Abstract
Activation-induced cytidine deaminase (AID) is essential for the DNA cleavage that initiates both somatic hypermutation (SHM) and class switch recombination (CSR) of the Ig gene. Two alternative mechanisms of DNA cleavage by AID have been proposed: RNA editing and DNA deamination. In support of the latter, AID has DNA deamination activity in cell-free systems that is assumed to represent its physiological function. To test this hypothesis, we generated various mouse AID mutants and compared their DNA deamination, CSR, and SHM activities. Here, we compared DNA deamination, CSR, and SHM activities of various AID mutants and found that most of their CSR or SHM activities were disproportionate with their DNA deamination activities. Specifically, we identified a cluster of mutants (H48A, L49A, R50A, and N51A) with low DNA deamination activity but relatively intact CSR activity. Of note is an AID mutant (N51A) that retained CSR function but lost DNA deamination activity. In addition, an APOBEC1 mutation at N57, homologous to N51 of AID, also abolished DNA deamination activity but retained RNA editing activity. These results indicate that DNA deamination activity does not represent the physiological function of AID.
Keywords: class switch, immunoglobulin gene, APOBEC1, somatic hypermutation, cytidine deaminases
The activation of B cells by antigen stimulation induces their extensive proliferation in germinal centers, giving rise to somatic hypermutation (SHM) and class switch recombination (CSR) in the Ig locus. Such DNA alterations confer the antibody memory required for effective vaccination. Activation-induced cytidine deaminase (AID) is specifically expressed in activated B cells and is required for both SHM and CSR (1, 2). AID is a member of the cytidine (C) deaminase family. It possesses a C deaminase domain that is indispensable for its physiological functions, and recombinant AID catalyzes the deamination of C in vitro (1). There is, however, a long-standing dispute about whether AID deaminates C to uridine (U) on DNA (DNA deamination model) (3–5) or on RNA (RNA editing model) (6–8). The DNA deamination model is based on the observations that AID induces a mutator phenotype in Escherichia coli and catalyzes the deamination of dC on single-stranded (ss) DNA in vitro (4, 5, 9, 10). However, AID's structural homology with APOBEC1 (1), a well established RNA editing C deaminase, suggests that it may edit mRNA to generate mRNAs encoding putative endonucleases or their guiding factors (7). This view is supported by the requirement for de novo protein synthesis (11, 12) and the nucleo-cytoplasmic shuttling of AID to achieve CSR (13). Thus, to clarify the mechanism by which AID promotes CSR, we examined the requirement for AID to deaminate dC on ssDNA to exert its physiological CSR activity. For this purpose, we looked for loss-of-deamination mutants of AID that could still mediate CSR, because such mutants should not exist if the DNA deamination activity is essential for AID function [supporting information (SI) Fig. S1].
Results and Discussion
DNA Deamination Activity of AID Mutants.
We aimed to investigate the correlation between three activities of AID, ssDNA deamination activity, CSR, and SHM. We chose a series of AID point mutants (alanine-replacements) at residues located outside the domains comprising the C deamination catalytic center, and those required for SHM-specificity, CSR-specificity, and nucleo-cytoplasm shuttling, to avoid mutants with obvious causes of physiological function loss (13–16). First, the mutant proteins were synthesized in vitro by using a wheat germ cell-free system. We analyzed the ssDNA deamination activity by using an in vitro reaction with improved sensitivity by Alexa-680 substarate labeling and infrared emission detection (Fig. S2). Among the mutants tested, one with alanine substituted for asparagine at position 51 (N51A) resulted in the complete loss of the ssDNA deamination activity, compared with the same amount of wtAID (Fig. 1A). Furthermore, although AID mutants at neighboring sites (D45A and R50A) showed a dose-dependent DNA deamination activity, N51A showed no enzymatic activity, even when the protein amount reached equimolar ratio to the substrate (Fig. 1B). Extended studies on the enzymatic activity of single and composite mutants within the region between D45 and C55 (Fig. 1C) identified a cluster of mutations between F46 and N51 that strongly reduced the DNA deamination activity, which ranged from complete loss (N51A) to severe reduction (G47A, H48A, L49A, and R50A). All mutants with double or triple substitutions, including N51A, had little or no DNA deamination activity.
Fig. 1.
