Abstract
μ opioid receptors are G protein–coupled receptors that mediate the pain-relieving effects of clinically used analgesics, such as morphine. Accumulating evidence shows that μ-δ opioid heterodimers have a pharmacologic profile distinct from those of the μ or δ homodimers. Because the heterodimers exhibit distinct signaling properties, the protein and mechanism regulating their levels have significant effects on morphine-mediated physiology. We report the characterization of RTP4, a Golgi chaperone, as a regulator of the levels of heterodimers at the cell surface. We show that the association with RTP4 protects μ-δ receptors from ubiquitination and degradation. This leads to increases in surface heterodimer levels, thereby affecting signaling. Thus, the oligomeric organization of opioid receptors is controlled by RTP4, and this governs their membrane targeting and functional activity. This work is the first report of the identification of a chaperone involved in the regulation of the biogenesis of a family A GPCR heterodimer. The identification of such factors as RTP4 controlling dimerization will provide insight into the regulation of heterodimers in vivo. This has implications in the modulation of pharmacology of their endogenous ligands, and in the development of drugs with specific therapeutic effects.
Keywords: enkephalin, G protein-coupled receptors, heroin, hetero-oligomerization
The dimerization of G protein–coupled receptors (GPCRs) is a widely studied phenomenon that can profoundly modify the pharmacology of interacting partners in vitro. Allosteric modulation of ligand binding, alteration in G protein activation, and coupling to a new signaling pathway are known to result from GPCR association (1, 2). Thus, a greater level of complexity could arise from in vivo receptor–receptor interactions, making dimers promising targets for the development of new drugs with more specific therapeutic effects (3). Early studies using heterologous expression explored the functional outcome resulting from the association of two identical (homodimers) or, in most cases, two distinct receptors (heterodimers). Recent studies have focused on a role for receptor association in their folding and maturation, that is, GPCR oligomer biosynthesis (4). Similar to the dimerization-dependent expression known for class C receptors (5), class A receptors have also been found to require dimerization for enhanced expression (6, 7). In addition, inefficient targeting of GPCR oligomers in vivo has been shown to be the cause of pathophysiology in some cases, thus emphasizing the importance of the proper receptor assemblage for normal delivery to the cell surface (8, 9). The mechanism underlying this phenomenon has not been well explored.
The three subtypes of opioid receptors, μ, δ, and κ, are known to form homodimers and heterodimers (10). μ opioid receptors mediate most of the pain-relieving effects of morphine, the prototypical analgesic used in clinics (11). The observation that antagonism of δ receptors or lack of δ receptors leads to a reduction in the tolerance that develops on chronic administration of morphine (12) suggests a possible link between tolerance and opioid receptor dimerization. Furthermore, a large component of morphine and 6′-GNTI analgesic potency has been linked to the presence of μ-δ (13) and δ-κ (14) receptor heterodimers, respectively. These findings, together with the observation that the development of tolerance correlates with the enhanced surface expression (through externalization) of δ receptors in dorsal root ganglion neurons expressing μ receptors (15), support the idea that mechanisms and/or proteins that modulate the level of μ-δ complexes serve as critical factors in the development of tolerance (16). Thus, the factors influencing the homodimer-to-heterodimer ratio become a key issue in determining the net effect of an opiate. Moreover, they could redirect the coupling of opioid receptors to a distinct signal transduction pathway (17, 18). But very little is known about the events leading to dimerization per se; it continues to be perceived as a phenomenon resulting from random collision of the partners. Dimerization likely occurs through a defined sequence of events, as has been shown for the subunit assembly of multiproteins complexes, such as AMPA receptors or G proteins (19, 20). Here, we show that association with δ receptors leads to the specific retention of μ receptors in the Golgi compartment, resulting in the decreased cell surface expression of both receptors. Coexpression of RTP4, a member of the receptor transport protein (RTP) family that is known to participate in the export of odorant and taste receptors (21, 22) leads to enhanced cell surface expression as well as decreased ubiquitination of receptors. Our results show that RTP4 regulates the proportion of μ-δ heterodimers, leading to changes in the extent of signaling by these receptors, and thus represents a critical factor influencing the action of exogenous and endogenous opioid ligands.
