Abstract
Homeostatic plasticity is thought to play an important role in maintaining the stability of neuronal circuits. During one form of homeostatic plasticity, referred to as synaptic scaling, activity blockade leads to a compensatory increase in synaptic transmission by stimulating in dendrites the local translation and synaptic insertion of the AMPA receptor subunit GluR1. We have previously shown that all-trans retinoic acid (RA) mediates activity blockade-induced synaptic scaling by activating dendritic GluR1 synthesis and that this process requires RARα, a member of the nuclear RA receptor family. This result raised the question of where RARα is localized in dendrites and whether its localization is regulated by RA and/or activity blockade. Here, we show that activity blockade or RA treatment in neurons enhances the concentration of RARα in the dendritic RNA granules and activates local GluR1 synthesis in these RNA granules. Importantly, the same RNA granules that contain RARα also exhibit an accumulation of GluR1 protein but with a much slower time course than that of RARα, suggesting that the former regulates the latter. Taken together, our results provide a direct link between dendritically localized RARα and local GluR1 synthesis in RNA granules during RA-mediated synaptic signaling in homeostatic synaptic plasticity.
Keywords: FMRP, GluR1, local protein translation, synaptic scaling
Homeostatic synaptic plasticity plays important roles in maintaining the stability of neural networks through the adjustment of global synaptic strength (1, 2). A particular form of homeostatic plasticity, referred to as synaptic scaling, modifies all synapses onto a neuron concurrently in a multiplicative fashion, with greater synaptic adjustment at stronger synapses than at weaker ones, allowing global changes of synaptic activity while preserving relative synaptic strength between individual synapses (3, 4). One well characterized form of synaptic scaling is the increase in synaptic strength induced by chronic blockade of neuronal activity with tetradotoxin (TTX) and the NMDA receptor antagonist APV. This form of synaptic scaling, manifested by an increase in synaptic glutamate receptor response, is mediated by the local synthesis and synaptic insertion of homomeric GluR1 receptors (5–7).
We recently found that all-trans retinoic acid (RA), the active form of retinoids in the brain, mediates activity blockade-induced synaptic scaling (8). We demonstrated that activity blockade—which induces homeostatic plasticity—strongly stimulates neuronal RA synthesis, which in turn activates dendritic GluR1 synthesis. We thus described a function of RA as a synaptic signal that operates during homeostatic plasticity to up-regulate synaptic strength by increasing the size of the postsynaptic glutamate receptor response. Additionally, we suggested that RARα may be critically involved in this form of RA signaling because RARα is present in dendrites, and knocking down RARα blocks synaptic scaling. These results were surprising, given our previous understanding of the function of RARα in transcription, and raised a number of questions. Where in the dendrites is RARα localized? Is its localization regulated? How does RA act on the localization of RARα, and how does this relate to the activation of GluR1 synthesis?
The actions of retinoids are primarily mediated by nuclear receptor proteins termed retinoic acid receptors (RAR-α, -β, -γ) and retinoid “X” receptors (RXR-α, -β, -γ). Like other members of the steroid receptor family, RAR and RXR are well known transcription factors. Although structurally similar, the ligand specificity differs between RAR and RXR in that RAR binds RA with high affinity, whereas RXR binds 9-cis-retinoic acid exclusively (9). Because 9-cis-RA is undetectable in vivo, the effects of retinoids on gene transcription are presumed to be mediated largely by RA interactions with RARs (10). In the mammalian brain, RARs exhibit distinct patterns of expression. Specifically, RARα is found in the cortex and hippocampus (11, 12), whereas RARβ is highly expressed in the caudate/putamen, nucleus accumbens, and olfactory tubercles (11, 12). RARγ is not detectable in the CNS (11) (but see ref. 12).
In the current study, we examined at the ultrastructural level the dynamic regulation of RARα localization by RA or activity blockade. Consistent with the transcription-independent action of RA during synaptic scaling (8), we found that RARα is present in neuronal dendrites in addition to its well accepted presence in the nucleus. In the dendrites, RARα is absent from RNA granules at basal conditions. RA treatment or activity blockade, however, stimulate rapid accumulation of RARα in RNA granules, where GluR1 synthesis is subsequently activated. These results suggest that the induction of local GluR1 synthesis in dendrites is associated with a RA-regulated change in the localization of RARα.
Results
RA Induces Local Translation in Neuronal Dendrites of a Reporter mRNA Containing the GluR1 UTRs.
