Abstract
An elaborate network of highly inducible phase 2 proteins protects aerobic cells against the cumulative damaging effects of reactive oxygen intermediates and toxic electrophiles, which are the major causes of malignancy and chronic degenerative diseases. Many chemical and phytochemical agents, all of which react with thiol groups, induce the phase 2 response through their reactivity with critical cysteine thiols of Keap1. We recently found that the anti-inflammatory potencies (suppression of iNOS and COX-2 expression) of a series of triterpenoids with Michael reaction centers were closely correlated with the potencies of these agents to induce the phase 2 response. We now report that representatives of seven recognized chemical classes of inducers, including isothiocyanates, bisbenzylidenes, arsenicals, heavy metals, and vicinal dithiols, showed highly correlated inducer and anti-inflammatory potencies spanning more than six orders of magnitude of concentrations in established cells and in primary mouse peritoneal macrophages. Potency measurements were expressed as the Dm values (median effect concentration) by use of the Median Effect Equation. Whereas the phase 2 induction required the functional integrity of both the repressor Keap1 and the transcription factor Nrf2, the effectiveness of inducers in blocking the up-regulation of iNOS by inflammatory cytokines was related to the nature of the cytokine and the inducer concentration. These studies identify suppression of inflammation as a consistent property of inducers of the phase 2 response and strongly suggest that this property is a central aspect of their chemoprotective actions.
Keywords: Keap1, NAD(P)H:quinone oxidoreductase, Nrf2, sulforaphane, inducible NOS
Chronic degenerative and neoplastic diseases are the dominant medical problems of aging populations, and development of effective strategies for their prevention or retardation is critical. The life-long cumulative effects of oxidative stress, toxicities of electrophiles, damaging effects of radiation (especially from the sun), and inflammation are largely responsible for the pathogenesis of age-related chronic diseases. Aerobic cells have developed an elaborate and functionally overlapping, highly inducible network of “phase 2” genes for their protection (1–8).
Phase 2 genes are regulated by a common molecular signaling pathway that depends on the transcription factor Nrf2. The mechanisms of regulation of phase 2 genes involve the cytoplasmic repressor Keap1, which is equipped with highly reactive cysteine residues that are the sensors of inducers (9). Under basal conditions, Keap1 binds and retains Nrf2 in the cytoplasm and targets it for ubiquitination and proteasomal degradation. When inducers modify specific and highly reactive cysteine residues of Keap1, it loses the ability to repress Nrf2, which translocates to the nucleus, binds to the antioxidant response elements (ARE) of phase 2 genes, and activates their transcription (5–7, 9). Much evidence points to the powerful protective effects of phase 2 genes: (i) their up-regulation protects cells, animals, and humans against a wide variety of damaging agents including oxygen- and nitrogen-based oxidants, toxicities of carcinogens and other electrophiles, and radiation (5–7, 10); (ii) when the phase 2 induction mechanism is disrupted, cells are much more susceptible to the damaging toxicities; and (iii) numerous anticarcinogens have been identified and isolated from natural sources by bioassays that monitor induction of Nrf2-dependent enzymes such as NAD(P)H:quinone oxidoreductase (NQO1) (11–13). The successful strategy is exemplified by the phase 2 bioassay-guided isolation of the isothiocyanate sulforaphane from broccoli (12).
Inducers belong to 10 distinct chemical classes that include Michael reaction acceptors, isothiocyanates, arsenic derivatives, vicinal dimercaptans, and heavy metals (4, 14–16). All inducers react with thiol groups, and their inducer potencies parallel the rate of their reactivity with thiols (17, 18).
We have recently observed a remarkable correlation among the potencies of a large series of triterpenoid Michael acceptors as phase 2 enzyme inducers and their inhibition of inflammatory responses in macrophages (19). Although participation of phase 2 inducers in combating oxidative stress, electrophile toxicity, and radiation damage has been studied extensively, their capacity to block inflammatory processes has received less attention. In this paper, we focus on the recent finding that inducers of phase 2 genes also promote the anti-inflammatory state. We examined whether the correlation between phase 2 inducer and anti-inflammatory potencies of triterpenoids extends to other classes of inducers. We report here that several different chemical classes of inducers of the phase 2 response also suppress LPS-evoked inflammation in an established RAW264.7 macrophage-like cell line and in primary mouse macrophages. Mobilization of macrophage is the primary response of tissues to inflammation. LPS, a component of Gram-negative bacterial cell walls, and other cytokines (e.g., TNF-α and IFN-γ) are potent stimulants of macrophage accumulation and induction of iNOS and COX-2, which are markers of inflammation. The potencies of a wide variety of chemical agents exerting these two types of activities are remarkably closely correlated over more than six orders of magnitude of concentration, suggesting strongly that the two responses share common or very similar molecular targets. Thus, anti-inflammatory activity is likely to be an important component of the chemoprotective actions of phase 2 inducers.
