemented with 1 μg/ml anti-CD40 antibody (G28.5, ATCC) and 200 units per ml of IL-4 (R&D Systems). Fibroblasts were purchased from TCS Cellworks and cultured as recommended.
RNA Extraction and qRT-PCR Analysis.
Total RNA was extracted from 2 × 106 cells by using a phenol/chloroform/GITC-based extraction kit (RNAwiz, Ambion). Genomic DNA contamination was removed by DNase I treatment (Turbo DNA-free Kit, Ambion); 5 μg of RNA was primed with random hexamers and reverse transcribed by using SuperScript RT II (Invitrogen). Quantitative RT-PCR was performed on a 7900HT PCR machine (Applied Biosystems) by using TaqMan Universal PCR Master mixture (Applied Biosystems). All primer sets utilized TaqMan technology (Roche Molecular Systems), designed by using Primer Express (Applied Biosystems). Data were analyzed by using the accompanying software (SDS 2.1). To allow transcript expression levels to be compared across the locus, all qRT-PCR assays were quantified against a standard sample of genomic DNA and normalized to the expression of an endogenous control gene, β2-microglobulin; β2-microglobulin expression routinely had a Ct value four cycles less than primer set A (i.e., it was ≈16 times more abundant). In Fig 1A, paired t tests were performed on the data to determine statistically significant differences between the expression of primer sets A and B vs. H and I, and P vs. H and I. We also used t tests to determine the significance of the difference between the stimulated and unstimulated data for primer sets K and L.
Strand-Specific RT-PCR.
RNA was isolated and DNase I treated as described above; 5 μg of RNA was denatured at 70°C for 2 min and annealed to 2 pmol of strand-specific primers, or no-primers, in RT buffer (Invitrogen) at 50°C for 2 min. SuperScript RT II (Invitrogen) was added, and cDNA was made by RT at 50°C for 2 h. Quantitative RT-PCR was performed as described above. Data were analyzed by comparing the Ct values of the No primer control and the sense or antisense primed RT reaction (ΔCt). The No primer control was set to an arbitrary value of 1 the relative amount of sense or antisense primed product calculated by 2ΔCt, this figure was expressed as a percentage of the total (sense plus antisense plus no primer) product. It should be noted that a comparative analysis of the different primer sets cannot be made, because the efficiency of the differently primed RT reactions could not be assessed.
ChIP.
Mononucleosomes and dinucleosomes were recovered from native chromatin and immuno-precipitated as previously described (63). Briefly, 5 × 107 cells were washed in PBS and lysed by swelling in detergent. Nuclei were isolated by centrifugation on a sucrose cushion. The nuclear pellet was resuspended in 1 ml MNase buffer and digested to mononucleosomes and dinucleosomes by incubation with MNase I (Amersham Bioscience). Soluble chromatin (mononucleosomes) was recovered after centrifugation to yield an S1 fraction; larger fragments (dinucleosomes) were released by dialysis overnight in 1 mM Tris·HCl, pH 7.5/0.2 mM EDTA (S2 fraction). Ten μg of fraction S1 and S2 were made to 1 ml in ChIP buffer (50 mM NaCl/50 mM Tris·HCl, pH 7.5/5 mM EDTA) and incubated overnight with 4 μg of antibody: anti-acetyl H3 (Upstate, 06-595), anti-acetyl H4 (Upstate, 06-866), anti-H3 K4 3m (Abcam, ab8580), anti-H3 K9 3m (Abcam, ab8898), or anti-H3 K27 3m (Upstate, 17-622); rabbit IgG was used as a control. ChIPs were incubated with 40 μl of protein A magnetic beads (Miltenyi) for 1 h before separation on magnetic microcolumns (Miltenyi). DNA was phenol/chloroform extracted, and ethanol precipitated before analysis by qPCR. Protease (Sigma) and phosphotase (Calbiochem) inhibitor cocktails and 5 mM sodium butyrate (Sigma) were added to all ChIP solutions; qPCR was performed as described for qRT-PCR. Each sample was quantified against a serial dilution of input DNA, giving a ratio of IP/Input for each modification at each location in the locus. To control for differences in the IP efficiency between individual experiments, the mean ratio of IP/input was calculated for all locations across the locus for each modification. This “mean IP efficiency” was then used to normalize all data to a constant value.
Supplementary Material
Acknowledgments.
We thank the staff at the Evelina Children's Hospital Guy's and St. Thomas' National Health Service Foundation Trust for their help with the collection of tonsils. We also thank Dr. M Gellert for critical reading of this manuscript. D.F. is supported by a Research Councils U.K. fellowship. This work was supported by Medical Research Council (MRC) Project Grant G0400106, Asthma U.K. funded core support for the MRC & Asthma U.K. Centre for Allergic Mechanisms of Asthma, and the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808462105/DCSupplemental.