In vitro DNA deamination activity of AID and its mutants. (A) Representative polyacrylamide gel electrophoresis analysis of the deamination activity by AID and its mutants. The 13- and 26-base bands indicate the product (deaminated and cleaved) ssDNA and substrate ssDNA, respectively. Western blot analysis shows that comparable amounts of AID protein were used. (B) Titration of AID and its mutants. All reactions contained 5 nM oligonucleotide and proteins at the indicated enzyme/substrate molar ratios, and each assay was repeated more than three times. (C) The relative deamination activity of each mutant was determined as a percentage of the wtAID activity at the enzyme concentration showing 50% cleavage by wtAID. Bars above columns represent mean ± SD. (D) Frequencies of RifR mutants generated after overnight culture of E. coli BL21 carrying an expression plasmid for AID, its mutants, or a vector control in the presence of isopropyl β-d-thiogalactoside. Each point represents the RifR colony number per 109 viable cells from an independent overnight culture. The median number of RifR colonies is indicated. Western blot analysis of whole lysates (107 viable cells) shows that the protein amounts of the mutant AIDs were not less than that of wtAID.
The ability of wtAID to cause a mutator phenotype in E. coli is reported to be a marker for its dC to dU deamination activity on ssDNA (5, 17). Therefore, we assayed the N51A mutant for its mutagenic potential in the E. coli system. The median number of rifampicin-resistant colonies induced by N51A expression was far less than that induced by wtAID and was comparable to that caused by vector alone or by a triple mutant in the catalytic center, KSS (H56K-C87S-C90S), which resulted in the total loss of any function (unpublished data) (Fig. 1D). Sequencing of the mutational hot-spot region of the rpoB gene in rifampicin-resistant clones revealed that the N51A mutation profile was indistinguishable from those of KSS and vector alone that may be attributable to an intrinsic mutagenic potential of E. coli as reported (5) (Fig. S3). These assessments of DNA deamination activity in the cell-free and E. coli systems clearly indicate that N51A possesses no DNA deamination activity.
Dissociation of DNA Deamination Activity and Physiological Function.
We next assessed the CSR activity of three mutants (D45A, R50A, and N51A) that carried normal, 20%, and no DNA deamination activity, respectively, compared with wtAID. We expressed a similar level of AID and its mutant proteins in AID−/− spleen B cells by retroviral gene transfer. We estimated the percentage of IgG1+-infected cells, 72 h after culturing, with LPS and IL-4 (Fig. 2A). Surprisingly, the deamination-defective mutant N51A showed half the WT level of CSR activity. Furthermore, although D45A had normal DNA deamination activity, it showed a very weak CSR activity. In contrast, R50A, which had only 20% of the wtDNA deamination activity, showed even stronger CSR activity than did wtAID. The relative CSR activities of the WT and mutant AID proteins were semiquantitatively confirmed by RT-PCR for γ1 switch transcripts (Fig. 2B). CSR activities of most (7 of 11) of the mutants were not proportional to DNA deamination activities, although the F46A, K52A, S53A, and C55A mutants showed a somewhat weak correlation between the two activities (Figs. 1C and 2C). Extremely surprising was the finding that a cluster of mutants, H48A, L49A, R50A, and N51A showed much higher relative CSR activity than expected from their DNA deamination activity. Mutations at residues between K52 and C55 revealed subtle effect on the CSR activity of AID, whereas all double and triple mutants showed severely reduced CSR activities. Collectively, all of the single mutants, especially at the residues between H48 and N51, showed striking discordance between their DNA deamination and CSR activities. The fact that CSR activity can be found in the N51A mutant without DNA deamination activity suggests that DNA deamination is not required for CSR.
Fig. 2.
CSR activities of AID mutants. (A) The N51A mutant of mouse AID showed a significant level of CSR. Representative FACS plots show the level of CSR as assessed by the percentage of IgG1+ cells among the infected AID−/− spleen cells (GFP+). The level of expression of AID for all infections was comparable is shown by GFP+ percentages in total lymphocyte gate. (B) RT-PCR determination of the level of γ1 switch transcripts from the same infected cells shown above confirmed the CSR activity of the mutant AID. (C) Summary of the CSR activity of all of the tested mutants as determined by FACS analysis. Bars above columns represent mean ± SD. More than three independent experiments were performed. (D) SHM activity of AID mutants. The point mutation frequency was determined by sequencing a GFP construct from infectants of each AID mutant. The genomic DNA used for this analysis was pooled from three independent experiments. (E) Comparison of the ssDNA deamination, CSR, and SHM activities of all of the tested mutants relative to WT AID (100%).