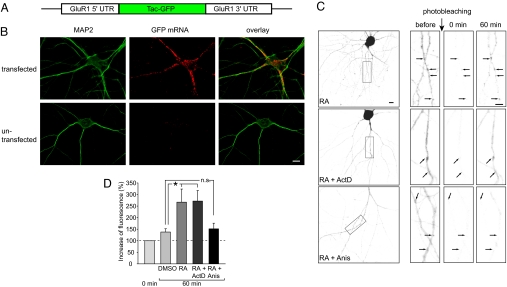
Dendritic targeting and translation of mRNAs are believed to be mediated by cis-regulatory elements in the 5′ and 3′ flanking the ORF. To examine whether GluR1 UTRs mediate RA-dependent translation control, we generated a reporter construct in which an ORF encoding a membrane-anchored GFP (Tac-GFP) is flanked by the 5′ and 3′ UTRs of the GluR1 mRNA (Fig. 1A). In hippocampal neurons transfected with this reporter construct, the Tac-GFP mRNA was targeted to dendrites (Fig. 1B). Twenty-four hours after transfection, GFP expression could be detected in the soma and dendrites. Although the GFP signal in the soma was usually saturated, the signal in dendrites showed discrete stationary “hotspots” on top of a diffuse background (Fig. 1C). We photobleached a 70 × 70-μm area covering the region of interest (boxed area in Fig. 1C) and its surround, and treated the cultured neuron with RA or DMSO. A marked increase in newly synthesized GFP in the dendritic hotspots was observed 30–60 min after RA treatment, whereas the recovery of diffuse background signal within this time frame was minimal (Fig. 1D). Consistent with the notion of local protein synthesis, this increase in newly synthesized GFP in the dendritic hotspots was sensitive to the translation inhibitor anisomycin but not to the transcription inhibitor actinomycin D (Fig. 1 C and D). The differences in the recovery of GFP signal at the hotspots are not due to treatment-related differences in protein diffusion because treated and untreated neurons exhibited a similar increase in GFP signal in dendritic regions outside of the hotspots (RA: 144.3 ± 11.4%, n = 13; RA plus ActD: 157.9 ± 20%, n = 18; RA plus anis: 166.9 ± 9.7%, n = 16; P > 0.1).
Fig. 1.
A GFP-reporter mRNA reveals RA-dependent regulation of dendritic GluR1 synthesis. (A) Schematic drawing of the reporter construct containing membrane-associated Tac-GFP and the 5′ and 3′ UTRs of the GluR1 mRNA. (B) In situ hybridization of the reporter mRNA encoding Tac-GFP in transfected hippocampal neurons (GFP-GluR1 UTR reporter mRNA, red; MAP2, green). (Scale bar: 10 μm.) (C) Representative grayscale images of a membrane-anchored Tac-GFP in neurons exposed to RA, RA plus ActD, or RA plus anisomycin before, immediately after (0 min), and 60 min after photobleaching. (Scale bar: 10 μm.) (D) Quantification of the increase in GFP signal after photobleaching (Anis, the translation inhibitor anisomycin; ActD, transcription inhibitor actinomycin D. *, P < 0.01).
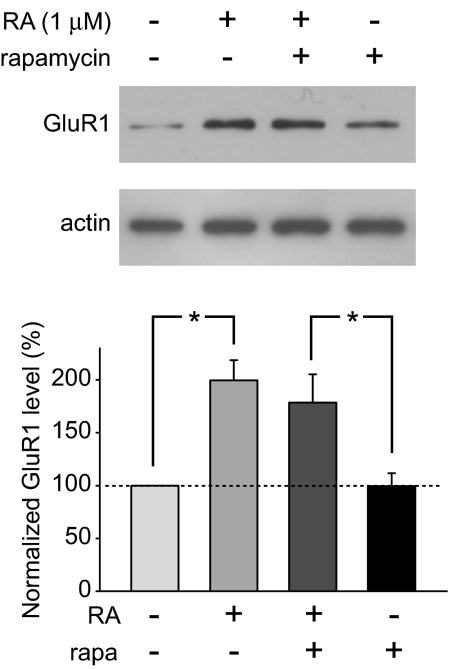
A recent study reported an effect of RA on spine morphogenesis in hippocampal neurons that requires translation of GluR1 protein through activation of mTOR signaling (13). We therefore examined the effect of the mTOR inhibitor rapamycin on RA-induced GluR1 synthesis in synaptoneurosomes prepared from rat hippocampus. Consistent with the effect we reported previously (8), 1 μM RA induced rapid synthesis of GluR1. However, addition of rapamycin 30 min before the RA treatment did not block RA-induced GluR1 synthesis (Fig. 2), indicating that mTOR-dependent signaling is not required for the RA-induced activation of GluR1 translation in neuronal dendrites.