Results
Comparison of Potencies of Compounds as Inducers of NQO1 and as Suppressors of iNOS Up-Regulation in Macrophages.
The two types of measurements were made on the same cells treated identically, thereby minimizing potential confounding effects such as differences in uptake and metabolism of chemical agents in different cell types. We first established a standardized procedure for up-regulating iNOS and for quantifying its inhibition. Previously IFN-γ was used as the proinflammatory cytokine in mouse RAW264.7 cells, and the abilities of compounds to inhibit induction of iNOS were compared with their potencies as inducers of NQO1 in murine hepatoma cells (19). With use of IFN-γ, the response of RAW264.7 cells to inducers of NQO1 was extremely low, which is consistent with the finding that IFN-γ induced Bach1 and repressed the ARE-dependent expression of HO-1 in human cells (20, 21), and that overexpression of Bach1 also repressed endogenous and transfected NQO1 gene expression (22). Bach1 belongs to the cap'n'collar b-Zip family of transcription factors that include Nrf2, and Bach1 presumably competes with Nrf2 for binding to the ARE (23), thereby suppressing transcription of phase 2 genes. In contrast, LPS had little effect on induction of NQO1 (data not shown), and thus, LPS was chosen to stimulate iNOS expression when NO production and NQO1 activity induction were measured on the same cells.
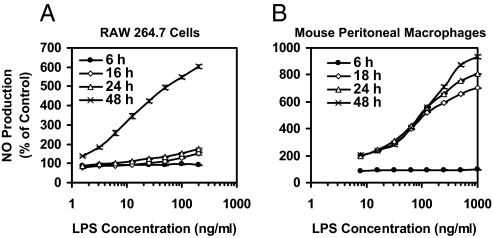
RAW264.7 macrophage-like cells produce very little NO in the first 24 h when stimulated by LPS (1–200 ng/ml), but NO production increased dramatically and dose-dependently in the subsequent 24 h (Fig. 1). We used a concentration of LPS (10 ng/ml) that was near the midpoint of the dose-response curve for RAW264.7 macrophages. Primary peritoneal macrophages responded more quickly but required higher LPS concentrations (100 ng/ml).
Fig. 1.
Time- and dose-response of iNOS activity (NO production) induced by LPS in macrophages. RAW264.7 cells (A) (2 × 104 per well) or mouse peritoneal macrophages (B) (105 per well) in 96-well plates were exposed to serial dilutions of LPS for up to 48 h. NO production was measured as nitrite accumulation in the medium. Controls contained no LPS. Results are expressed as means ± SEM (n = 8).
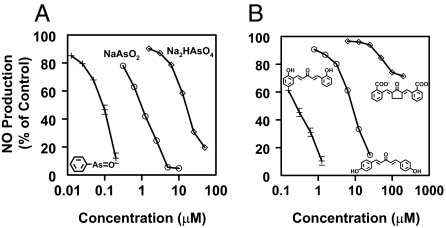
For potency comparisons, we chose 19 phase 2 inducers belonging to 7 structurally very different chemical classes (Table 1). Within each class, we selected compounds that were similar in structure but differed considerably in potency. Fig. 2 shows concentration-effect plots for inhibition of LPS-stimulated NO formation in RAW264.7 macrophages for two classes of compounds: (i) arsenic derivatives—trivalent sodium arsenite (CD 0.21 μM in Hepa 1c1c7 murine hepatoma cells), pentavalent sodium arsenate (CD 12 μM), and phenyl arsenoxide (CD 0.057 μM) and (ii) bisbenzylidene Michael acceptors—bis(2-hydroxybenzylidine)acetone (CD 0.2 μM), bis(4-hydroxybenzylidine)acetone (CD 14 μM), and bis(2-carboxybenzylidine)cyclopentanone (CD 65 μM). In these examples, the potency ratio between the most and least potent compounds for induction of NQO1 in Hepa1c1c7 murine hepatoma cells was 200- to 300-fold.
Table 1.