Results
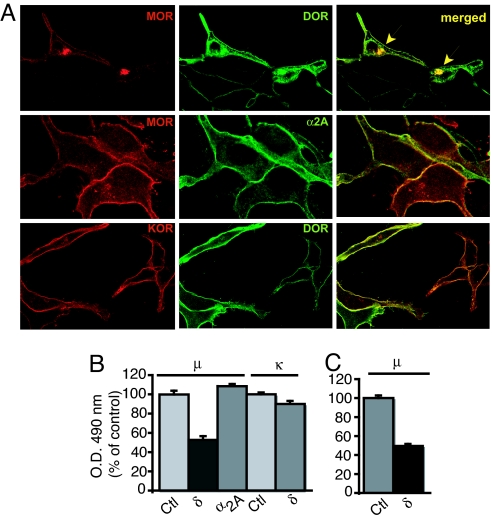
Because very little is known about the cell biological mechanisms underlying cell surface expression of family A GPCR heterodimers, we used the μ-δ opioid receptor heterodimer as a model system and examined the events and proteins involved in their efficient cell surface expression. We found that coexpression of myc-tagged δ receptors with FLAG-tagged μ receptors led to increased retention of both receptors in an intracellular compartment (Fig. 1). We also observed a similar phenomenon using HA-tagged μ coexpressed with FLAG-tagged δ receptors (data not shown), indicating that the interaction was not influenced by the type of epitope tag. In contrast, coexpression of μ receptors with α2A adrenergic receptors or of δ receptors with κ receptors did not lead to intracellular retention of any of these receptors (Fig. 1A). These results suggest that the increased intracellular retention of μ by coexpression with δ receptors exhibits receptor type selectivity, which could lead to a reduction in the cell surface expression of the heterodimer. We examined this using a whole cell binding assay (data not shown) or surface enzyme-linked immunosorbent assay (ELISA) (Fig. 1 B and C). We find that coexpression with myc-δ receptors leads to a ≈50% reduction in the cell surface expression of FLAG-μ receptors in both HEK293 (Fig. 1B) and Neuro2A cells (Fig. 1C). μ receptor expression was not altered by coexpression with HA-tagged α2A adrenergic receptors (Fig. 1B). Similarly, δ receptor expression was not altered by coexpression with FLAG-tagged κ receptors (Fig. 1B). Taken together, these results suggest that μ-δ heterodimerization leads to increased intracellular retention and decreased cell surface expression of the heterodimer.
Fig. 1.
Coexpression of μ and δ receptors leads to loss of cell surface μ receptors in HEK293 cells. (A) Cell surface expression of receptors shows that coexpression with myc-tagged δ receptors induces a large retention of FLAG-tagged μ receptors in an intracellular compartment. Coexpression of HA-tagged α2AR with μ receptor or δ receptor with FLAG-tagged κ does not lead to intracellular retention supporting the specificity of μ-δ interactions. (B) Quantitation of cell surface expression of receptors shows that coexpression with myc-tagged δ receptors but not with HA-tagged α2A receptors induces a large decrease in FLAG-tagged μ receptor expression. Coexpression of δ receptor does not alter the cell surface expression of FLAG-tagged κ receptors in HEK293 cells. (C) Quantitation of cell surface expression of δ receptors cotransfected with μ receptors in Neuro2A cells. Values are expressed as mean ± standard error of the mean (SEM) (n = 3).
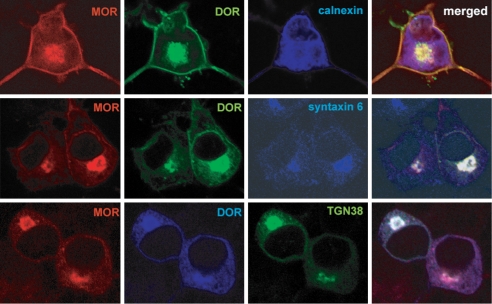
Next, we examined the compartment of localization of μ and δ receptors in Neuro2A cells and found that the receptors colocalized with two markers of the Golgi apparatus, syntaxin 6 and TGN38, but not with calnexin, the marker of the endoplasmic reticulum (ER) (Fig. 2). In agreement with this, we found μ receptors in a mature (endoglycosidase H–resistant) form in either the absence or the presence of δ receptors [supporting information (SI) Fig. S1]. These data suggest that expression of δ receptors with μ receptors leads to the retention of a significant portion of both receptors in the Golgi apparatus.
Fig. 2.
Coexpression of μ and δ receptors leads to intracellular rentention of both receptors in the Golgi apparatus. Immunofluoresence images of Neuro2A cells cotransfected with μ (Left) and δ receptors (Middle). Labeling with anti-syntaxin 6 antibody (Middle) as well as TGN38 (Lower) shows that intracellular μ-δ receptors colocalize with the marker for Golgi apparatus and not with that of ER compartment (anti-calnexin antibody; Upper).
