Abstract
Nucleotide excision repair (NER) is a major cellular defense mechanism against DNA damage. We have investigated the role of Mms19 in NER in the yeast Saccharomyces cerevisiae. NER was deficient in the mms19 deletion mutant cell extracts, which was complemented by the NER/transcription factor TFIIH, but not by purified Mms19 protein. In mms19 mutant cells, protein levels of the core TFIIH component Rad3 (XPD homologue) and Ssl2 (XPB homologue) were significantly reduced by up to 3.5- and 2.2-fold, respectively. The other four essential subunits of the core TFIIH, Tfb1, Tfb2, Ssl1, and Tfb4, and the TFIIK subunits Tfb3, Kin28, and Ccl1 of the holo TFIIH were not much affected by Mms19. Elevating Rad3 protein concentration by overexpressing the protein from a plasmid under the GAL1 promoter control restored proficient NER in mms19 mutant cells, as indicated by complementation for UV sensitivity. Overexpression of Ssl2 had no effect on repair. Overexpression of Rad3, Ssl2, or both proteins, however, could not correct the temperature-sensitive growth defect of mms19 mutant cells. These results show that Mms19 functions in NER by sustaining an adequate cellular concentration of the TFIIH component Rad3 and suggest that Mms19 has distinct and separable functions in NER and cell growth, thus implicating Mms19 protein as a novel multifunctional regulator in cells.
Keywords: DNA damage, DNA repair, repair regulation, transcription factor
Nucleotide excision repair (NER) is a major cellular defense mechanism against cytotoxicity and mutagenesis induced by UV radiation and a variety of chemical carcinogens (1). Deficient NER results in the human hereditary disease xeroderma pigmentosum (XP). NER involves three classes of proteins: indispensible, subpathway-specific, and accessory NER proteins. Most NER proteins are indispensible for repair, such as the yeast Rad1, Rad2, Rad4, Rad10, and Rad14 (XPF, XPG, XPC, ERCC1, and XPA in humans, respectively), and TFIIH. Losing any of the indispensable NER proteins results in complete inactivation of NER. Rad7, Rad16, Rad26, and Rad28 are pathway-specific NER proteins in that the Rad7/Rad16 complex is specifically required for global genome repair; whereas Rad26 and Rad28, Cockayne syndrome B (CSB) and A (CSA) in humans, respectively, are specific for transcription-coupled repair. Missing one of these genes inactivates either global genome repair or transcription-coupled repair, leaving the other NER subpathway intact. Accessory NER proteins include Rad23 (HHR23A/B in humans), Tfb5 (trichothiodystrophy A or TTD-A in humans), and Mms19. These proteins modify the activity of NER through different mechanisms. In the absence of an accessory NER protein, some residual levels of repair remain, leading to moderate rather than severe cellular sensitivity to DNA-damaging agents.
In the NER pathway, the most complex protein factor is TFIIH, a large protein complex indispensable for both NER and RNA polymerase II transcription. For repair, TFIIH is required to unwind the duplex DNA around the lesion with ATP hydrolysis (2–5), such that dual incisions can occur on the 3′ side of the damage by Rad2 (XPG) (6, 7) and on the 5′ side of the damage by the Rad1–Rad10 (XPF–ERCC1) complex (8–10) at the junction of single-stranded and double-stranded DNA in the helix-opened region. The holo TFIIH is composed of two subcomplexes: core TFIIH and TFIIK/CAK. Whereas core TFIIH is sufficient for the NER function, holo TFIIH is essential for transcription. Core TFIIH consists of six essential subunits, Ssl2/Rad25, Rad3, Tfb1, Tfb2, Ssl1, and Tfb4 in yeast (XPB, XPD, p62, p52, p44, and p34, respectively, in humans), and an accessory and nonessential subunit Tfb5; whereas TFIIK/CAK is composed of three essential subunits, Tfb3, Kin28, and Ccl1 in yeast (MAT1, CDK7, and cyclin H, respectively, in humans) (1). Tfb5 interacts with the core TFIIH subunit Tfb2, but not with other NER proteins (11). In NER, Tfb5 plays an accessory role (11, 12), likely by stabilizing the core TFIIH architecture (11). Inherited defects in TFIIH activity can lead to three human hereditary diseases, XP, combined XP and CS, and combined XP and TTD. Whereas XP is easily understood by deficient NER, CS and TTD are more complex diseases that are best understood by defects in NER and subtle deficiencies in transcription (13–15).
The mechanistic role of Mms19 is one of the least understood aspects of NER. Yeast mms19 deletion mutant cells are moderately sensitive to DNA-damaging agents and are temperature-sensitive for growth (16). Both transcription-coupled and global genomic NER in mms19 mutant cells are deficient (17). The human MMS19 gene was cloned (18, 19), and the gene product is capable of physical interactions with the XPB (Ssl2 homologue) and XPD (Rad3 homologue) subunits of human TFIIH (18). Extracts of the yeast mms19 deletion mutant cells exhibit a thermolabile defect in RNA polymerase II transcription in vitro, which led to the proposal that Mms19 is an upstream regulator of TFIIH activity (16). However, nothing is known about how TFIIH activity might be regulated by Mms19, and whether such regulation constitutes the Mms19 function in NER. Therefore, we have investigated the role of Mms19 in NER in the yeast S. cerevisiae. In this article, we demonstrate that Mms19 is required to sustain an adequate cellular concentration of the TFIIH component Rad3 for repair and show that Rad3 up-regulation is not responsible for Mms19 function in cell growth, suggesting distinct and separable functions for Mms19 in NER and cell growth.