Potencies of chemically distinct families of compounds in elevating NQO1 activity and in inhibiting LPS-stimulated iNOS activity in RAW 264.7 cells, expressed as Dm values, and their potencies as inducers of NQO1 in murine hepatoma cells (Hepa 1c1c7), expressed as CD (concentrations required to double) values
| Chemical Class | Compound | CD, μM in Hepa 1c1c7 |
Dm, μM in RAW 264.7 (Rank order)† |
|
|---|---|---|---|---|
| Induction of NQO1 | Induction of NQO1 | Inhibition of iNOS | ||
| Isothiocyanates | RS-Sulforaphane | 0.22 | 1.86 (5) | 0.44 (6) |
| Benzyl isothiocyanate | 2.7 | 27.3 (11) | 2.23 (10) | |
| Hexyl isothiocyanate | In* | 1080 (18) | 12.4 (14) | |
| Triterpenoids | TP-225 | 0.0003 | 0.0035 (1) | 0.0011 (1) |
| TP-155 | 0.001 | 0.012 (2) | 0.0053 (2) | |
| TP-151 | 0.0023 | 0.054 (3) | 0.033 (3) | |
| Bis(benzylidenes) | Bis(2-hydroxybenzylidene)acetone | 0.2 | 7.58 (8) | 0.26 (5) |
| Bis(4-hydroxybenzylidene)acetone | 14 | 91.2 (14) | 7.33 (12) | |
| Bis(2-carboxylbenzylidene)cyclopentanone | 65 | 792 (17) | 508 (18) | |
| Arsenicals | Phenyl arsenoxide | 0.057 | 0.27 (4) | 0.066 (4) |
| Sodium arsenite | 2.1 | 7.37 (7) | 0.87 (8) | |
| Sodium arsenate | 12 | 403 (16) | 15.0 (15) | |
| Heavy Metals | Mercuric chloride | 0.72 | 62.4 (12) | 18.8 (16) |
| Cadmium chloride | 10.5 | 10.1 (9) | 5.29 (11) | |
| Mercaptans | 2,3-Dimercaptopropanol (BAL) | 24 | 75.4 (13) | 8.14 (13) |
| Dihydrolipoic acid | 40 | 274 (15) | 94.6 (17) | |
| Propane-1,3-dithiol | In* | In (19) | 898 (19) | |
| Chelates | BAL/HgCl2 (10:1) | 0.10 | 2.85 (6) | 0.45 (7) |
| BAL/HgCl2 (1:1) | 0.28 | 21.4 (10) | 1.32 (9) | |
TP-225, 2-cyano-3,12-dioxooleane-1,9(11)-dien-28-onitrile; TP-155, methyl-2-cyano-3,12-dioxooleane-1,9(11)-dien-28-oate; TP-151, 2-cyano-3,12-dioxooleane-1,9(11)-dien-28-oic acid.
*Inactive (In) is defined as less than a 20% increase in the induction ratio (treated/control) at the highest concentration at which there was less than 50% cell toxicity.
†The potency order ranks for each type of measurement are given in parentheses.
Fig. 2.
Effect of potent phase 2 inducers on iNOS activity (NO production). RAW264.7 cells (2 × 104 per well) in 96-well plates were incubated for 24 h and treated with LPS (10 ng/ml) in the simultaneous presence of serial dilutions of arsenic derivatives (A) or bis(benzylidene) derivatives (B) for an additional 48 h. NO production was then measured as nitrite accumulation in the medium. Control cells were treated with LPS only. Results are expressed as means ± SEM. (n = 8).
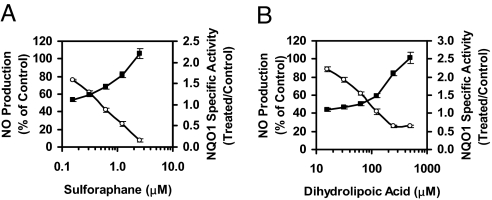
As expected, induction of NQO1 and inhibition of up-regulation of iNOS were negatively correlated processes, as shown for the two representative agents, sulforaphane (an isothiocyanate) (Fig. 3A) and dihydrolipoic acid (a vicinal dithiol) (Fig. 3B). For each compound, the two effects occurred within the same concentration ranges, which differed by more than two orders of magnitude.
Fig. 3.
Chemically distinct families of compounds dose-dependently induce NQO1 activity while inhibiting LPS-stimulated NO production. RAW264.7 cells (2 × 104 per well) were grown for 24 h in 96-well plates and exposed to LPS (10 ng/ml) in the simultaneous presence of serial dilutions of sulforaphane (A) and dihydrolipoic acid (B) for 48 h. NO production was then measured as nitrite accumulation in the medium (open circles) and NQO1 activity (filled squares) was determined in cell lysates. Control cells were treated with LPS only. Results are expressed as means ± SEM. (n = 8).
Methods for Correlation of Potencies as Inducers of NQO1 and Suppressors of iNOS Up-Regulation by LPS.