Fig. 2.
mTOR signaling is not required for RA-induced GluR1 synthesis. Hippocampal synaptoneurosomes were pretreated with rapamycin (200 nM) or DMSO for 30 min before the addition of RA. GluR1 synthesis in RA-treated synaptoneurosomes was evident in both DMSO and rapamycin pretreated groups (n = 3; *, P < 0.02).
Dendritic GluR1 Synthesis in Neuronal RNA Granules.
Local protein synthesis in dendrites requires mRNA and the translation machinery, both of which are trafficked into dendrites in RNA granules, which are large RNA–protein complexes that serve not only as mRNA trafficking units but also as local storage compartments for mRNAs and the translation machinery (14, 15). The presence of the 30S and 50S ribosomal subunits, but the lack of certain translation factors, in RNA granules (14, 15) suggests that these mRNA storage sites can be translationally activated upon recruitment of these translation factors. To investigate the possible role of RA in protein translation in RNA granules, we performed standard and ImmunoGold EM studies on cultured neurons and examined the localization of RARα and GluR1 as a function of RA treatment or synaptic activity blockade.
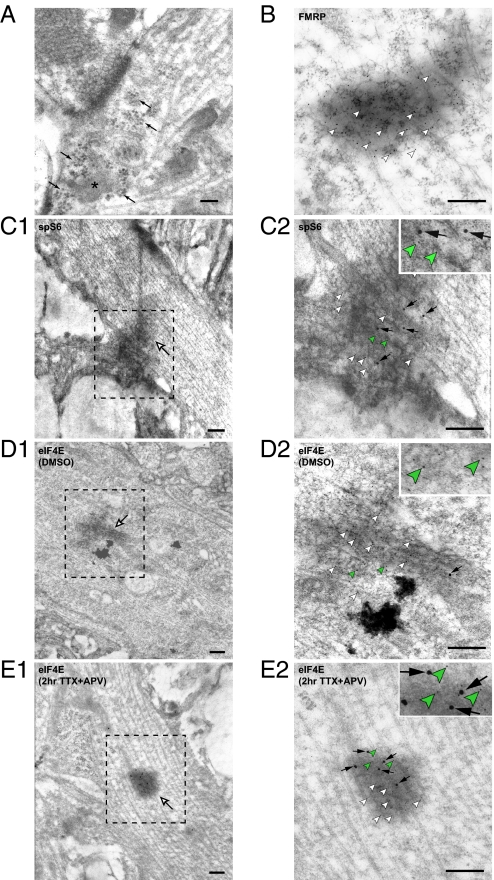
Consistent with previous findings (16), we found in our cultured hippocampal neurons polyribosomes in dendrites where they are sometimes near the synapse (Fig. 3A, arrows). Electron-dense (dark) complexes without a clearly defined membranous boundary (Fig. 3A, asterisk) were occasionally associated with the polyribosomes, possibly representing RNA granules. FMRP (fragile-X mental retardation protein), a known marker for RNA granules, is highly and specifically enriched in these dark complexes, as shown by ImmunoGold labeling using an FMRP antibody (Fig. 3B). The average number of FMRP gold particles found inside each electron-dense granule was 26.3 ± 5.2, whereas only 1.7 ± 0.3 particles were observed in similar-sized dendritic regions outside the granules (n = 20 RNA granules). To show that these FMRP-containing structures are translationally competent, we examined the presence of critical translation factors such as ribosomal protein S6 and eIF4E (eukaryotic initiation factor 4E), which initiates cap-dependent protein translation. Consistent with previous reports (15), S6 is found in RNA granules at basal conditions (Fig. 3 C1 and C2). eIF4E was shown to be normally absent from FMRP-rich RNA granules but to be recruited into these granules upon translation activation (17). Indeed, under basal (DMSO-treated) conditions, eIF4E was either absent or present at low levels in the RNA granules (Fig. 3 D1 and D2), indicative of RNA granules in a translationally dormant state. Blocking neuronal activity with TTX and APV for 2 hours, which induces local protein synthesis in neuronal dendrites (6), increased the amount of eIF4E immunolabeling in FMRP-rich RNA granules (Fig. 3 E1 and E2) (gold particles per granule: 2.5 ± 0.65, n = 10 for DMSO-treated group; 6.36 ± 1.06, n = 14 for TTX plus APV-treated group; P < 0.01), suggesting that dendritic RNA granules become translationally active upon activity blockade.