Results
Deficient NER in mms19 Mutant Extracts Results from TFIIH Abnormality.
To gain insights into the role of Mms19 in NER, we performed in vitro repair assays in yeast cell-free extracts. In this assay, repair was measured by 32P-dCMP incorporation into the repair patch during repair DNA synthesis of plasmid containing N-2-acetylaminofluorene (AAF) adducts (Fig. 1, +AAF DNA bands). An undamaged plasmid larger in size was used in the same reaction as the internal control for nonspecific DNA synthesis (Fig. 1, −AAF DNA bands). Repair products were separated by electrophoresis on an argarose gel and visualized by autoradiography of the gel (Fig. 1 Lower). To show similar DNA recovery from various repair reactions, the gel was stained with ethidium bromide and photographed before autoradiography (Fig. 1 Upper). Under the conditions used, AAF DNA adducts are repaired specifically by the NER pathway in yeast cell-free extracts (20, 21).
Fig. 1.
Complementation of deficient NER in mms19 mutant cell extracts with purified TFIIH. (Upper) Ethidium bromide-stained gel. (Lower) Autoradiograph of the gel. (A) In vitro NER was performed in 50–200 μg of cell-free extracts of the various yeast strains as indicated. (B) Complementation experiments were performed by mixing the mms19 mutant extract with that of the rad3 mutant (lane 3) or rad14 deletion mutant (lane 4), respectively, using 100 μg of extract protein each. Repair in the WT (lane 1) and the mms19 mutant (lane 2) extracts was separately performed with 200 μg of extract protein each. (C) In vitro NER was performed in yeast cell-free extracts (200 μg) of the WT (lane 1) or the mms19 mutant strain in the absence (lanes 1 and 2) or presence of various amounts of purified TFIIH as indicated. +AAF, AAF-damaged pUC18 DNA; −AAF, undamaged pGEM3Zf DNA.
In cell-free extracts of the WT strain, proficient in vitro NER resulted in damage-specific radio-labeling of the AAF-containing plasmid, as compared with the internal control of undamaged DNA (Fig. 1 Lower, lanes 1) [supporting information (SI) Fig. S1A]. As expected, repair in the rad3 mutant (the rad3–2 allele, G461R) (22) or rad14 deletion mutant extracts was deficient (bottom panel of Fig. 1A, lanes 2–7), (Fig. S1A). As shown in Fig. 1A (lanes 8–10) and Fig. S1A, in vitro NER in the mms19 deletion mutant extracts was deficient. The deficient repair was complemented by rad14 mutant extracts (Fig. 1B, lane 4), but could not be complemented by the rad3 mutant extracts (Fig. 1B, lane 3) (Fig. S1B). We have previously shown that deficient NER in the rad3 mutant extracts reflects deficient function of TFIIH, as the mutant extract is complemented by purified TFIIH, but not by purified Rad3 protein (23). Thus, it is likely that TFIIH activity in the mms19 mutant extract is deficient. To test this possibility, we complemented the in vitro NER with purified TFIIH. As shown in Fig. 1C (lanes 3–5) and Fig. S1C, TFIIH restored proficient NER in the mms19 mutant extracts. In contrast, purified yeast Mms19 protein (Fig. S2A) was unable to complement deficient NER in mms19 deletion mutant extracts (Fig. S2 B and C). Complementation was not observed even when excessive amounts of Mms19 protein (up to 100 ng) were added to the in vitro repair reaction (Fig. S2 B and C). Under identical reaction conditions, in vitro complementation was observed when 1 ng of Tfb5 or 10 ng of Rad2 protein was added to the respective deletion mutant extracts (11, 24). These results show that deficient NER in mms19 mutant extracts results from TFIIH abnormality and suggest that Mms19 protein does not directly participate in the biochemistry of NER.
Requirement for Mms19 in Sustaining an Adequate Concentration of Rad3 and Ssl2 Proteins in Cells.