Among the innumerable methods for analyzing dose-response relations, the Median Effect Equation of Chou (24, 25) is very useful for obtaining highly quantitative results. The equation fa/fu = (D/Dm)m, where fa is the fraction of a process that is affected, fu is the fraction unaffected (i.e., 1 − fa), D is the dose of compound required to produce the effect fa, and Dm is the concentration at which a 50% effect is obtained (i.e., fa = fu). The advantage of this analysis is that it provides a linear transformation of all experimental observations in the determination of Dm, in contrast to conventional methods of expressing inhibitory potencies (IC50, LD50) which often rely on interpolation between values near the midpoint of effectiveness. To our knowledge, such Dm values have been used in the past only for quantifying inhibitory processes. Application of the Median Effect Equation to the induction of NQO1 activity required additional considerations [see supporting information (SI) Text and Fig. S1].
Structure-Activity Comparisons of Inducer and Anti-inflammatory Potencies.
All of the 19 inducers of NQO1 in murine hepatoma cells also induced this enzyme in RAW264.7 macrophages and dose-dependently inhibited LPS-stimulated NO production. Table 1 compares the median effect concentrations (Dm) in these two types of assays in macrophages and with the CD values for induction of NQO1 determined in murine hepatoma cells (14, 15). The potencies (Dm) spanned more than six orders of magnitude in concentration, ranging from the triterpenoid TP-225, which was the most potent inhibitor of iNOS (Dm for NQO1 induction = 0.0035 μM; Dm for iNOS inhibition = 0.0011 μM), to the least potent compound examined, propane-1,3-dithiol (Dm for NQO1 induction = inactive; Dm for iNOS inhibition = 898 μM).
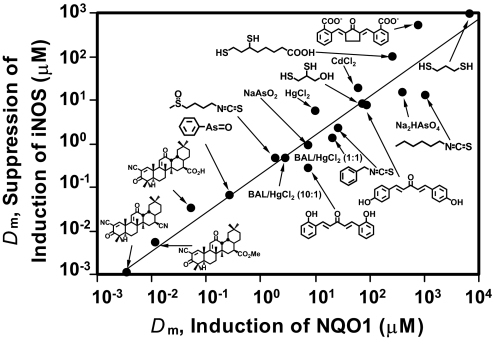
Interestingly, in RAW264.7 cells, the Dm values for inhibition of iNOS are consistently lower than those for induction of NQO1 and are very similar to the CD values in Hepa1c1c7 cells, perhaps reflecting the specialization and sensitivity of the two cell types to pro-inflammatory stimuli (RAW264.7) and inducers of drug metabolism (Hepa1c1c7), respectively. Strikingly, the rank orders of potencies of the 19 compounds (Table 1) of inhibition of iNOS up-regulation and NQO1 induction in RAW264.7 cells were highly correlated over more than six orders of magnitude with an r2 value of 0.93 and a slope of 0.88 (Fig. 4). The P value for trend was 0.023, and the Spearman's ρ value was 0.37, corresponding to a P value of 0.020.
Fig. 4.
Correlation of potencies of 19 compounds for suppression of iNOS induction by LPS and as inducers of NQO1 in RAW264.7 cells, expressed as Median Effect (Dm) values given in Table 1. The linear correlation coefficient is r2 = 0.93 and the slope is m = 0.88.
Despite differences in the absolute magnitudes of the potencies in suppressing iNOS up-regulation by LPS and in inducing NQO1 in murine macrophages, the extremely close correlation between rank orders of the potencies of extraordinarily diverse chemical compounds, belonging to seven very different chemical classes, agrees with and expands our observations on a large series of triterpenoid Michael reaction acceptors compared in different cell lines (19). This result strongly suggests that the anti-inflammatory and phase 2 induction pathways are probably closely linked functionally and mechanistically.
Protection of Macrophages Against Oxidative Stress by Inducers of Phase 2 Response.
Induction of the phase 2 response protects against reactive oxygen species (ROS) arising from exogenous oxidants and oxidative cycling in many cell lines, including ARPE-19 retinal pigment epithelial cells (26, 27) and U937 leukemia cells (19). We examined this protection and its dependence on nrf2 gene function by measuring formation of fluorescent products from the oxidation-sensitive dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in RAW264.7 cells and peritoneal macrophages derived from WT and nrf2−/− mice that were exposed to tert-butyl hydroperoxide (Table 2). The levels of ROS observed are clearly suppressed by the nine inducers tested (belonging to four different chemical classes). The median effect concentrations required to reduce ROS parallel the inducer potencies of these compounds in RAW264.7 cells and primary peritoneal macrophages (see Table 1). In marked contrast, there is no protection by six of the nine compounds in peritoneal macrophages derived from nrf2−/− mice which, as expected, did not induce the phase 2 response (data not shown). The protective effects of the three dithiols (Table 2) in nrf2−/− macrophages probably result from direct scavenging of ROS by the thiol groups of these compounds, i.e., their direct antioxidant properties.