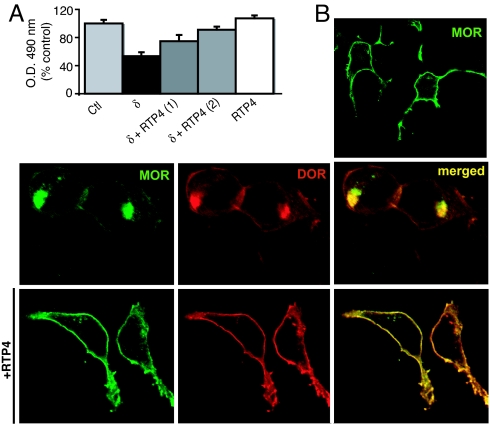
Most of the chaperones described so far aid in the folding and export of receptors from the ER. Proteins of the REEP and RTP family have been reported to greatly enhance the membrane targeting of odorant and taste receptors that otherwise are difficult to express in heterologous cells and tend to accumulate in Golgi apparatus (21, 22). We tested two of the RTP family members, RTP4 and RTP2, and found that the increasing expression of RTP4 led to a concomitant increase in the cell surface expression of both μ and δ receptors (Fig. 3A), whereas expression of RTP2 did not (data not shown). Cell surface expression of μ receptors alone was not altered by RTP4. Confocal imaging revealed that expression of RTP4 in μ-δ cells led to enhanced cell surface localization and decreased Golgi localization of μ-δ receptors (Fig. 3B). Taken together, these results suggest that RTP4 acts intracellularly to prevent accumulation of μ-δ receptors, allowing them to be targeted to the cell surface.
Fig. 3.
Expression of RTP4 prevents the retention of μ-δ receptors in HEK293 cells. (A) Quantitation of cell surface expression of μ receptors cotransfected with δ receptors without or with RTP4. Expression of increasing amounts of RTP4 cDNA (1 or 2 μg) leads to a concomitant increase in the level of cell surface μ expression. Data are given as mean ± SEM (n = 3). (B) Immunofluoresence labeling of μ (green) and δ (red) receptors transfected without (Upper) and with (Lower) RTP4. The intracellular accumulation of both receptors (yellow, Upper Right) is decreased when RTP4 is coexpressed, leading to increased cell surface localization of both μ and δ receptors (Lower).
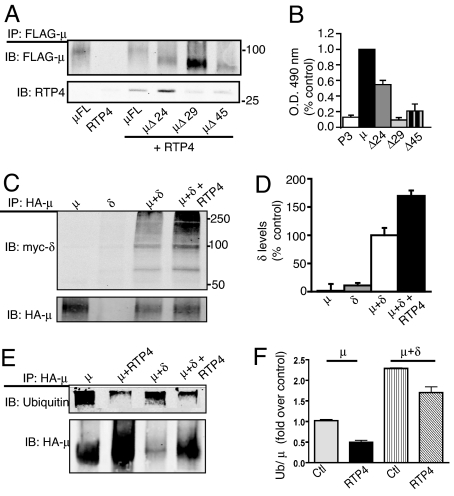
We then examined the direct interaction of RTP4 with μ and δ receptors. To facilitate these studies, we N-terminally tagged RTP4 with the FLAG epitope and coexpressed it with either μ or δ receptors. We found that RTP4 could be coimmunoprecipitated with both of these receptors (Fig. 4 and Fig. S2A). We also found that this interaction occurs at the cell surface, because incubating cells with either anti-myc or anti-FLAG antibodies before lysis and isolation of interacting complexes gave similar results (Fig. 4A and Fig. S2A). Next, we used a series of μ receptor C-terminal deletions (Fig. 4A) to show that the C-terminal 29 aa are necessary for interaction with RTP4, because the interaction was lost with the truncation of 29 or 45 residues but not with the truncation of 24 residues, indicating that a critical region within the C-tail is necessary for interaction with RTP4 (Fig. 4 A and B). We examined the localization of FLAG-RTP4 by confocal imaging. In cells expressing μ-δ receptors, RTP4 exhibited a colocalization with μ-δ receptors at the cell surface, whereas when expressed alone, RTP4 exhibited an intracellular localization (Fig. S3). This suggests a chaperone-like role for RTP4 in the cell surface targeting of μ-δ receptors. Next, we directly examined the ability of RTP4 to associate with μ-δ heterodimers by coimmunoprecipitation. We found a significant increase in the amount of δ receptors in μ receptor immunoprecipitates on coexpression with RTP4 (Fig. 4 C and D). The finding of an interaction of RTP4 with the heterodimer under conditions in which immunoprecipitation was carried out by incubating cells with antibody before lysis (Fig. 4 C and D) implies that RTP4 remains associated with μ-δ receptors at the cell surface. This indicates that RTP4 associates with the heterodimer in an intracellular compartment and facilitates its trafficking to the cell surface.
Fig. 4.