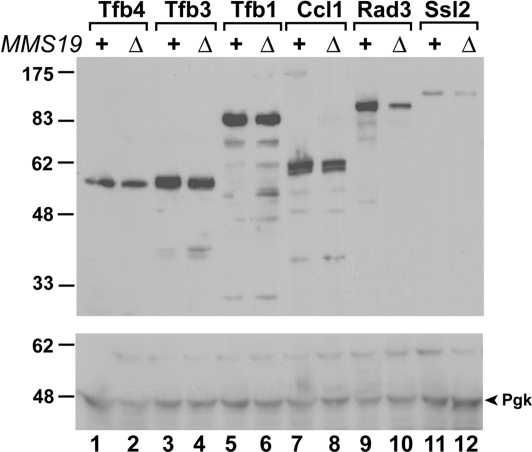
The TFIIH abnormality in mms19 mutant cells may simply result from a reduction in TFIIH concentration. To examine this possibility, we determined cellular levels of the individual subunits of TFIIH in the presence or absence of the Mms19 protein. The tandem affinity purification (TAP)-tagged SSL2, RAD3, TFB1, TFB2, SSL1, TFB4, CCL1, TFB3, and KIN28 strains were used. In each yeast strain, the corresponding chromosomal gene was tagged with the TAP epitope sequence at the 3′ end, resulting in a gene product containing the TAP tag at its C terminus. Cellular levels of the tagged protein in each strain can be readily detected by Western blot analysis using TAP-specific antibodies. The MMS19 gene in each of these nine TAP-tagged strains was deleted to create another nine strains. Then, cellular levels of the Ssl2, Rad3, Tfb1, Tfb2, Ssl1, Tfb4, Ccl1, Tfb3, and Kin28 proteins were determined by Western blot analysis in these isogenic strains in the presence or absence of the Mms19 protein. The TAP sequence adds 21 kDa to the tagged protein. 3-Phosphoglycerate kinase (Pgk) was used as a loading control for normalizing equal loading between the WT extract and its corresponding mms19 deletion mutant extract (Fig. 2, compare lanes 1 and 2, lanes 3 and lane 4, lanes 5 and 6, lanes 7 and 8, and lanes 9 and 10). As shown in Fig. 2A, the Rad3 (110 kDa) (compare lanes 5 and 6) and Ssl2 (116 kDa) (compare lanes 7 and 8) protein levels were reduced by 1.5- and 1.7-fold (after normalizing against the Pgk loading control), respectively, in mms19 deletion mutant cells from the level in WT cells. In contrast, Tfb1 (93 kDa), Ccl1 (66 kDa) (Fig. 2A, lanes 1–4), Tfb2 (79 kDa) (Fig. 2A, lanes 9 and 10), Ssl1 (73 kDa), Tfb4 (58 kDa), Tfb3 (59 kDa), and Kin28 (56 kDa) (Fig. 2B) protein levels (the full-length bands) were not reduced by deletion of MMS19. Quantitative protein ratios between the WT and the mms19 mutant cells for Tfb1, Ccl1, Tfb2, Ssl1, Tfb4, Tfb3, and Kin28 (after normalizing against the loading control) were 0.9, 1.0, 0.9, 0.7, 0.9, 1.0, and 0.9, respectively.
Fig. 2.
Protein levels of the TFIIH subunits in mms19 mutant cells. The TAP-tagged yeast SSL2, RAD3, TFB1, TFB2, SSL1, TFB4, CCL1, TFB3, and KIN28 strains containing the MMS19 gene (MMS19, +) or mms19 deletion (MMS19, Δ) were grown in rich medium. Cell extracts (50 μg of extract protein each) were separated by electrophoresis on a 10% SDS-polyacrylamide gel. The TAP-tagged proteins and the loading control Pgk (arrowhead) were identified by two-step Western blot analysis as described in Materials and Methods. Immuno-reactive protein bands were visualized by ECL detection on x-ray film. (A) Tfb1, Ccl1, Rad3, Ssl2, and Tfb2 proteins. (B) Ssl1, Tfb4, Tfb3, and Kin28 proteins. Protein size markers in kDa are indicated on the right.
The experiments shown in Fig. 2 were performed with cells grown in rich medium. Because the repair defects of mms19 deletion mutant cells are more severe when growing in minimum medium (see below), we examined the Rad3 and Ssl2 protein levels again with the mutant cells grown in minimum medium. As shown in Fig. 3 (compare lanes 9 and 10 and lanes 11 and 12), Rad3 and Ssl2 were reduced to a greater extent, by 3.5- and 2.2-fold (after normalizing against the loading control), respectively, in mms19 deletion mutant cells. Other TFIIH subunits examined, Tfb4, Tfb3, Tfb1, and Ccl1, were much less affected (Fig. 3, lanes 1–8), yielding a protein ratio between the WT and the mms19 mutant cells of 0.6, 1.1, 1.1, and 1.4, respectively. These results show that Mms19 is required to sustain an adequate concentration of Rad3 and Ssl2 proteins in yeast cells.
Fig. 3.
Rad3 and Ssl2 protein levels in mms19 mutant cells grown in minimum medium. The TAP-tagged yeast TFB4, TFB3, TFB1, CCL1, RAD3, and SSL2 strains containing the MMS19 gene (MMS19, +) or mms19 deletion (MMS19, Δ) were grown in minimum medium to an OD600 of ≈2. Cell extracts were prepared from equal numbers of cells. Equal amounts of the cell extracts were separated by electrophoresis on a 10% SDS-polyacrylamide gel. The TAP tagged proteins and the loading control Pgk (arrowhead) were identified by two-step Western blot analysis as described in Materials and Methods. Immuno-reactive protein bands were visualized by ECL detection on x-ray film. Protein size markers in kDa are indicated on the left.