Table 2.
Potencies of chemically distinct compounds in suppression of ROS in different cells, expressed as Dm values
| Compound | Median effect concentration, Dm, μM |
||
|---|---|---|---|
| RAW 264.7 | WT macrophages | nrf2−/− macrophages | |
| RS-Sulforaphane | 3.14 | 9.48 | In |
| Hexyl isothiocyanate | 17.5 | 148 | In |
| Bis(2-hydroxybenzylidene)acetone | 0.60 | 3.64 | In |
| Bis(4-hydroxybenzylidene)acetone | In | In | In |
| Sodium arsenite | 3.78 | 13.9 | In |
| Sodium arsenate | 137 | 31.4 | In |
| 2,3-Dimercaptopropanol | 7.78 | 187 | 17.8 |
| Propane-1,3-dithiol | 136 | 232 | 28.4 |
| BAL/HgCl2 (1:1) | 0.61 | 0.57 | 0.022 |
After 24 h of incubation with test compounds, the cells were exposed to 20 μM DCFH-DA for 30 min and then challenged with 500 μM tert-butyl hydroperoxide for 30 min. The fluorescence intensity was measured. Controls contained no test compounds, and the potencies (Dm) were determined by Median Effect Equation of Chou. Inactive (In) is defined as less than 10% suppression of ROS.
Does Suppression of LPS-Induced iNOS in Primary Macrophages Require Nrf2?
Much evidence indicates that induction of the phase 2 response plays a key role in cellular protection against carcinogens largely by detoxifying oxidants, electrophiles, and blocking the effects of UV radiation. Thus, the present studies examined whether the Keap1/Nrf2/ARE pathway, which is essential for phase 2 gene induction, is also obligatory for suppression of inflammatory responses. We compared inhibition of LPS-dependent up-regulation of iNOS by phase 2 inducers in peritoneal macrophages from WT and nrf2−/− mice. In contrast to RAW264.7 cells, both WT and nrf2−/− mouse peritoneal macrophages produced large amounts of NO dose-dependently in response to LPS in the first 24 h, and the NO production remained at a high level for at least 48 h (Fig. 1B). Surprisingly, when WT and nrf2−/− peritoneal macrophages stimulated by LPS (100 ng/ml) were treated for 24 h with seven phase 2 inducers belonging to five different chemical classes, all of the test compounds inhibited NO production with similar potencies irrespective of their Nrf2 status (Table 3). As expected, induction of NQO1 was not observed in nrf2−/− cells, indicating that suppression of LPS up-regulation of iNOS by phase 2 inducers does not require Nrf2 or induction of phase 2 enzymes.
Table 3.
Potencies of chemically distinct compounds in elevating NQO1 activity and in inhibiting LPS-stimulated iNOS activity in different cells, expressed as Dm values
| Compound | Median effect concentration, Dm, μM |
|||||
|---|---|---|---|---|---|---|
| RAW 264.7 |
WT macrophages |
nrf2−/− macrophages |
||||
| Induction of NQO1 | Inhibition of iNOS | Induction of NQO1 | Inhibition of iNOS | Induction of NQO1 | Inhibition of iNOS | |
| RS-Sulforaphane | 1.86 | 0.44 | 21.5 | 1.54 | In | 0.81 |
| Triterpenoid TP-225 | 0.0035 | 0.0011 | 0.21 | 0.21 | In | 0.2 |
| Bis(2-hydroxybenzylidene)acetone | 7.58 | 0.26 | 12.3 | 1.67 | In | 1.97 |
| Bis(4-hydroxybenzylidene)acetone | 91.2 | 7.33 | In | 87.7 | In | 32.9 |
| Phenyl arsenoxide | 0.27 | 0.066 | 0.35 | 0.03 | In | 0.061 |
| Sodium arsenite | 7.37 | 0.87 | 14.8 | 3.85 | In | 9.32 |
| 2,3-Dimercaptopropanol (BAL) | 75.4 | 8.14 | 100 | 30.8 | In | 46.6 |
Macrophages were incubated with serial dilution of test compounds in the presence of 10 ng/ml LPS for 48 h (RAW 264.7), or 100 ng/ml LPS for 24 h (WT and nrf2−/− peritoneal macrophages). NO production was then measured as nitrite accumulation in the medium, and NQO1 activity was determined in cell lysates from the same wells. Control cells were treated with LPS only. The potencies (Dm) were determined by Median Effect Equation of Chou. Inactive (In) is defined as less than 10% induction of NQO1.