AID is well known to induce mutations in artificial target genes in nonlymphoid cells (18–20). We assessed the SHM activity of AID mutants by using a murine fibroblast cell line with an artificial GFP gene construct carrying a premature stop codon (20). This GFP reversion assay showed that D45A, R50A, and N51A all had relatively weak SHM activity compared with wtAID, at comparable protein amounts (Fig. S4). Seven days after retroviral gene transfer, we extracted the genomic DNA and sequenced the GFP gene, as described (16) (Fig. 2D). All of the mutants showed <20% of the SHM activity of wtAID except for R50A (30%), C55A (34%), and S53A (120%).
DNA deamination, CSR, and SHM activities of each AID mutant relative to wtAID are summarized in Fig. 2E. The results indicated that most of the mutants lost the three activities disproportionately except for S53A (all intact) and double/triple mutants including N51A (all lost). This dissociation of the three activities is more clearly presented by scatter plots of the mutants' deamination activities with their CSR and SHM activities (Fig. S5 and Fig. S6, respectively). It is striking that some AID mutants showed a strong DNA deamination activity in vitro, but their SHM activities were negligible. Of note are the single and double mutants of D45A (D45A-F46A and D45A-R50A), which had extremely low SHM activity despite having strong DNA deamination activity. However, whether or not the DNA deamination is involved in SHM is equivocal because the loss of SHM in the presence of the DNA deamination can be also explained by defects in other functions such as cofactor interaction. In addition, CSR and SHM activities of many AID mutants were affected independently, suggesting that AID may employ different mechanisms for CSR and SHM in consistence with reports (14–16).
Titration of DNA Deamination and CSR Activities in B Cells.
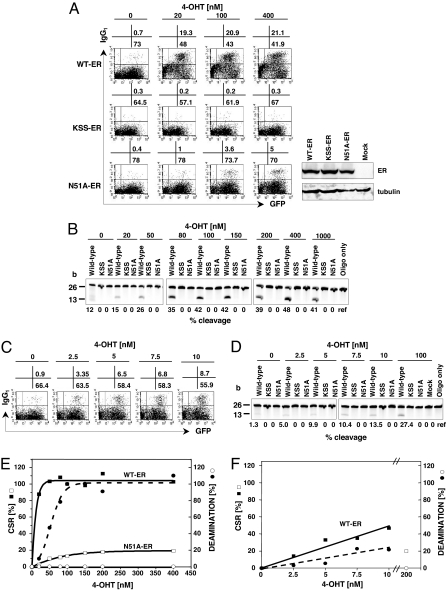
Because our findings were quite unexpected from the assumption that dC to dU deamination by AID initiates both CSR and SHM, we considered several possible explanations. First we had to exclude any experimental bias by the comparison of the activities of AID proteins from different expression systems (one synthesized in vitro and the other in mammalian B-cells for the deamination activity and CSR assay, respectively). For example, there may be B cell factors that stimulate DNA deamination activity. To address these possibilities, it appeared essential to titrate precisely the CSR activity of the wtAID and its corresponding deamination activity by using AID proteins in B cells. We assessed the CSR and DNA deamination activities of wtAID, N51A, and KSS fused to human estrogen receptor (ER) by titration of 4-hydroxytamoxifen concentration (4-OHT) as described (12). CSR activity of both wtAID-ER and N51A-ER showed a clear dose dependency on 4-OHT concentration (Fig. 3 A and E). The maximum level of CSR activity we observed for N51A under these conditions (48 h after 4-OHT addition) was 20% of wtAID-ER. Although we were able to demonstrate a significant level of dose-dependent DNA deamination activity for wtAID-ER, no deamination activity was detected for N51A-ER even at saturating 4-OHT concentrations for CSR (Fig. 3 B and E). KSS-ER had neither CSR nor DNA deamination activity. In an independent experiment, we assessed the deamination activity of wtAID-ER at 4-OHT concentrations that give rise to the CSR activity equivalent to N51A (≈20% wtAID) (Fig. 3 C and D). Obviously, the DNA deamination activity of wtAID-ER was readily detectable at these 4-OHT concentrations (Fig. 3 E and F). The results confirmed that N51A has no DNA deamination activity yet catalyzes CSR, indicating that DNA deamination is not required for CSR.