Fig. 3.
Characterization of neuronal RNA granules. (A) Polyribosomes (black arrows) are present in neuronal dendrites. RNA granule-like electron-dense dark complexes lacking a clear membranous boundary (asterisk) are sometimes associated with polyribosomes. (Scale bar: 100 nm.) (B) FMRP protein (5-nm gold, white arrowheads) is enriched in these dendritic dark complexes. (Scale bar: 200 nm.) (C1) Ribosomal protein S6 (15-nm gold) is present in dendritic RNA granules (white arrow). (Scale bar: 200 nm.) (C2) The image of the RNA granule in C1 under higher magnification. The 5-nm (white and green arrowheads) and 15-nm gold particles (black arrows) label FMRP and S6 respectively. (Scale bar: 200 nm.) (D–E) Double immunolabeling of FMRP (5-nm gold, white and green arrowheads) and eIF4E (15-nm gold, black arrows in D2 and E2) in dendritic RNA granules (white arrows in D1 and E1). (D1) The eIF4E protein level in RNA granules is undetectable under basal conditions. (D2) The image of the RNA granule in D1 at higher magnification. (E1) eIF4E is recruited into RNA granules (black arrow) upon translation activation by treatment with TTX and APV. (E2) The image of the RNA granule in E1 at higher magnification. (Scale bars: D–E, 200 nm.)
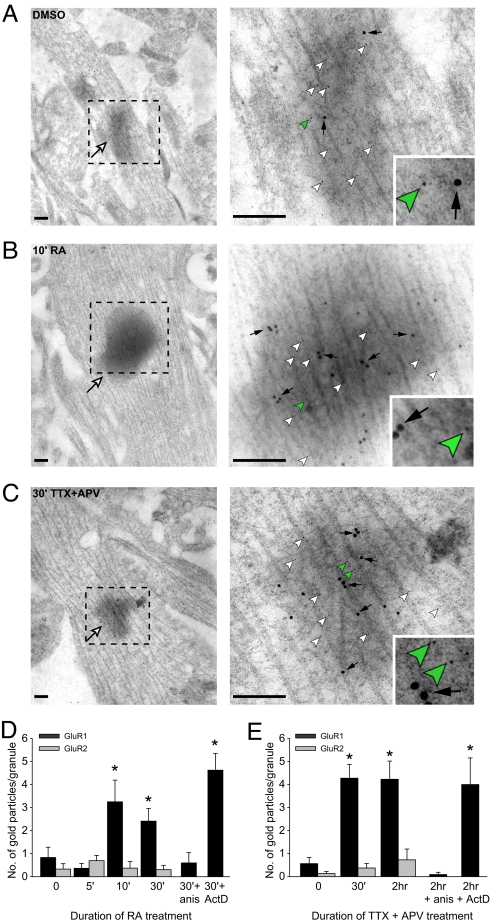
We next performed double ImmunoGold labeling experiments with FMRP and GluR1 antibodies to directly examine GluR1 synthesis in FMRP-containing RNA granules. Under basal conditions or after DMSO treatment, a low background level of GluR1 proteins was present in these RNA granules (Fig. 4A). Treating neurons with 1 μM RA rapidly increased GluR1 protein level within 10 min (Fig. 4B), and this elevated level of GluR1 in RNA granules persisted for at least 30 min (Fig. 4D). Consistent with our previous findings that blocking neuronal activity promotes RA synthesis in neurons (8), blocking neuronal activity with TTX and APV for 30 min also significantly increased GluR1 protein level in these RNA granules (Fig. 4C), and the increase persisted for at least 2 h (Fig. 4E). The slower time course of GluR1 accumulation in RNA granules after activity blockade in comparison with direct RA treatment likely reflects the time required for the production and local accumulation of RA triggered by reduced activity. A translation inhibitor, anisomycin, blocked the observed changes induced by activity blockade or RA, indicating that the increase in GluR1 protein level in RNA granules is due to local synthesis of GluR1 proteins (Fig. 4 D and E). Presence of a transcriptional inhibitor, actinomycin D, did not affect the RA-induced increase. Consistent with our previous findings that GluR2 translation is not regulated by RA or activity blockade (8), protein levels of GluR2 remained low and unchanged under all these conditions (Fig. 4 D and E), although GluR2 mRNA has been found in neuronal dendrites (18).