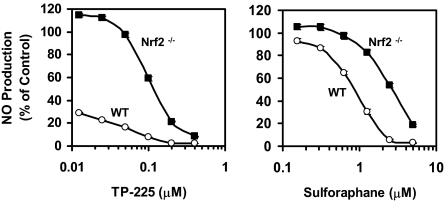
Nonetheless, when we stimulated primary macrophages with both IFN-γ and TNF-α in place of LPS, two potent phase 2 inducers (sulforaphane and the triterpenoid TP-225) were much less effective inhibitors of NO production in Nrf2−/− macrophages than they were in WT macrophages (Fig. 5). This is consistent with our previous findings with mouse embryo fibroblasts (MEF) treated with both IFN-γ and TNF-α, in which a potent triterpenoid inducer suppressed NO production in WT but not nrf2−/− MEF (19). This apparent paradox may be explained if LPS and TNF-α induce iNOS expression mainly through the NF-κB signaling pathway, whereas IFN-γ utilizes the JAK/STAT pathway. In addition, a synergistic regulation of gene transcription by IFN-γ and LPS/ TNF-α that involves complex interactions between NF-κB and STAT families of transcription factors has been demonstrated in various cell types (28, 29). Our findings therefore suggest that both Nrf2-dependent and -independent pathways lead to inhibition of the up-regulation of iNOS by phase 2 inducers.
Fig. 5.
Effects of potent phase 2 inducers on iNOS activity (NO production) in WT and Nrf2 −/− macrophages. Cells (105 cells per well) in 96-well plates were exposed to serial dilutions of triterpenoid TP-225 (Left) and sulforaphane (Right), in the presence of both IFN-γ and TNF-α, both at 10 ng/ml each for 24 h. Then NO production was measured as nitrite accumulation in the medium. Control cells were treated with IFN-γ and TNF-α only. Results are expressed as means ± SEM (n = 8).
Discussion
Several studies have suggested that the Keap1/Nrf2/ARE pathway is involved in immune and inflammatory processes in addition to its critical role in phase 2 induction. Yoh et al. (30) showed that aged Nrf2-deficient female mice developed lupus-like autoimmune responses. Moreover, nrf2 knockout mice showed prolonged inflammation during cutaneous wound healing (31) and displayed enhanced bronchial inflammation and susceptibility to severe airway inflammation and asthma (32, 33). The Keap1/Nrf2/ARE pathway has also been shown to modulate redox-sensitive inflammatory gene expression in endothelial cells (34). Also in Nrf2-deficient mouse embryonic fibroblasts exposed to both inflammatory cytokines INF-γ and TNF-α, we observed that the Keap1/Nrf2/ARE pathway is essential for the anti-inflammatory effects of triterpenoids (19). Others have reported that Nrf2 deficiency also resulted in augmented lung inflammation in response to nonlethal challenge with LPS or TNF-α, suggesting that Nrf2 suppressed inflammation by inhibiting NF-κB activation through regulation of redox balance (35), and that activation of Nrf2-dependent compensatory antioxidative pathways by a triterpenoid (CDDO-Im) protects from LPS-induced inflammatory response and mortality (36). Very recently, hepatocyte-specific conditional Keap1 knockout and nrf2-WT mice, but not nrf2-knockout mice, pretreated with CDDO-Im were found to be highly resistant to Con A-mediated inflammatory liver injury (37). All these studies suggest the important role of the Keap1/Nrf2/ARE pathway in protecting against the damaging effects of inflammation.
A fundamental aspect of the mechanism of the phase 2 response is the chemical reaction of inducers with critical and highly reactive thiol groups of cysteine residues of Keap1 (38, 39). Further evidence that Keap1 is the primary sensor and target for inducers was provided by spectroscopic demonstration that Keap1 reacts directly with inducers of many different types including sulforaphane, bis(2-hydroxybenzylidene)acetone, and triterpenoid TP-225 (19, 38). The extremely close correlation between the potencies of many compounds in inducer and anti-inflammatory assays raises the question whether both processes also depend on Keap1 or on another common mechanism involving modification of reactive cysteine thiols. Although macrophages are difficult to obtain from Keap1 null mice, studies with double knockout keap1−/−::nrf2−/− mice should be informative.