Restoration of Proficient NER in mms19 Mutant Cells Specifically by Elevating Cellular Concentration of the Rad3 Protein.
If insufficient Rad3 or Ssl2 is the major cause of the deficient NER in the mms19 mutant, then, such repair deficiency should be corrected by elevating the cellular concentration of the corresponding protein. Accordingly, we constructed plasmids in which the RAD3 or SSl2 gene was under the control of the inducible GAL1 promoter and transformed the respective construct into the mms19 mutant strain. Protein overexpression from the plasmid was induced by galactose. In vivo NER was measured by a quantitative UV sensitivity assay. As shown in Fig. 4A, UV sensitivity of mms19 mutant cells was complemented by overexpressing the Rad3 protein from the plasmid. When cells were grown in minimal medium, the mms19 mutant strain was much more sensitive to UV radiation (Fig. 4B). For example, the mutant cells were 200-fold more sensitive to UV radiation at 30 J/m2 when grown in minimal medium than in rich medium (compare Fig. 4 B and A). The enhanced UV sensitivity of the mms19 mutant cells growing in minimal medium was again complemented by overexpressing the Rad3 protein from the plasmid (Fig. 4B). In contrast, overexpression of Ssl2 did not complement the UV sensitivity of mms19 mutant cells (Fig. 4B). Overexpression of Ssl2 in combination with Rad3 also did not affect complementation of the mms19 mutant by Rad3 (Fig. 4B). Similarly, overexpressing the TFB2 gene, which codes for another component of the core TFIIH, from the plasmid under the control of the GAL/1/10 promoter had no effect on the UV sensitivity of the mms19 mutant strain (Fig. S3). Overexpressing the Rad3 protein from the plasmid also had no effect on WT cells (Fig. S3).
Fig. 4.
Effect of Rad3 overexpression on UV sensitivity of mms19 mutant cells. Rad3 overexpression from the plasmid construct was achieved by growing cells in minimal medium containing 0.5% sucrose and 2% galactose (induction medium) for 14–16 h at 30°C. After dilution, cells were plated onto the rich (YP) induction medium (A) or minimal induction medium (B) plates. The uncovered plates were irradiated with UV light at the indicated doses. Surviving colonies were counted after incubation at 30°C for 2–5 days. Survival rates are expressed relative to those of nonirradiated cells with the standard deviations shown as error bars. (A) UV sensitivity assay in rich medium. Yeast strains are the WT BY4742 containing the empty vector (WT) (○) and mms19Δ containing the empty vector (mms19) (▵) or the RAD3 plasmid (mms19/Rad3) (▴). (B) UV sensitivity assay in minimal medium. Yeast strains are the WT BY4742 containing two empty vectors (○) and mms19Δ containing two empty vectors (mms19) (▵), the SSL2 plasmid and an empty vector (mms19/Ssl2) (◇), the RAD3 plasmid and an empty vector (mms19/Rad3) (▴), or the SSL2 and RAD3 plasmids (mms19/Ssl2 + Rad3) (⧫).
Overexpression of Rad3, Ssl2, Tfb2, Rad3 and Ssl2 together, or Rad3 and Tfb2 together from plasmids in either minimum or rich medium was confirmed by Western blot analysis (Fig. S4). Furthermore, expression of a given protein was similar with respect to whether the protein was expressed alone or simultaneously with another protein, in rich medium or minimum medium, in WT or mms19 mutant cells (Fig. S4). More degradation products were detected when Ssl2 was overexpressed in mms19 mutant cells as compared with the WT cells (Fig. S4, compare lanes 7, 8, 12, and 15 with lanes 9 and 10). However, this phenomenon was not observed with overexpression of Rad3 or Tfb2 (Fig. S4).
Taken together, these results show that proficient NER is restored specifically by elevating cellular concentration of the Rad3 protein.
Separation of Mms19 Functions in DNA Repair and Cell Growth.
In mms19 mutant cells, lower TFIIH activity as a result of lowered Rad3 protein concentration results in deficient NER (see above). Because TFIIH is essential for RNA polymerase II transcription, lower TFIIH activity might lead to compromised expression of some critical genes for cell growth, giving rise to the temperature-sensitive phenotype of the mms19 mutant strain. If this hypothesis is correct, then elevating the Rad3 protein level in the mms19 mutant strain should also correct its growth defect. Thus, we determined whether the temperature-sensitive growth of mms19 mutant cells can be complemented by overexpressing the Rad3 protein from the plasmid construct under the control of the GAL1 promoter.