Fig. 3.
Determination of CSR and ssDNA deamination activities of ER-tagged wtAID and its mutants (KSS and N51A). (A) FACS plots after titration of 4-OHT. One day after retroviral infection and stimulation with LPS and IL-4, 4-OHT at increasing concentrations was added to each culture and the percentage of IgG1+ cells was determined 48 h later. Western blots represent the protein amounts after addition of 100 nM 4-OHT. (B) Polyacrilamide gel electrophoresis for determination of the DNA deamination activity of whole-cell lysates from the same experimental set used for CSR assay. Protein was extracted from cells incubated with 4-OHT at indicated concentrations by Dounce homogenization in PBS containing 10 μM ZnCl2 and 1% Nonidet P-40. Deamination assays were performed as described in Fig. 1. Percent cleavage was calculated by using densitometry. (C) FACS plots from an independent set of experiment for determination of wtAID-ER CSR activity at lower 4-OHT concentrations. (D) Deamination activity for wtAID-ER, KSS-ER, and N51A-ER determined in the same experiment as C. (E) Curve-fitted graph describing the relative activities of wtAID-ER (solid symbols) and N51A-ER (open symbols) based on the experiment described in A and B after subtraction of the levels at 0 nM 4-OHT. (F) Curve-fitted graph describing the relative activities of wtAID-ER based on the experiment described in C and D after subtraction of the levels at 0 nM 4-OHT. The activities of N51A-ER at 200 nM 4-OHT are taken from E.
Dissociation of DNA Deamination and Physiological Function in APOBEC1.
APOBEC1, another member of the C deaminase family, has DNA deamination activity in vitro, although it is well established to be a RNA editing enzyme of ApoB 100 mRNA (21). Because N51 in AID is homologous to N57 in APOBEC1 (Fig. S7), we examined DNA deamination and RNA editing activities of the N57A mutant of APOBEC1. As shown in Fig. 4 A and B, N57A almost lost DNA deamination activity even at the enzyme to substrate ratio of 7.5. Nonetheless, N57A converted the specific C in an ApoB 100 mRNA fragment into U with ≈20% efficiency of the WT APOBEC1 (Fig. 4C). This finding further supports our notion that DNA deamination activity of C deaminase does not necessarily represent its physiological function.
Fig. 4.
In vitro DNA deamination and RNA editing activities of APOBEC1 mutant. (A) Representative polyacrylamide gel electrophoresis analysis of deamination activity by AID, APOBEC1, and its mutant. The experimental condition is the same as in Fig. 1A. (B) Titration of AID, APOBEC1 and its mutant proteins for deamination activity. The experimental condition is the same as in Fig. 1B except for the molar ratio of enzyme to substrate. Each assay was repeated more than three times. (C) Apolipoprotein B RNA editing activities of AID, APOBEC1, and its mutant were determined by sequencing from two independent experiments. An RNA fragment (239 b)containing C at nt 6,666 of human apoB100 cDNA was synthesized in vitro and incubated at 30°C for 2 h with the indicated proteins synthesized in vitro. RNA was extracted, reverse-transcribed, and amplified by using PCR for cloning. p, frequency of mutations was compared with AID+ACF (Fisher's exact test).
Overexpressed APOBEC3G, which helps protect cells from HIV infection, deaminates C on cDNAs of the HIV retrovirus genome (22). However, this DNA deamination function is not required for the protection of host cells from HIV infection (23). One possible interpretation is that the DNA deamination activity of APOBEC3G emerges when it is overexpressed. The molecular mechanism underlying the anti-HIV function of APOBEC3G is still unknown (24). It is still completely uncertain whether the DNA deamination activity of C deaminase family members, including AID, APOBEC1, and APOBEC3G, contributes to their physiological functions.