Although it is tempting to attribute the extremely close correlation between the potencies of compounds as inducers of Nrf2-dependent genes and their suppression of iNOS to the possibility that modification of critical cysteine residues of Keap1 is common to both signaling pathways, it is also possible that other cysteine-dependent redox systems that control inflammation may be involved. Thus, NF-κB, which is known to be up-regulated by sulforaphane (40), is a DNA-binding transcription factor that remains sequestered in the cytoplasm as an inactive complex with its inhibitory counterpart IκBs. When exposed to inflammatory stimuli, such as cytokines and microbial infection, IκBs are rapidly phosphorylated and degraded, which results in the release of free NF-κB dimers (p50 and p65) that translocate to the nucleus for the transcription of target genes. IκB kinase (IKK) is the protein kinase responsible for IκB phosphorylation and degradation in response to proinflammatory stimuli (41, 42). Structurally, IKK subunits have cysteine residues located at functionally important sites such as Cys-179 at the activation loop of IKKβ (43). Cysteine residues are also conserved in the DNA-binding domain (Cys-62 in p50 and Cys-38 in p65) of NF-κB heterodimer (44). Thiol-reactive agents may directly inhibit IKK kinase activity or NF-κB DNA-binding by affecting the redox state of critical cysteine residues in the IKK kinase activation loop and NF-κB DNA-binding loop. Indeed, several thiol-reactive compounds such as cyclopentenone prostaglandin (15d-PGJ2), arsenite, and two synthetic triterpenoids have been shown to suppress the NF-κB pathway through binding to Cys-179 of IKKβ, Cys-38 of p65, or Cys-62 of p50 (43, 45–48). Further, 15d-PGJ2 binds to specific cysteines of Keap1 thereby activating the Keap1-Nrf2/ARE pathway and can also exert anti-inflammatory actions by inhibiting the NF-κB pathway (49, 50).
In conclusion, despite the extraordinarily close potency correlation among compounds that are chemically diverse in inducing the Keap1/Nrf2/ARE response and suppressing the up-regulatory response of inflammatory cytokines, it is not clear that the primary sensors for both responses are necessarily identical. What is clear, however, is that all inducers of the phase 2 response examined have anti-inflammatory activity, and inducer potency is a reliable and highly useful predictor of anti-inflammatory activity.
Materials and Methods
Chemicals.
Structures are shown in Fig. 4. Triterpenoids were a gift of M. B. Sporn (Department of Pharmacology and Toxicology, Dartmouth Medical School). Synthetic RS-sulforaphane and benzyl isothiocyanate were from LKT Laboratories. Bis(benzylidene)acetones were synthesized (15). 2,3-Dimercaptopropanol (BAL) was from Alfa Aesar. All other chemicals were from Sigma-Aldrich. BAL-mercury chelates were prepared by incubating 1:1 and 10:1 molar ratios of BAL/HgCl2 at 25°C for 30 min in dimethyl sulfoxide solution (51).
Cell Culture.
Murine macrophage-like RAW264.7 cells were obtained from the American Type Culture Collection and cultured at 37°C in DMEM containing 10% heat-inactivated (55°C for 30 min) FBS.
Cell Treatment.
RAW264.7 cells (20,000 cells per well) were plated in 96-well plates, grown for 24 h, and exposed to serial dilutions of test compounds in the presence of 10 ng/ml LPS. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 48 h.
Preparation of Peritoneal Macrophages from nrf2−/− Mice.
nrf2−/− mice on C57BL/6 background were initially established by Itoh et al. (52). WT C57BL/6 mice were purchased from Charles River Laboratories and used as a comparative control. Macrophages were harvested by lavage from the peritoneal cavity with Dulbecco's phosphate buffered saline (DPBS) 4 days after an i.p. injection of thioglycolate broth (Brewer, 4%). Isolated cells were plated into 96-well plates at a density of 105 cells per well in RPMI medium 1640 containing 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. After 3 h of incubation at 37°C in 5% CO2, nonmacrophage cells were washed out by DPBS. The remaining macrophages were exposed immediately to serial dilutions of test compounds in the presence of 100 ng/ml LPS or 10 ng/ml of both IFN-γ and TNF-α for 24 h.
Measurement of iNOS Induction.
Nitrite concentration in culture supernatants of RAW264.7 cells and peritoneal macrophages, measured as an indicator of iNOS induction, were determined by the Griess reaction (53). Briefly, 100 μl of each supernatant was mixed with 100 μl of Griess reagent [1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl)ethylenediamide dihydrochloride]. Absorbance was measured at 550 nm and compared with a standard curve of sodium nitrite. The nitrile values of cells treated with LPS but without test compounds were used as controls.
Assay of NAD(P)H-Quinone Acceptor Oxidoreductase (NQO1) Activity.
After the Griess reaction assays for nitrite, the culture media were discarded, and cells were washed three times with DPBS. Cell lysates were prepared, and the specific activities of NQO1 were determined (11, 54). Cells treated with LPS or IFN-γ and TNF-α but without test compounds were used as controls.
Detection of Intracellular ROS.