References
- 1.Max EE. In: Fundamentals of Immunology. Paul WE, editor. Philadelphia: Lippincott; 1998. p. 111. [Google Scholar]
- 2.Lorenz M, Jung S, Radbruch A. Switch transcripts in immunoglobulin class switching. Science. 1995;267:1825–1828. doi: 10.1126/science.7892607. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri J, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuberger MS, Harris RS, Di Noia J, Petersen-Mahrt SK. Immunity through DNA deamination. Trends Biochem Sci. 2003;28:305–312. doi: 10.1016/S0968-0004(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 8.Stavnezer J. Molecular processes that regulate class switching. Curr Top Microbiol Immunol. 2000;245:127–168. doi: 10.1007/978-3-642-59641-4_6. [DOI] [PubMed] [Google Scholar]
- 9.Yu K, Huang FT, Lieber MR. DNA substrate length and surrounding sequence affect the activation-induced deaminase activity at cytidine. J Biol Chem. 2004;279:6496–6500. doi: 10.1074/jbc.M311616200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, et al. A selective defect in IgG2b switching as a result of targeted mutation of the I gamma 2b promoter and exon. EMBO J. 1993;12:3529–3537. doi: 10.1002/j.1460-2075.1993.tb06027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu K, et al. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 12.Fugmann SD, Schatz DG. RNA AIDS DNA. Nat Immunol. 2003;4:429–430. doi: 10.1038/ni0503-429. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 14.Peng HZ, et al. Nonimmunoglobulin gene hypermutation in germinal center B cells. Blood. 1999;93:2167–2172. [PubMed] [Google Scholar]
- 15.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MS, Kanegai CM, Doerr JR, Wall R. Somatic hypermutation of the B cell receptor genes B29 (Igbeta, CD79b) and mb1 (Igalpha, CD79a) Proc Natl Acad Sci USA. 2003;100:4126–4131. doi: 10.1073/pnas.0735266100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashe HL, et al. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribnau J, et al. Intergenic transcription and developmental remodeling of chromatin subdomains in the human beta-globin locus. Mol Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- 19.Rogan DF, Cousins DJ, Staynov DZ. Intergenic transcription occurs throughout the human IL-4/IL-13 gene cluster. Biochem Biophys Res Commun. 1999;255:556–561. doi: 10.1006/bbrc.1999.0241. [DOI] [PubMed] [Google Scholar]
- 20.Rogan DF, et al. Analysis of intergenic transcription in the human IL-4/IL-13 gene cluster. Proc Natl Acad Sci USA. 2004;101:2446–2451. doi: 10.1073/pnas.0308327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolland DJ, et al. Antisense intergenic transcription in V(D)J recombination. Nat Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 22.Bolland DJ, et al. Antisense intergenic transcription precedes Igh D-to-J recombination and is controlled by the intronic enhancer Emu. Mol Cell Biol. 2007;27:5523–5533. doi: 10.1128/MCB.02407-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdel A, Moazed D. RNAi-directed assembly of heterochromatin in fission yeast. FEBS Lett. 2005;579:5872–5878. doi: 10.1016/j.febslet.2005.08.083. [DOI] [PubMed] [Google Scholar]
- 24.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Whang N, Wuerffel R, Kenter AL. AID-dependent histone acetylation is detected in immunoglobulin S regions. J Exp Med. 2006;203:215–226. doi: 10.1084/jem.20051774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski DA, Stavnezer J. Stimuli that enhance IgA class switching increase histone 3 acetylation at Salpha, but poorly stimulate sequential switching from IgG2b. Eur J Immunol. 2007;37:240–251. doi: 10.1002/eji.200636645. [DOI] [PubMed] [Google Scholar]
- 27.Fear DJ, et al. Transcription of Ig germline genes in single human B cells and the role of cytokines in isotype determination. J Immunol. 2004;173:4529–4538. doi: 10.4049/jimmunol.173.7.4529. [DOI] [PubMed] [Google Scholar]
- 28.Campana D, et al. Human B cell development I Phenotypic differences of B lymphocytes in the bone marrow and peripheral lymphoid tissue. J Immunol. 1985;134:1524–1530. [PubMed] [Google Scholar]
- 29.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Downs JA, Nussenzweig MC, Nussenzweig A. Chromatin dynamics and the preservation of genetic information. Nature. 2007;447:951–958. doi: 10.1038/nature05980. [DOI] [PubMed] [Google Scholar]