Cells were grown at the permissive temperature (30°C) with Rad3 overexpressed from the plasmid construct. After serial dilutions, cells were spotted onto minimal medium plates to allow growth at either 30°C or 37°C (the restrictive temperature) with continued Rad3 overexpression. As shown in Fig. 5Left, mms19 mutant cells were viable at 30°C as indicated by similar numbers of colonies, but grew slower than the WT as evidenced by smaller colony sizes (e.g., the 10−4 dilution spots). Surprisingly, elevating cellular concentration of the Rad3 protein had no effect on the defective cell growth of the mms19 mutant strain at 37°C, as was the case of the negative control that overexpressed Tfb2 from the plasmid (Fig. 5 Right). Additionally, we also overexpressed Ssl2 alone or in combination with Rad3 from the plasmid under the control of the GAL1 promoter. Again, defective cell growth at 37°C in the absence of Mms19 was not corrected, as was the case that overexpressed both Tfb2 and Rad3 from the plasmid (Fig. 5 Right). The mms19 mutant cells grew slightly faster at 30°C when Ssl2 was overexpressed from the plasmid, although they still grew slower than the WT cells (Fig. 5 Left) (e.g., the 10−2 and 10−3 dilution spots). We also consistently observed that mms19 mutant cells grew slightly faster at 30°C when Rad3 was overexpressed, although the effect was less pronounced than that when Ssl2 was overexpressed (e.g., the 10−2 dilution spots in Fig. 5 and data not shown).
Fig. 5.
Effect of Rad3 overexpression on temperature-sensitive growth of mms19 mutant cells. Overexpression of Rad3, Ssl2, or Tfb2 protein or their combination as indicated on the right from the respective plasmid construct was achieved by growing cells in minimal medium containing 0.5% sucrose and 2% galactose (induction medium) for 14–16 h at 30°C. After serial dilutions as shown at the bottom, cells were spotted onto the minimal induction medium plates and grown at 30°C (Left) or 37°C (Right). Yeast strains are the WT BY4742 containing two empty vectors (WT) and the RAD3 and SSL2 plasmids (WT/Rad3 + Ssl2); and mms19Δ containing one empty vector (mms19), the RAD3 plasmid and an empty vector (mms19/Rad3), the SSL2 plasmid and an empty vector (mms19/Ssl2), the RAD3 and TFB2 plasmids (mms19/Rad3 + Tfb2), the TFB2 plasmid and an empty vector (mms19/Tfb22), and the RAD3 and SSL2 plasmids (mms19/Rad3 +Ssl2).
As was the case for in vivo NER reflected by UV sensitivity, mms19 mutant cells exhibited significantly less severe defect for growth when growing in rich medium as compared with minimal medium at 37°C (data not shown). In rich medium, mms19 mutant cells showed severe growth defect only when the incubation temperature was elevated to 39°C (Fig. S5). Again, such growth defect at 39°C in rich medium was not corrected by overexpression of Rad3, Ssl2, or both together (Fig. S5). The third phenotype of mms19 mutant cells is methionine auxotroph, i.e., the mutant cells are deficient in methionine biosynthesis (16). This defect also was not corrected by Rad3 overexpression (data not shown). These results show that lowered cellular concentration of the Rad3 protein is not the cause of defective cell growth of mms19 mutant cells. Therefore, the Mms19 protein has distinct and separable functions in NER and cell growth.
Discussion
Accessory NER proteins regulate/modulate the activity of NER through different mechanisms. Mms19 is such an accessory NER protein. We found that Mms19 functions in NER by sustaining an adequate cellular concentration of the TFIIH component Rad3.
The NER/transcription factor TFIIH is structurally and functionally complex. Studies on Tfb5 and its human homologue TTD-A (11, 12) led us to propose that this small protein functions as an architectural stabilizer of the core TFIIH (11). More recently, Ito et al. (25) reported that the human TFIIH is stabilized by XPG (Rad2 homologue), which was viewed as the molecular basis of the CS features associated with some XPG patients. Our present results revealed a novel mechanism of TFIIH regulation: up-regulation of the Rad3 protein concentration by Mms19. Without Mms19, the Rad3 protein level is much reduced in cells. As a result, it is expected that the cellular TFIIH concentration is reduced. Insufficient TFIIH levels would result in deficient NER as observed with the mms19 deletion mutant cells. Elevating the depressed Rad3 protein level by molecular techniques, thus bypassing the Mms19 regulation, indeed restored proficient NER in mms19 mutant cells. Therefore, Mms19 regulates TFIIH, and thus the NER pathway, via the Rad3 protein. Rad3 stabilization by Mms19 is a likely mechanism for its up-regulation in cells.
Unlike Tfb5 and XPG, purified Mms19 protein has no effect on deficient NER in mms19 deletion mutant extracts. Furthermore, elevating Rad3 protein concentration from its depressed levels in mms19 mutant cells resulted in proficient NER, even though Mms19 protein is still missing in these cells. Thus, it is unlikely that Mms19 may contribute to TFIIH stabilization, like TFB5 and XPG. Hence, the elaborate TFIIH protein complex is regulated by at least three proteins via three different mechanisms.
Overexpressing Rad3 alone is sufficient to restore deficient repair of mms19 mutant cells. Although cellular Ssl2 is also reduced without Mms19, its overexpression had no effect on deficient NER in mms19 mutant cells. Overexpression of Ssl2 together with Rad3 in the mutant cell also had no effect on the Rad3-mediated repair complementation. Therefore, Rad3 appears to be the sole NER target of Mms19 regulation. We observed that mms19 mutant cells overexpressing Rad3 were slightly more sensitive to UV radiation than the WT cells (Fig. 4). A possible role for Mms19 was suggested in recombination (16) and DNA damage tolerance (26). Such a role could be responsible for the slight UV sensitivity of mms19 mutant cells containing overexpressed Rad3.