Finally, why is the DNA deamination activity preferentially lost whereas the CSR activity is relatively intact in the mutants clustered between G48 and N51? Because they have the catalytic activity, a likely explanation is that the mutations in the G48-N51 region cause defect in a step before catalysis, such as binding to ssDNA, which may not be required for CSR. However, it is difficult to pin point the affected step because there may be several steps in the DNA deamination reaction. AID has been shown to bind not only DNA but also RNA (25), although the interaction of AID alone with both kinds of nucleic acids in vitro is not sequence specific (9, 26). According to the recent report, the AID mutants (S38A and S43P) show a high level of deamination activity at two non-hot-spot motifs, GGC and CGC, where AID is relatively inactive (27). However, N51A does not show any ssDNA deamination on these sequences (data not shown). If DNA deamination is not required for CSR, the alternative explanation for the function of AID, i.e. RNA targeting, is likely, although the question will remain open until the actual RNA target of AID is identified.
Materials and Methods
Mice.
AID knockout mice on a C57BL/6 background (8) were maintained in our animal facility under specific pathogen-free conditions. Spleen cells were obtained from these animals at 2–3 months of age. All mouse protocols were approved by the Institute of Laboratory Animals, Faculty of Medicine, Kyoto University (Kyoto, Japan).
AID Protein Preparation.
cDNAs of mouse AID and its various mutants with 9-His-tag at C-termini were cloned into pEU-MCS vector for in vitro transcription and translation. AID and its mutant proteins were synthesized in vitro by using wheat germ cell extracts (Cell Free Science) according to the manufacturer's recommendations with minor modifications. In vitro translation was performed for 28 h at 17°C with a supplement of 10 μM ZnCl2. At the end of synthesis, AID concentrations in wheat germ extracts were measured as described in this paragraph. In vitro-synthesized wtAID protein was partially purified from the wheat germ extract by using Ni+ beads (Qiagen) according to the manufacturer's protocols. The purified wtAID protein and 1 μg control mouse IgG antibody were run on 15% SDS polyacrylamide gel electrophoresis (PAGE), followed by Coomassie brilliant blue staining. The amount of wtAID (25 kDa) was estimated by comparison with that of the light chain of the antibody (333 ng) by using densitometry. The amounts of AID mutants in wheat germ extracts were determined by comparison with known amounts of the purified wtAID, described above, with densitometry of Western blots by using an anti-His monoclonal antibody.
Deamination Assay.
The Uracil DNA Glycosylase (UDG)-coupled deamination assay has been described (10). Alexa-flour 680-labeled single-stranded oligonucleotide (Alexa-5′-TTTTTTTTTTTAGCGTTTTTTTTTTT-3′) was purchased from Japan BioService. Briefly, 0.1 pmol of labeled substrate was incubated with AID in crude wheat-germ extract, 0.4 unit UDG, and 1 μg RNase for 60 min at 37°C in PBS supplemented with 10 μM ZnCl2 in a volume of 20 μl. The reaction was followed by incubation at 95°C for 8 min after addition of NaOH to 150 mM to cleave the alkali-labile abasic site. Samples were electrophoresed on 15% denaturing acrylamide gels and visualized by using Odyssey (Li-Cor). Cleavage ratio was determined by band quantitation by using Odyssey.
Frequency and Sequences of Mutations in E. coli.
The mouse AID and its mutant cDNAs were cloned into pET 23d vector (Novagen). The mutation assay was performed by using E. coli strain BL21 transformed with the vector alone or the expression construct. Fluctuation assay and mutation analysis of rpoB gene were performed as described (5). Within the 132-bp region analyzed, we detected mutations in 61 of 81, 81 of 84, 83 of 88, and 78 of 81 RifR colonies transformed with the vector control, AID, KSS, and N51A, respectively.
CSR Assay in AID−/− Spleen B Cells.
CSR assay was performed as described (16). RT-PCR was done as described (8).
SHM Assay in NIH 3T3-pI Clone 19 Cells.
SHM assay in mouse fibroblast harboring GFP with premature stop codon (28) as an artificial substrate for SHM was performed as described (16, 20).
4-OHT Titration for Determination of CSR and Deamination Activity.
ER-tagged constructs were obtained by cloning of the ER cDNA into pMSCV-wtAID-ires-GFP, pMSCV-KSS-ires-GFP, and pMSCV-N51A-ires-GFP. One day after infection of AID−/− cells stimulated with LPS and IL-4, cells were pooled, divided equally to add different concentrations of 4-OHT, and assayed for CSR and DNA deamination. Percentages of switched IgG1+ cells were determined 48 h later. On the same day, whole-cell lysates were prepared through Dounce homogenization in PBS containing 10 μM ZnCl2 and 1% Nonidet P-40. ssDNA deamination assay was performed as described above by using 3 μg whole cell lysate per reaction.