RTP4 interacts with μ and δ receptors. (A) Western blotting analysis shows that RTP4 coimmunoprecipitates with full-length (μ FL) or Δ24 but not with Δ29- and Δ45-truncated μ receptors. In panels A, B and D cells were incubated with antibodies to the epitope tag before the lysis step so as to enable the isolation of cell surface receptors. (B) Quantitation of the coimmunoprecipitation of RTP4 with mutant μ receptors. The data with wild type μ receptors is taken as 1.0; data represents mean ± SEM (n = 3). P3 represents cells transfected with control plasmid, pCDNA3. (C) Western blot analysis shows that increased myc-tagged δ receptors are detected after HA-tagged μ immunoprecipitation in the presence of RTP4. (D) Quantitation of coimmunoprecipitation data, expressed as mean ± SEM (n = 3). (E) Western blot analysis shows the level of ubiquitination of μ receptors when coexpressed with δ receptors; coexpression of RTP4 leads to a decrease in the extent of ubiquitination. (F) Quantification of the ubiquitination data, expressed as mean ± SEM (n = 3). All signals were normalized to the value with μ alone taken as 1.0.
One mechanism by which RTP4 mediates increases in the level of heterodimers could be through a decreased rate of degradation (and not due to transcription-mediated increases in levels, because the expression of these receptors in heterologous cells is controlled by an exogenous expression system). Recent studies have described ubiquitination-mediated proteasomal degradation as a way to regulate the level of GPCRs (23, 24). Consequently, we examined the ubiquitination state of μ receptors when coexpressed with δ receptors (Fig. 4 E and F). The extent of ubiquitination of μ receptors was increased when coexpressed with δ receptors (Fig. 4 E and F). Furthermore, expression of RTP4 led to a significant decrease in the extent of ubiquitination of μ receptors (Fig. 4 E and F), and δ receptors (Fig. S4), in the μ-δ complex. The fact that μ-δ heterodimers are highly ubiquitinated suggests that they are likely to be efficiently degraded through the proteasomal degradation pathway.
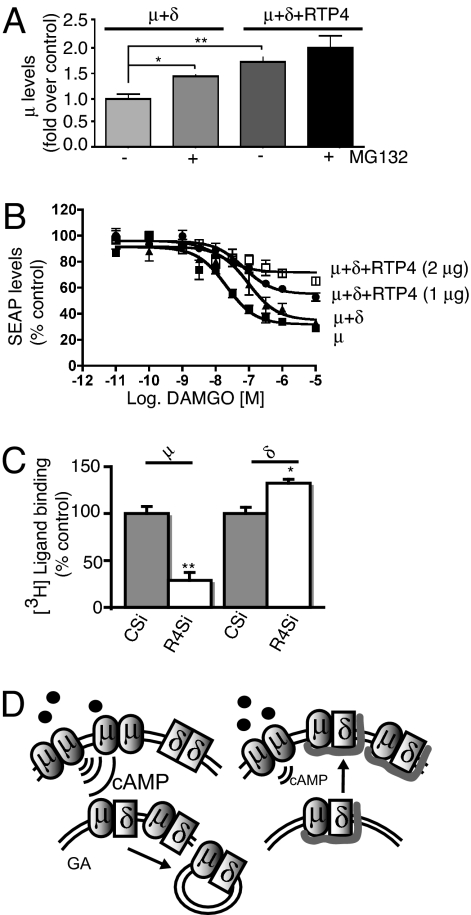
We directly examined proteasomal-mediated degradation using MG132, a proteasome inhibitor. We find that treatment with MG132 did not significantly affect the relative levels of μ receptors (when expressed alone); coexpression of RTP4 did not modulate this effect (Fig. S5). In contrast, the treatment with MG132 significantly increased the relative levels of μ receptors (when coexpressed with δ receptors); this effect was seen only in the absence of RTP4 (Fig. 5A). Taken together, these results suggest a basal level of ubiquitination of μ receptors (with no significant receptor degradation). In the presence of δ, the level of μ receptor ubiquitination is enhanced (with enhanced receptor degradation). Expression of RTP4 protects the μ-δ heterodimer from degradation, directly demonstrating a role of RTP4 in modulating heterodimer levels. This is consistent with our findings that expression of δ receptors led to increased retention of μ receptors in an intracellular compartment (and decreased cell surface expression), and that RTP4 was able to reverse this effect (Figs. 1 and 2). Results from the glycosylation studies also support such a notion; an immature form (with high mannose content) of the μ receptor was detected only when RTP4 was expressed in μ-δ cells (Fig. S1B). This is presumably due to rapid degradation of the immature form of the receptor in the absence of RTP4. Thus, RTP4-mediated regulation of μ-δ ubiquitination/degradation is a likely mechanism by which the level of heterodimer expression is modulated in vivo.
Fig. 5.