Fig. 4.
RA and activity blockade induce an increase in GluR1 immunoreactivity in dendritic RNA granules. (A–C) Double ImmunoGold labeling of FMRP (5-nm gold, arrowheads) and GluR1 (15-nm gold, black arrows) in dendritic RNA granules (white arrows) observed at low (Left) and high (Right) magnifications. Insets show zoomed-in images of the 5-nm gold particles next to the green arrowheads. (Scale bars: 200 nm.) (A) Basal conditions. (B) RA treatment (10 min). (C) Activity blockade with TTX and APV (30 min). (D) Quantification of GluR1 and GluR2 gold particles in RNA granules after RA treatment. RA treatment for 10 min or 30 min selectively increased GluR1 levels and was blocked by anisomycin (anis) but not actinomycin D (ActD; n = 15 RNA granules per group from two experiments. *, P < 0.05). (E) Quantification of GluR1 and GluR2 gold particles in RNA granules under activity blockade. TTX and APV treatment for 30 min and 2 h selectively increased GluR1 levels and was blocked by anisomycin but not actinomycin D (n = 30 RNA granules per group from three experiments. *, P < 1 × 10−5).
Activity Blockade or RA Treatment Induces the Presence of RARα in RNA Granules.
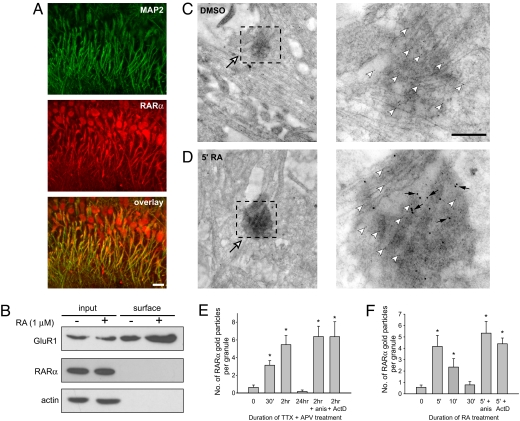
Retinoid signaling is mediated by its nuclear receptors, the RARs and the RXRs, both of which consist of isotypes α, β, and γ. RARα is expressed at high levels in hippocampal CA1-CA3 pyramidal cells (11, 12), RARβ is not expressed in hippocampus, and RARγ is primarily expressed in the dentate gyrus granule neurons (12). Using biochemical methods, we have previously found that RARα protein is present in synaptoneurosomes (8). Immunocytochemistry of adult hippocampal slices further revealed its subcellular localization in neuronal dendrites (Fig. 5A), indicative of a possible nonnuclear function of RARα.
Fig. 5.
RARα is present in dendrites and translocates into RNA granules in response to RA or activity blockade. (A) RARα was localized in the nuclei and dendrites (indicated by MAP2 staining) of hippocampal pyramidal neurons. (Scale bar: 20 μm.) (B) RARα protein is not expressed at neuronal plasma membrane. Whereas RA treatment increased GluR1 surface expression detected with surface protein biotinylation, no RARα was detected on the cell surface under basal or RA-treated conditions. (C and D) Double ImmunoGold labeling of FMRP (5-nm gold, white arrowheads) and RARα (15-nm gold, black arrows) in dendritic RNA granules (white arrows) observed at low (Left) and high (Right) magnifications. (Scale bar: 200 nm.) (C) RARα was present at undetectable levels in RNA granules under basal condition. (D) RA stimulation rapidly increased RARα in RNA granules within 5 min of treatment. (E) Prolonged activity blockade induced transient increase of RARα proteins in RNA granules and was not blocked by anisomycin or actinomycin D, indicating translocation but not translation of RARα (n = 20 RNA granules per group from three experiments. *, P < 0.005). (F) RA treatment induced a similar transient translocation of RARα proteins into RNA granule with a faster time course and was not blocked by anisomycin or actinomycin D (n = 20 RNA granules per group from three experiments. *, P < 0.005).
It was suggested that RARα is present at the neuronal cell surface, i.e., functions as a cell-surface receptor (13). To test this possibility, we performed surface protein biotinylation in our cultured neurons. We did not detect any surface expressed RARα under either basal (DMSO-treated) or RA-treated conditions (Fig. 5B). Under these same experimental conditions, a significant increase in surface GluR1 expression induced by RA treatment was detected, which is consistent with our previous findings (8) and validates our experimental procedures.