Macrophages were treated with serial dilutions of the test compounds for 24 h, and then incubated with 20 μM 2′,7′-DCFH-DA for 30 min (55). After washing with PBS twice, the cells were challenged with 500 μM tert-butyl hydroperoxide for 30 min, and fluorescence intensity was measured in a microplate reader with excitation at 485 nm and emission at 535 nm.
Statistical Analysis.
All calculations were performed with STATA 10.0 (Stata Corporation, College Station, TX). The Np trend used is a nonparametric test for trend across ordered groups and is an extension of the Wilcoxon rank sum test.
Supplementary Material
Acknowledgments.
We thank J. W. Fahey for assistance with the statisti-cal analysis, S. Stein for preliminary experiments, and T. C. Chou for advice on the use of the Median Effect Equation. Triterpenoids were provided by M. B. Sporn, T. Honda, and G. Gribble (Dartmouth School of Medicine, Hanover, NH), bis-benzylidene Michael acceptors were provided by R. E. Bozak and R. J. Hicks (California State University, Hayward, CA), and nrf2−/− mice were provided by T. W. Kensler (Johns Hopkins University Bloomberg School of Public Health). This work was supported by National Institutes of Health Grants CA06793 and CA93780, American Cancer Society Grant RSG-07–157-01-CNE, the American Institute for Cancer Research, the Lewis B. and Dorothy Cullman Foundation, and the W. Patrick McMullan Family Fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808346105/DCSupplemental.
References
- 1.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radical Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 2.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. BioFactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 3.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 4.Holtzclaw WD, Dinkova-Kostova AT, Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: The quest for the elusive sensor that responds to inducers. Adv Enzyme Regulation. 2004;44:335–367. doi: 10.1016/j.advenzreg.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Molec Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 8.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 10.Talalay P, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci USA. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Talalay P, Cho C-G, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YH, Pezzuto JM. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Methods Enzymol. 2004;382:380–414. doi: 10.1016/S0076-6879(04)82021-4. [DOI] [PubMed] [Google Scholar]
- 14.Prestera T, Zhang Y, Spencer SR, Wilczak CA, Talalay P. The electrophile counterattack response: Protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 15.Dinkova-Kostova AT, Abeygunawardana C, Talalay P. Chemoprotective properties of phenylpropenoids, bis(benzylidene)cycloalkanones, and related Michael reaction acceptors: Correlation of potencies as phase 2 enzyme inducers and radical scavengers. J Med Chem. 1998;41:5287–5296. doi: 10.1021/jm980424s. [DOI] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 17.Spencer SR, Wilczak CA, Talalay P. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990;50:7871–7875. [PubMed] [Google Scholar]
- 18.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinkova-Kostova AT, et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamuro T, et al. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 21.Udono-Fujimori R, et al. Expression of heme oxygenase-1 is repressed by interferon-gamma and induced by hypoxia in human retinal pigment epithelial cells. Eur J Biochem. 2004;271:3076–3084. doi: 10.1111/j.1432-1033.2004.04241.x. [DOI] [PubMed] [Google Scholar]
- 22.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 23.Oyake T, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 25.Chou T-C, Martin N. Paramus, NJ: ComboSyn; 2005. CompuSyn for Drug Combinations. A Computer Software for Quantitation of Synergism and Antagonism, and the Determination of IC50, ED50 and LD50 Values. [PC software and user's guide] [Google Scholar]
- 26.Gao X, Dinkova-Kostova AT, Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: The indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci USA. 2001;98:15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paludan SR. Synergistic action of pro-inflammatory agents: Cellular and molecular aspects. J Leukoc Biol. 2000;67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Ganster RW, Guo Z, Shao L, Geller DA. Differential effects of TNF-alpha and IFN-gamma on gene transcription mediated by NF-kappaB-Stat1 interactions. J Interferon Cytokine Res. 2005;25:707–719. doi: 10.1089/jir.2005.25.707. [DOI] [PubMed] [Google Scholar]
- 30.Yoh K, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Braun S, et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangasamy T, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rangasamy T, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen XL, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 35.Thimmulappa RK, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006a;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thimmulappa RK, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006b;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osburn WO, et al. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinkova-Kostova AT, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakabayashi N, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 41.Baeuerle PA. IkappaB-NF-kappaB structures: At the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 42.Karin M. How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 43.Kapahi P, et al. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 44.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 45.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 46.Straus DS, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 48.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 49.Itoh K, et al. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putzer RR, et al. Mercurials and dimercaptans: Synergism in the induction of chemoprotective enzymes. Chem Res Toxicol. 1995;8:103–110. doi: 10.1021/tx00043a014. [DOI] [PubMed] [Google Scholar]
- 52.Itoh K, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 53.Suh N, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- 54.Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 55.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of ROS formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.