Functional impact of RTP4 expression. (A) Coexpression with RTP4 leads to decreased degradation of μ-δ receptors as determined by quantification of HA-tagged μ receptors coexpressed with δ receptors without or with RTP4 treated without or with 50 μM MG132 at 37°C for 3 h before harvesting. (B) Coexpression with RTP4 leads to alteration in μ-δ signaling, as detected by the pCRE-SEAP assay. Coexpression of RTP4 leads to a decrease in signaling by DAMGO, as demonstrated by the decreased efficacy of DAMGO. Data are expressed as mean ± SEM (n = 3). (C) Whole cell binding in SKNSH cells transfected with control or RTP4-directed siRNA. The receptor levels were measured in cells 48 h after siRNA transfection using 3H-DAMGO (for μ) or 3H-Deltorphin (for δ). Data are given as mean ± SEM (n = 3), **, P < 0.001 and *, P < 0.05 versus control siRNA. (D) A model for the role of RTP4 in modulating opioid receptor levels. (Left) In the absence of RTP4, μ-δ heterodimers are largely retained in the Golgi apparatus (GA) and eventually routed to degradation pathways. (Right) In the presence of RTP4, the heterodimers are stabilized and routed to the membrane, leading to a reduced population of receptor homodimers.
We next examined the functional significance of the modulation of the heterodimer by RTP4 using a cAMP-sensitive gene reporter assay (secreted alkaline phosphatase [SEAP] assay). We found that coexpression of δ receptors with μ receptors led to a ≈6-fold decrease in the potency of the μ agonist, [D-A612NMe-Phe4-Gly-015]-enkephalin (DAMGO) (Fig. 5B); the decrease in the extent of Gi-mediated signaling by the heterodimer is in accordance with our previous study showing a decrease in G protein coupling (using the [35S]-GTPγS binding assay) as a consequence of μ-δ heterodimerization (13). We also found that the increasing expression of RTP4 (leading to an increased cell surface expression of μ-δ receptors) led to a concomitant decrease in signaling, as evidenced by the reduced efficacy of DAMGO (Fig. 5B). This decreased efficacy suggests a noncompetitive allosteric-type modulation of μ-δ receptor function by RTP4; such an allosteric mechanism has been proposed in the case of other protein–protein interactions modulating GPCR function (25). We found no significant change in the extent of coupling of μ or δ receptors when RTP4 was expressed individually with either of these receptors alone (Fig. S6). Taken together, RTP4 expression led to an increase in the μ-δ levels, which in turn resulted in decreased μ-δ heterodimer-mediated G protein signaling; this is consistent with our previous observation that μ-δ heterodimerization led to a switch in signaling from a Gi-mediated pathway to a β-arrestin–mediated pathway (18).
We next examined the role of RTP4 in modulating the levels of endogenous μ-δ heterodimers. For this, we used a human neuroblastoma cell line, SKNSH, that we previously showed to express μ-δ heterodimers. Because these cells express RTP4 (Fig. S7), we used an siRNA-mediated knockdown of RTP4 to evaluate its role on the level of opioid receptors. The levels of RTP4 RNA were reduced by ≈50% after 48 h of transfection compared with the scrambled siRNA control (Fig. S7). The levels of μ-δ receptors were altered significantly under these conditions. A small but significant increase in δ receptor binding sites and a substantial decrease in μ receptor binding sites occurred (Fig. 5C). We also found a significant decrease in the heterodimer level as determined using a μ-δ heterodimer–specific antibody (Gupta, Mulder, Gomes, Harkany, and Devi, unpublished data). These results are consistent with the notion that reducing the amount of RTP4 affects the level of heterodimers and that the decrease in μ receptors results in an increased level of uncomplexed δ receptors. Thus, the endogenous modulation of the levels of RTP4 would have functional implications, because decreases in the RTP4 level would lead to decreases in the μ-δ heterodimer level (and increases in the δ receptor level), leading to changes in the extent of signaling by endogenous or exogenous opiate ligands.
Discussion
Here, we show that RTP4 binds to opioid receptors and regulates their cell surface expression. In the absence of RTP4, the trafficking of heterodimers is highly inefficient, leading to an accumulation of μ-δ complexes in the Golgi apparatus that are targeted to the degradation pathways (Fig. 5D, Left). We propose that RTP4 participates in the proper folding of the μ-δ heterodimer, allowing it to be routed out of the Golgi apparatus to the cell surface (Fig. 5D, Right). Thus, RTP4 is able to affect the relative signaling of μ receptors in cells endogenously expressing all three partners.