Besides NER deficiency, mms19 deletion mutant cells exhibit additional cellular defects such as cell growth, as exhibited by a temperature-sensitive growth phenotype and a methionine auxotroph (16). Because Mms19 regulates TFIIH through Rad3, one would suspect that lowered concentration and thus activity of TFIIH in mms19 mutant cells may lead to a transcription deficiency that ultimately is responsible for other cellular defects such as temperature-sensitive growth. The observation that mms19 mutant cell extracts show a thermal labile defect in RNA polymerase II transcription in vitro (16) adds further to the suspicion that temperature-sensitive growth of the mutant cells is also a result of insufficient Rad3 and thus TFIIH concentration in cells, as is the case for NER. This does not prove to be the case. When TFIIH deregulation was corrected by overexpressing the Rad3 protein or both the Rad3 and Ssl2 proteins in mms19 mutant cells, the NER defect was corrected, but not the temperature-sensitive growth and methionine biosynthesis defects. Therefore, the NER function of Mms19 is apparently separated from its function in cell growth. A similar conclusion was made recently by Hatfield et al. (27) from deletion analysis of the human MMS19.
TFIIH is known to be thermally unstable. Incubating yeast nuclear extracts at 47°C for 4–7 min inactivates TFIIH activity for in vitro transcription (28). The thermal labile transcription observed in mms19 mutant extracts most likely reflected a lower concentration of TFIIH as a result of reduced Rad3 protein level, rather than a direct regulation of TFIIH activity through posttranslational protein modification by Mms19 as originally proposed (16). Thus, adding purified TFIIH, but not purified Mms19, led to complementation of the deficient in vitro transcription (16). At the permissive temperature, mms19 deletion mutant cells do not show severe defects in transcription as indicated by viable cell growth. Furthermore, in vitro transcription in mms19 mutant cell extracts is apparently normal at 25°C (16). In contrast, NER is severely deficient even at the permissive temperatures as indicated by UV sensitivity and deficient repair in the mutant cell extracts. Because the NER deficiency is caused by insufficient TFIIH via reduced Rad3 level, we conclude that NER may be more sensitive to fluctuations in TFIIH concentration than transcription is in cells. Thus, TFIIH may be in greater excess for transcription than for NER, and/or TFIIH may be preferentially recruited for transcription over NER in cells.
Our results suggest that Rad3/TFIIH regulation is not the sole mechanism for all cellular deficiencies of the mms19 mutant cells. Accordingly, we hypothesize that Mms19 is a novel multifunctional regulator in cells, affecting several important cellular processes that include NER and cell growth. Supporting this hypothesis, Wu et al. (29) reported that human MMS19 protein is an AF-1-specific coactivator of estrogen receptor. Consistent with our hypothesis, Mms19 is a large protein (118 kDa) predicted to contain two globular domains and conserved HEAT (Huntingtin, Elongation factor 3, protein phosphatsase 2A, TOR1) repeats (19). Recent deletion analysis by Hatfield et al. (27) indicated that the HEAT repeat of the human MMS19 is important for its complementation activities in yeast mms19 deletion mutant cells. It is possible that Mms19-mediated up-regulation of a target protein may also be used in regulating additional cellular processes such as cell growth.
Materials and Methods
Materials.
Purified yeast TFIIH was provided by Roger Kornberg (Stanford University, Stanford, CA) (30). Purified yeast Mms19 protein was obtained from Enzymax. N-acetoxy-N-2-acetylaminofluorene (AAAF, the activated form of AAF) was obtained from the Midwest Research Institute (Kansas City, MO). An ECL kit was purchased from Amersham Pharmacia Biotech. Affinity-purified rabbit polyclonal anti-TAP antibody was purchased from Open Biosystems. Mouse monoclonal anti-Pgk antibody was purchased from Invitrogen.
S. cerevisiae Strains.
BY4741 was purchased from ATCC. BY4742mms19Δ and TAP-tagged RAD3, TFB1, TFB2, TFB3, TFB4, SSL1, SSL2, KIN28, and CCL1 strains in the BY4741 genetic background were obtained from Open Biosystems via Brian Rymond (University of Kentucky). The C-terminal TAP tag consists of a calmodulin binding peptide, a TEV cleavage site, and two IgG binding domains of Staphylococcus aureus protein A, adding 21 kDa to the tagged protein (31).
BY4741mms19Δ and mms19 deletion in the TAP-tagged strains were created by deleting the MMS19 coding region via homologous recombination using the KanMX as the selection marker. Briefly, genomic DNA was isolated from the BY4742mms19Δ mutant strain. Two primers, 710upM (CCTCCAAGACATCCTGGCCTGAAG) and 406dnM (GGGCACATTAACGGACC-ATGAGAGG), were used to amplify the genomic mms19Δ-KanMX module of the BY4742mms19Δ strain by PCR. The resulting 2.7-kb PCR product contained 1.6 kb of the KanMX selection marker flanked by 710 bp upstream and 406 bp downstream of the genomic DNA homologous to the targeting locus of MMS19. BY4741, and the nine TAP-tagged strains were transformed with the purified DNA fragment (0.5 μg each). Transformed cells were selected on minimal medium plates containing geneticin (200 μg/ml). Deletion of the chromosomal MMS19 gene was initially confirmed by PCRs and was further confirmed by the phenotype of temperature-sensitive growth on minimal medium plates at 37°C.