RNA Editing Assay.
cDNAs of rat APOBEC1 and its mutant with 9-His-tag at the C-terminus and human APOBEC1 complementation factor (ACF) were cloned into pEU-MCS vector and proteins were synthesized by in vitro transcription and translation as described above. For in vitro apoB RNA editing activity, 50-μl editing reactions containing 1 ng of in vitro transcribed human apoB100 RNA (nucleotides 6,541–6,780; GenBank NM 000384) and whole wheat germ extracts containing equal amounts of AID, APOBEC1, and its mutant, in addition to lysates containing ACF, were incubated at 30°C for 2 h in PBS and ZnCl2 10 nM. Editing efficiency at C6666 of apoB RNA was evaluated by sequencing after RT-PCR and cloning.
Supplementary Material
Acknowledgments.
We thank A. Kawamura and H. Hijikata for technical support, H. Nagaoka, I. Okazaki, N.A. Begum, and T. Doi for reading the manuscript and providing critical comments, Y. Endo, T. Sawasaki, and K. Okawa for critical suggestions in protein preparation, and Y. Shiraki and T. Nishikawa for technical assistance in preparing the manuscript. This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grant-in-Aid 17002015 for Specially Promoted Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806641105/DCSupplemental.
References
- 1.Muramatsu M, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 2.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri J, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 5.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 6.Honjo T, Nagaoka H, Shinkura R, Muramatsu M. AID to overcome the limitations of genomic information. Nat Immunol. 2005;6:655–661. doi: 10.1038/ni1218. [DOI] [PubMed] [Google Scholar]
- 7.Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. doi: 10.1016/S0065-2776(06)94001-2. [DOI] [PubMed] [Google Scholar]
- 8.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 9.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu K, Huang FT, Lieber MR. DNA substrate length and surrounding sequence affect the activation-induced deaminase activity at cytidine. J Biol Chem. 2004;279:6496–6500. doi: 10.1074/jbc.M311616200. [DOI] [PubMed] [Google Scholar]
- 11.Begum NA, et al. De novo protein synthesis is required for activation-induced cytidine deaminase-dependent DNA cleavage in immunoglobulin class switch recombination. Proc Natl Acad Sci USA. 2004;101:13003–13007. doi: 10.1073/pnas.0405219101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi T, Kinoshita K, Ikegawa M, Muramatsu M, Honjo T. De novo protein synthesis is required for the activation-induced cytidine deaminase function in class-switch recombination. Proc Natl Acad Sci USA. 2003;100:2634–2638. doi: 10.1073/pnas.0437710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, et al. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc Natl Acad Sci USA. 2004;101:1975–1980. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell. 2003;12:501–508. doi: 10.1016/s1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 16.Shinkura R, et al. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat Immunol. 2004;5:707–712. doi: 10.1038/ni1086. [DOI] [PubMed] [Google Scholar]
- 17.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, et al. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature. 2002;415:802–806. doi: 10.1038/nature714. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki IM, Kinoshita K, Muramatsu M, Yoshikawa K, Honjo T. The AID enzyme induces class switch recombination in fibroblasts. Nature. 2002;416:340–345. doi: 10.1038/nature727. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa K, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- 21.Navaratnam N, et al. Evolutionary origins of apoB mRNA editing: Catalysis by a cytidine deaminase that has acquired a novel RNA-binding motif at its active site. Cell. 1995;81:187–195. doi: 10.1016/0092-8674(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 22.Harris RS, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 23.Bishop KN, Holmes RK, Malim MH. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: Not simply editing? Trends Biochem Sci. 2007;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larijani M, et al. AID associates with single-stranded DNA with high affinity and a long complex half-life in a sequence-independent manner. Mol Cell Biol. 2007;27:20–30. doi: 10.1128/MCB.00824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham P, et al. Impact of phosphorylation and phosphorylation-null mutants on the activity and deamination specificity of activation-induced cytidine deaminase. J Biol Chem. 2008;283:17428–17439. doi: 10.1074/jbc.M802121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. Increased transcription levels induce higher mutation rates in a hypermutating cell line. J Immunol. 2001;166:5051–5057. doi: 10.4049/jimmunol.166.8.5051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.