Some previous studies have shown that GPCR dimers can be formed as early as in the ER compartment (6, 26), and that this association is sustained throughout the maturation process in the Golgi apparatus and aids their cell surface targeting (27). Although μ and δ receptors associate to form homodimers and heterodimers in the ER, the heterodimers are retained in the Golgi apparatus, presumably due to the inefficient trafficking of this receptor combination. This could be due to major changes in receptor conformation triggered by heterodimerization of μ and δ receptors, as suggested by the markedly altered receptor pharmacology (13) and conformation of the N-terminal regions (28). Consequently, proteins involved in the trafficking of receptors to the cell surface may not recognize the altered conformation, leading to intracellular retention. A study examining inducible overexpression of δ receptors on μ levels found a reciprocal relationship (increases in δ levels paralleled decreases in μ levels),(29), supporting the occurrence of intracellular retention. It is likely that a motif recognized by accessory proteins when μ receptors associate with δ receptors is masked, preventing its routing to the cell surface. In the case of GABAb receptors, the reciprocal scenario appears to be true when the association of GABAbR2 with GABAbR1 leads to the masking of a motif involved in the retention of GABAbR1 into the ER and the Golgi apparatus, leading to export of the heterodimer to the cell surface (5). RTP4 has been proposed to be anchored to the membrane by a transmembrane segment at its very C-terminal end (21). The largely intracellular structure of RTP4 allows it to bind to receptors through their C-terminal tails, as has been shown for M opioid receptor, although the precise residues involved in the interaction remain to be determined. Binding of RTP4 to this motif would prevent dimer retention and/or serve as an adaptor protein for other proteins involved in routing toward the plasma membrane.
RTP4 has been shown to facilitate the export of odorant (21) and taste (22) receptors to the cell surface. We found that RTP4 is able to export μ-δ heterodimers by (i) countering the accumulation of μ-δ receptors in the Golgi apparatus, (ii) interacting with both receptors, and (iii) decreasing the ubiquitination of the heterodimers. Thus, we propose that RTP4 could work as a chaperone protein that promotes the dimerization of GPCRs. The fact that the μ-δ complex cannot traffic properly and thus is routed toward degradation pathways suggests that RTP4 functions as a chaperone to stabilize and transport the heterodimer complex. A study examining the pharmacologic chaperoning activities of opiate drugs found that the extent of ubiquitination of δ receptors was decreased significantly by these drugs (30). We found that μ or δ receptor–selective lipophilic opiate ligands significantly decrease the extent of ubiquitination of the heterodimer and enhance the level of their cell surface expression (Fig. S8). Taken together, these studies suggest that the maturation of opioid receptor heterodimers can be facilitated by opiates that function as pharmacologic chaperones and that RTP4 functions as an endogenous chaperone of μ-δ heterodimers.
Most of the reported chaperones to be involved in GPCR maturation appear to be predominantly intracellular, implying a transient interaction with the receptors (31). We found that RTP4 exhibits membrane localization and colocalization/association with μ-δ heterodimers, suggesting that the interaction of RTP4 with the receptor heterodimer is stable and persists after targeting of the complex to the cell surface. More studies are needed to explore the nature of this interaction and sites/domains of the receptor and of RTP4 involved in this interaction. Finally, because RTP4 is associated with the heterodimer at the cell surface, and because the heterodimers exhibit altered pharmacology, it is important to examine the extent of RTP4's involvement in modulating heterodimer pharmacology and heterodimer-mediated distinct signaling reported previously (17, 18). It is also important to examine the extent to which ubiquitination at different sites affects receptor exocytosis, endocytosis, and intracellular trafficking of the heterodimer, as has been seen for other GPCRs (32).
An increasing number of studies of dimerization in the maturation and/or targeting of GPCRs to the cell surface are associated with human diseases. Thus, the intracellular association of misfolded receptors (resulting from a mutated allele or single-nucleotide polymorphism) with the wild-type or common isoform of the receptor results in the intracellular retention of both receptors, preventing their cell surface expression (8, 9). In the case of opioid receptors, deletion of μ receptors has been shown to lead to an increase in cell surface expression and efficient coupling of δ receptors (33). This could also explain the enhanced δ receptor–mediated analgesia observed in animals lacking μ receptors in a neuropathic pain model (34). Chaperone proteins such as RTP4 likely play a significant role in regulating the relative levels of δ receptors uncomplexed with μ receptors, thus significantly affecting the relative contribution of each receptor homodimer or μ-δ heterodimer to opiate pharmacology. These examples emphasize the need for a better understanding of the mechanisms of maturation, targeting, and organization of GPCR oligomers. The identification of factors such as RTP4 that regulate dimerization will provide insight into how GPCR dimers affect the pharmacology of their endogenous ligands and/or of drugs with therapeutic potential.
Materials and Methods
Cell Culture and Transfection.