In Vitro NER.
In vitro NER was performed as described (11). Repair products were separated by electrophoresis on a 1% agarose gel, and repair synthesis was visualized by autoradiography.
Western Blot Analysis.
Yeast TAP-tagged strains and their mms19Δ derivatives were grown to an OD600 of ≈2. Cells were homogenized in a buffer containing 20 mM Hepes-KOH (pH 7.6), 10 mM MgSO4, 10 mM EGTA, 20% glycerol, 5 mM DTT, and protease inhibitors (32) at 4°C with zirconium beads in a miniBeadbeater. Clear whole-cell extracts were obtained after centrifugation at 20,000 × g for 15 min at 4°C in a microcentrifuge. Cell extracts (50 μg of extract protein) from each strain were separated by electrophoresis on a 10% SDS-polyacrylamide gel. The TAP-tagged protein and Pgk (loading control) were identified by two-step Western blot analysis. First, the membrane was probed by using anti-TAP antibody followed by incubation with horseradish peroxidase-labeled secondary antibody. Immuno-reactive protein bands were visualized by ECL detection on x-ray film. Second, the membrane was stripped by incubation at 50°C for 30 min in a buffer containing 62.4 mM Tris·HCl (pH 6.7), 100 mM 2-mercaptoethanol, and 2% (wt/vol) SDS. The membrane was reprobed by using anti-Pgk antibody followed by ECL detection on x-ray film. Protein bands were quantitated by scanning densitometry of the film with SigmaGel software (Sigma) for analysis.
Measurement of UV Sensitivity.
Different combinations of plasmids pEGUh6-RAD3, pEGLh6-TFB2, pEGLh6-SSL2, and the empty vector pEGLh6 or pEGUh6 were transformed into TAP-tagged RAD3 strain (WT) and its isogenic mms19 deletion mutant strain. Transformed cells were grown at 30°C in minimum media containing 2% sucrose and the required amino acids. At an OD600 of ≈1, protein expression from the plasmids was induced by diluting the culture 10-fold in minimum media containing 2% galactose, 0.5% sucrose, and the required amino acids. After growing for 14–16 h at 30°C, cells were appropriately diluted and plated onto rich (YP, 2% Bacto-peptone and 1% yeast extract) or minimum medium plates containing 0.5% sucrose and 2% galactose. The uncovered plates were irradiated with short-wave UV at the indicated doses. Surviving colonies were scored after 2–5 days of incubation at 30°C. UV survival was calculated by dividing surviving colonies after UV treatment by those without UV treatment.
Supplementary Material
Acknowledgments.
We thank Roger Kornberg for purified yeast TFIIH and Brian Rymond for the TAP-tagged yeast strains, which were made possible by the bioinformatic infrastructure funds provided by National Science Foundation Infrastructure Award EPS-0132295 (to B.R.). This work was supported by National Institutes of Health Grant CA67978.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.B.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710736105/DCSupplemental.
References
- 1.Friedberg EC, et al. DNA Repair and Mutagenesis. Washington, DC: Am Soc Microbiol; 2006. [Google Scholar]
- 2.Evans E, Fellows J, Coffer A, Wood RD. Open complex formation around a lesion during nucleotide excision repair provides a structure for cleavage by human XPG protein. EMBO J. 1997;16:625–638. doi: 10.1093/emboj/16.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans E, Moggs JG, Hwang JR, Egly JM, Wood RD. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 1997;16:6559–6573. doi: 10.1093/emboj/16.21.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mu D, Wakasugi M, Hsu DS, Sancar A. Characterization of reaction intermediates of human excision repair nuclease. J Biol Chem. 1997;272:28971–28979. doi: 10.1074/jbc.272.46.28971. [DOI] [PubMed] [Google Scholar]
- 5.Wakasugi M, Reardon JT, Sancar A. The noncatalytic function of XPG protein during dual incision in human nucleotide excision repair. J Biol Chem. 1997;272:16030–16034. doi: 10.1074/jbc.272.25.16030. [DOI] [PubMed] [Google Scholar]
- 6.Habraken Y, Sung P, Prakash L, Prakash S. Structure-specific nuclease activity in yeast nucleotide excision repair protein Rad2. J Biol Chem. 1995;270:30194–30198. doi: 10.1074/jbc.270.50.30194. [DOI] [PubMed] [Google Scholar]
- 7.O'Donovan A, Davies AA, Moggs JG, West SC, Wood RD. XPG endonuclease makes the 3′ incision in human DNA nucleotide excision repair. Nature. 1994;371:432–435. doi: 10.1038/371432a0. [DOI] [PubMed] [Google Scholar]
- 8.Tomkinson AE, Bardwell AJ, Bardwell L, Tappe NJ, Friedberg EC. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature. 1993;362:860–862. doi: 10.1038/362860a0. [DOI] [PubMed] [Google Scholar]
- 9.Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994;265:2082–2085. doi: 10.1126/science.8091230. [DOI] [PubMed] [Google Scholar]