- 31.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bannister AJ, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 33.Carninci P, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 34.Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlot T, Li G, Alt FW. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proc Natl Acad Sci USA. 2008;105:3843–3848. doi: 10.1073/pnas.0712291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YJ, Fu XH, Liu DP, Liang CC. Opening the chromatin for transcription. Int J Biochem Cell Biol. 2004;36:1411–1423. doi: 10.1016/j.biocel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Mills FC, Harindranath N, Mitchell M, Max EE. Enhancer complexes located downstream of both human immunoglobulin Calpha genes. J Exp Med. 1997;186:845–858. doi: 10.1084/jem.186.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinaud E, et al. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 2001;15:187–199. doi: 10.1016/s1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 39.Hampsey M, Reinberg D. RNA polymerase II as a control panel for multiple coactivator complexes. Curr Opin Genet Dev. 1999;9:132–139. doi: 10.1016/S0959-437X(99)80020-3. [DOI] [PubMed] [Google Scholar]
- 40.Garrett FE, et al. Chromatin Architecture near a Potential 3′ End of the Igh Locus Involves Modular Regulation of Histone Modifications during B-Cell Development and In Vivo Occupancy at CTCF Sites. Mol Cell Biol. 2005;25:1511–1525. doi: 10.1128/MCB.25.4.1511-1525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian M, Alt FW. Transcription-induced cleavage of immunoglobulin switch regions by nucleotide excision repair nucleases in vitro. J Biol Chem. 2000;275:24163–24172. doi: 10.1074/jbc.M003343200. [DOI] [PubMed] [Google Scholar]
- 42.Shinkura R, et al. The influence of transcriptional orientation on endogenous switch region function. Nat Immunol. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- 43.Julius MA, et al. Translocated c-myc genes produce chimeric transcripts containing antisense sequences of the immunoglobulin heavy chain locus in mouse plasmacytomas. Oncogene. 1988;2:469–476. [PubMed] [Google Scholar]
- 44.Apel TW, et al. Two antisense promoters in the immunoglobulin mu-switch region drive expression of c-myc in the Burkitt's lymphoma cell line BL67. Oncogene. 1992;7:1267–1271. [PubMed] [Google Scholar]
- 45.Morrison AM, et al. Deregulated PAX-5 transcription from a translocated IgH promoter in marginal zone lymphoma. Blood. 1998;92:3865–3878. [PubMed] [Google Scholar]
- 46.Pokholok DK, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein BE, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Luo Z, Scharff MD. Differential regulation of histone acetylation and generation of mutations in switch regions is associated with Ig class switching. Proc Natl Acad Sci USA. 2004;101:15428–15433. doi: 10.1073/pnas.0406827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice JC, et al. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 50.Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006;26:9185–9195. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiencke JK, Zheng S, Morrison Z, Yeh RF. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene. 2008;27:2412–2421. doi: 10.1038/sj.onc.1210895. [DOI] [PubMed] [Google Scholar]
- 52.Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci USA. 2006;103:15782–15787. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 54.Skok JA, et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 55.Goldmit M, et al. Epigenetic ontogeny of the Igk locus during B cell development. Nat Immunol. 2005;6:198–203. doi: 10.1038/ni1154. [DOI] [PubMed] [Google Scholar]
- 56.Bradley SP, et al. The histone methyltransferase Suv39h1 increases class switch recombination specifically to IgA. J Immunol. 2006;177:1179–1188. doi: 10.4049/jimmunol.177.2.1179. [DOI] [PubMed] [Google Scholar]
- 57.Jang N, Stewart G, Jones G. Polymorphisms within the PHF11 gene at chromosome 13q14 are associated with childhood atopic dermatitis. Genes Immun. 2005;6:262–264. doi: 10.1038/sj.gene.6364169. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. Positional cloning of a quantitative trait locus on chromosome 13q14 that influences immunoglobulin E levels and asthma. Nat Genet. 2003;34:181–186. doi: 10.1038/ng1166. [DOI] [PubMed] [Google Scholar]
- 59.Woo CJ, Martin A, Scharff MD. Induction of somatic hypermutation is associated with modifications in immunoglobulin variable region chromatin. Immunity. 2003;19:479–489. doi: 10.1016/s1074-7613(03)00261-9. [DOI] [PubMed] [Google Scholar]
- 60.Dedeoglu F, et al. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int Immunol. 2004;16:395–404. doi: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 61.Ito S, et al. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc Natl Acad Sci USA. 2004;101:1975–1980. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCloskey N, et al. The extrafollicular-to-follicular transition of human B lymphocytes: Induction of functional globotriaosylceramide (CD77) on high threshold occupancy of CD40. Eur J Immunol. 1999;29:3236–3244. doi: 10.1002/(SICI)1521-4141(199910)29:10<3236::AID-IMMU3236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods Mol Biol. 2004;287:99–120. doi: 10.1385/1-59259-828-5:099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.