HEK293, Neuro2A and SKNSH cells were grown in Dulbecco's modified Eagle medium supplemented with 5% fetal bovine serum. For all transfections, lipofectamine 2000 (Invitrogen) was used on 60%–80% confluent cells in a six-well plate according to the manufacturer's protocol. For expression in HEK293 and Neuro2A cells, FLAG-tagged μ receptor cDNA was cotransfected with or without cDNAs for myc-tagged δ, GFP-tagged δ, mRTP4, FLAG-tagged mRTP4, and HA-tagged α2A, with pcDNA3 used to keep the total amount of transfected DNA constant in all cases. When performing triple cotransfections, 1 μg of μ or δ cDNA, 0.5 to 2 μg of RTP4 cDNA, or 1 μg of TGN38-GFP cDNA was used. For sequential expression of μ and δ receptors, the μ-receptor cDNA was transfected 24 h before the transfection of δ receptor cDNA. The FLAG epitope was introduced by polymerase chain reaction (PCR) on the N-terminal tail of mRTP4 using the forward oligonucleotide TTAAGCTTCCATGGACTACAAGGACGATGATGACGCCCTGTTCCCCGATGACTTCAG and the reverse oligonucleotide AATCTAGATTATCTAGTGAAAAGACTAAAAAG. The amplified fragment was cloned into pcDNA3 using the introduced HindIII and XbaI sites. For decreasing the endogenous levels of RTP4 in SKNSH cells, 4 μg per well of control siRNA or siRNA to human RTP4 (Dharmacon) was used, and cells were used 24 or 48 h after transfection.
ELISA and Immunostaining.
First, ≈5 × 105 cells/well were plated on a 96-well plate 24 h after transfection. The next day, the wells were washed with phosphate-buffered saline (PBS) and incubated with either polyclonal anti-FLAG (Sigma) or monoclonal anti-myc (Cell Signaling) antibodies in PBS/0.5% BSA on ice for 2 h. ELISA was performed as described in ref. 28. For immunostaining, cells were plated 24 h after transfection on glass cover slips coated with poly-D-lysine. The next day, cells were fixed with methanol and incubated with polyclonal anti-FLAG and monoclonal anti-myc or monoclonal anti-HA (12CA5) antibodies in PBS/0.5% bovine serum albumin (BSA) at room temperature for 1 h. For subcellular studies in Neuro2A cells, anti-calnexin (Sigma) and anti-syntaxin 6 (Stressgen) antibodies were used as primary antibodies. Cells were then incubated for 1 h at room temperature with secondary Texas red or Cy5 anti-rabbit antibodies and Alexa 488 anti-mouse or Alexa 680 anti-mouse antibodies. When performing triple labeling of μ, δ, and RTP4, myc-tagged antibody conjugated to fluorescein (Cell Signaling) was used to detect δ receptors. Cover slips were mounted on Superfrost/Plus slides (ThermoFisher), and fluorescence was observed using a Leica TCS SP1 confocal microscope.
Coimmunoprecipitation and Western Blotting.
HEK293 cells were transfected with an equal amount of myc-tagged δ and HA-tagged μ or FLAG-tagged wild-type or Δ24-, Δ29-, or Δ45-truncated μ cDNA with or without mRTP4 or FLAG-tagged RTP4 cDNA. Then, 48 h after transfection, cells were washed with PBS and incubated with 5 μg of anti-HA, anti-myc (Cell Signaling), or anti-FLAG (M2, Sigma) monoclonal antibodies for 2 h on ice. Cells were washed three times and lysed in a buffer containing 1% Nonidet P-40, 10% glycerol, 300 mM NaCl, 1.5 mM MgCl2, 1 mM CaCl2, 50 mM Tris-Cl (pH 7.4), and protease inhibitor mixture (Sigma) for 2 h. The supernatant collected after 20 min of centrifugation was then incubated overnight at 4°C with 10% vol/vol of ImmunoPure Immobilized Protein A/G (Pierce). The beads were washed three times in lysis buffer, and elution was performed in 100 μl of 2X Laemmli buffer at 70°C for 15 min. Samples were separated on 8% or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western blotting using anti-FLAG, anti-myc polyclonal, anti-HA, or anti-ubiquitin monoclonal antibodies. Detection was performed after incubation with IRDye 680 or 800 secondary antibodies on the Odyssey Imaging System (Li-Cor). For deglycosyation, the immunoprecipitated receptors were treated with either endoglycosidase H or peptide N-glycosidase F according to manufacturer's protocol (New England Biolabs).
SEAP Assay.