- 10.Sijbers AM, et al. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Kou H, Wang Z. Tfb5 interacts with Tfb2 and facilitates nucleotide excision repair in yeast. Nucleic Acids Res. 2007;35:861–871. doi: 10.1093/nar/gkl1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coin F, et al. p8/TTD-A as a repair-specific TFIIH subunit. Mol Cell. 2006;21:215–226. doi: 10.1016/j.molcel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Laine JP, Egly JM. When transcription and repair meet: A complex system. Trends Genet. 2006;22:430–436. doi: 10.1016/j.tig.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fousteri M, Mullenders LH. Transcription-coupled nucleotide excision repair in mammalian cells: Molecular mechanisms and biological effects. Cell Res. 2008;18:73–84. doi: 10.1038/cr.2008.6. [DOI] [PubMed] [Google Scholar]
- 16.Lauder S, et al. Dual requirement for the yeast MMS19 gene in DNA repair and RNA polymerase II transcription. Mol Cell Biol. 1996;16:6783–6793. doi: 10.1128/mcb.16.12.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombaerts M, Tijsterman M, Verhage RA, Brouwer J. Saccharomyces cerevisiae mms19 mutants are deficient in transcription-coupled and global nucleotide excision repair. Nucleic Acids Res. 1997;25:3974–3979. doi: 10.1093/nar/25.20.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seroz T, et al. Cloning of a human homolog of the yeast nucleotide excision repair gene MMS19 and interaction with transcription repair factor TFIIH via the XPB and XPD helicases. Nucleic Acids Res. 2000;28:4506–4513. doi: 10.1093/nar/28.22.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queimado L, et al. Cloning the human and mouse MMS19 genes and functional complementation of a yeast mms19 deletion mutant. Nucleic Acids Res. 2001;29:1884–1891. doi: 10.1093/nar/29.9.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wu X, Friedberg EC. A yeast whole cell extract supports nucleotide excision repair and RNA polymerase II transcription in vitro. Mutat Res. 1996;364:33–41. doi: 10.1016/0921-8777(96)00019-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, et al. The RAD7, RAD16 and RAD23 genes of S. cerevisiae: Requirement for transcription-independent nucleotide excision repair in vitro and interactions between the gene products. Mol Cell Biol. 1997;17:635–643. doi: 10.1128/mcb.17.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naumovski L, Chu G, Berg P, Friedberg EC. RAD3 gene of Saccharomyces cerevisiae: Nucleotide sequence of wild-type and mutant alleles, transcript mapping, and aspects of gene regulation. Mol Cell Biol. 1985;5:17–26. doi: 10.1128/mcb.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, et al. Transcription factor b (TFIIH) is required during nucleotide excision repair in yeast. Nature. 1994;368:74–76. doi: 10.1038/368074a0. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Liu S, Zhang Y, Wang Z. Roles of Rad23 protein in yeast nucleotide excision repair. Nucleic Acids Res. 2004;32:5981–5990. doi: 10.1093/nar/gkh934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, et al. XPG stabilizes TFIIH, allowing transactivation of nuclear receptors: Implications for Cockayne syndrome in XP-G/CS patients. Mol Cell. 2007;26:231–243. doi: 10.1016/j.molcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Chanet R, Heude M. Characterization of mutations that are synthetic lethal with pol3–13, a mutated allele of DNA polymerase delta in Saccharomyces cerevisiae. Curr Genet. 2003;43:337–350. doi: 10.1007/s00294-003-0407-2. [DOI] [PubMed] [Google Scholar]
- 27.Hatfield MD, et al. Identification of MMS19 domains with distinct functions in NER and transcription. DNA Repair. 2006;5:914–924. doi: 10.1016/j.dnarep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Feaver WJ, Gileadi O, Kornberg RD. Purification and characterization of yeast RNA polymerase II transcription factor b. J Biol Chem. 1991;266:19000–19005. [PubMed] [Google Scholar]
- 29.Wu X, Li H, Chen JD. The human homologue of the yeast DNA repair and TFIIH regulator MMS19 is an AF-1-specific coactivator of estrogen receptor. J Biol Chem. 2001;276:23962–23968. doi: 10.1074/jbc.M101041200. [DOI] [PubMed] [Google Scholar]
- 30.Svejstrup JQ, Feaver WJ, LaPointe J, Kornberg RD. RNA polymerase transcription factor IIH holoenzyme from yeast. J Biol Chem. 1994;269:28044–28048. [PubMed] [Google Scholar]
- 31.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 32.Xin H, et al. The human RAD18 gene product interacts with HHR6A and HHR6B. Nucleic Acids Res. 2000;28:2847–2854. doi: 10.1093/nar/28.14.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.