Transfection of receptors with or without RTP4 was performed as described above for ELISA. First, ≈7 × 105 cells/well were plated 24 h after transfection onto a polyD-lysine–coated 96-well plate. The CAMP responsive element-secreted alkaline phosphatase plasmid (0.3 μg/well) was transfected 6 h later. On the next day, cells were stimulated for 6 h in media without serum with 10 μM forskolin and prespecified concentrations of DAMGO (Fig. 5B). Then 15 μl of supernatant was transferred into a second 96-well plate and the levels of alkaline phosphatase activity were quantitated as described in ref. 35.
Qualitative Reverse-Transcription PCR and Binding in SKNSH.
Either 24 h or 48 h after transfection of control siRNA or siRNA to RTP4, cells were collected and total RNA was extracted using the RNeasy kit (Qiagen). An equivalent amount of each RNA sample was used to perform reverse transcription (RT) using SuperScript II (Invitrogen). 1 μl of each product was used for quantitative RT-PCR using the SYBERgreen kit (Qiagen) and a LightCycler (Roche). Human RTP4 oligonucleotides were ATGGTTGTAGATTTCTGGACTTG (forward) and CTCTCTGTTGGTATTGCTTCC (reverse); human GAPDH oligonucleotides were GAAGGTGAAGGTCGGAGTC (forward) and GAAGATGGTGATGGGATTTC (reverse). Relative differences in the level of RNA were analyzed using the 2e(-ΔΔCT) method (36). For binding studies, cells were collected and incubated with 10 nM of 3H-DAMGO or 3H-Deltorphin in 50 mM Tris-Cl (pH 7.4) containing 0.32 M sucrose at room temperature for 1 h as described in ref. 13. Nonspecific binding was determined using 5 μM of D-phe-cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-Nll or Deltorphin. The cells were collected by filtration and radioactivity determined using a Wallac model 1209 beta counter.
Supplementary Material
Acknowledgments.
The authors thank Dr. Ivone Gomes for critically reading the manuscript and Drs. E. Illegens and R. Margolskee for providing the mouse RTP4 and RTP2 cDNAs. These studies were supported by National Institutes of Health Grants DA08863 and DA19251 (to L.A.D.) and R24 CA095823 (to the MSSM-Microscopy Shared Resource Facility).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804106105/DCSupplemental.
References
- 1.Devi LA. Heterodimerization of G-protein–coupled receptors: Pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 2.Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric properties of G protein-coupled receptor oligomers. Pharmacol Ther. 2007;115:410–418. doi: 10.1016/j.pharmthera.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Decaillot FM, Devi LA. Targeting opioid receptor heterodimers: Strategies for screening and drug development. AAPS J. 2006;8:E153–159. doi: 10.1208/aapsj080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein–coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Marshall FH, White J, Main M, Green A, Wise A. GABA(B) receptors function as heterodimers. Biochem Soc Trans. 1999;27:530–535. doi: 10.1042/bst0270530. [DOI] [PubMed] [Google Scholar]
- 6.Salahpour A, et al. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 7.Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J Biol Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- 8.Kaykas A, et al. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol. 2004;6:52–58. doi: 10.1038/ncb1081. [DOI] [PubMed] [Google Scholar]
- 9.Calebiro D, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- 10.Gomes I, Filipovska J, Jordan BA, Devi LA. Oligomerization of opioid receptors. Methods. 2002;27:358–365. doi: 10.1016/s1046-2023(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 11.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 12.Nitsche JF, et al. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes I, et al. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldhoer M, et al. A heterodimer-selective agonist shows in vivo relevance of G protein–coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan JS, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Fan T, et al. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- 18.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: Beta-arrestin2–mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Behrens M, et al. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem. 2006;281:20650–20659. doi: 10.1074/jbc.M513637200. [DOI] [PubMed] [Google Scholar]
- 23.Milojevic T, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 24.Petaja-Repo UE, et al. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 25.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein–coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 26.Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280:28663–28674. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- 27.Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: Real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem. 2006;281:27109–27116. doi: 10.1074/jbc.M604390200. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, et al. Conformation state-sensitive antibodies to G-protein–coupled receptors. J Biol Chem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law PY, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor–G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 30.Petaja-Repo UE, et al. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein–coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J Cell Biol. 2007;177:905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walwyn W, et al. Induction of delta opioid receptor function by up-regulation of membrane receptors in mouse primary afferent neurons. Mol Pharmacol. 2005;68:1688–1698. doi: 10.1124/mol.105.014829. [DOI] [PubMed] [Google Scholar]
- 34.Qiu C, Sora I, Ren K, Uhl G, Dubner R. Enhanced delta-opioid receptor-mediated antinociception in mu-opioid receptor–deficient mice. Eur J Pharmacol. 2000;387:163–169. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- 35.Decaillot FM, et al. Opioid receptor random mutagenesis reveals a mechanism for G protein–coupled receptor activation. Nat Struct Biol. 2003;10:629–636. doi: 10.1038/nsb950. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.