We next performed double ImmunoGold labeling of RARα in cultured hippocampal neurons. Neurons in DMSO-treated cultures showed low-level diffuse labeling of RARα protein in the dendrites (Fig. 5C Left), without specific localization in RNA granules (Fig. 5C Right, E, and F). Treatment with 1 μM RA induced, within 5 min after the onset of treatment, a large increase in the RARα protein level in FMRP-containing RNA granules (Fig. 5 D and F). The same increase was observed upon TTX and APV treatment, albeit with a slower time course (Fig. 5E). RARα accumulation in the RNA granule is not transcription- or translation-dependent because it was not blocked by anisomycin or actinomycin D (Fig. 5 E and F), suggesting that RA and activity blockade causes either a rapid translocation of RARα proteins into existing RNA granules or a rapid assembly of RNA granules containing RARα.
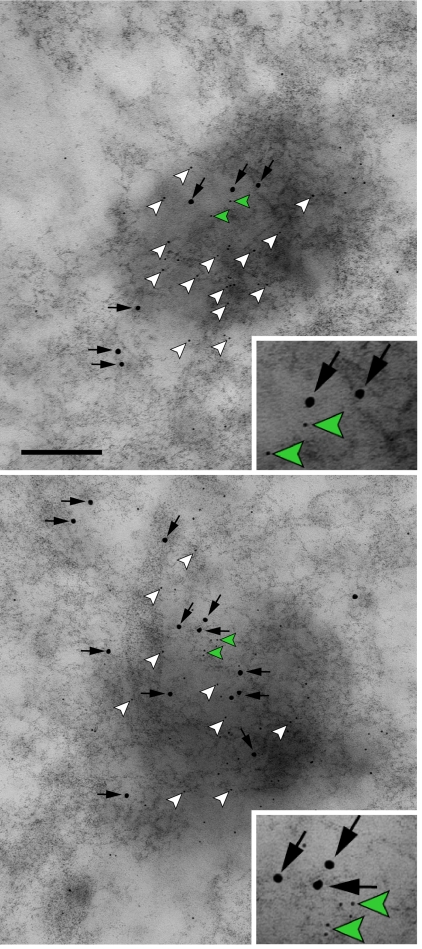
GluR1 and RARα protein exhibited intriguingly different time courses with which they accumulated in the RNA granule upon RA treatment. The RARα level peaked 5 min after RA treatment, whereas the GluR1 level increased with a highly reproducible 10-min delay (Figs. 4D and 5F), suggesting that RARα accumulates in RNA granules before the onset of GluR1 translation through a mechanism yet to be identified. In our previous study, we reported that RARα is required for dendritic GluR1 synthesis-dependent homeostatic synaptic plasticity (8). To determine whether dendritic RARα is involved in local translation of GluR1 in RNA granules, we performed double ImmunoGold labeling of RARα and GluR1 in neurons treated with TTX and APV for 2 h, which fully activates local translation of GluR1 (Fig. 6). We found that these two proteins colocalized in all 25 RNA granules examined, suggesting a role for RARα in GluR1 translation in RNA granules.
Fig. 6.
GluR1 translation is activated in RARα-positive RNA granules. Double ImmunoGold labeling of RARα (5-nm gold, arrowheads) and GluR1 (15-nm gold, black arrows) in dendritic RNA granules from neurons after 2 h of TTX and APV treatment. Insets show zoomed-in images of the 5-nm gold particles next to the green arrowheads. Two individual examples are displayed. (Scale bar: 200 nm.)
The RARα protein level in the RNA granule declined rapidly after the 5-min peak under RA treatment (Fig. 5F), and the same decline was also observed after 24 h of TTX and APV treatment (Fig. 5E), in contrast to the sustained GluR1 levels. This observation suggests that RARα may be required for the initial activation of GluR1 translation but is not required for maintaining the translationally active state of the RNA granule. Alternatively, it may represent a brief wave of translation in the RNA granule, which is turned off by a negative-feedback mechanism.
Discussion
The involvement of dendritic protein translation in synaptic plasticity is well documented (19). Several molecular players have been suggested to play roles in homeostatic synaptic plasticity, including α/β CaMKII (20), the immediate-early gene Arc/Arg 3.1 (21), TGF-β (22), and glia-derived TNFα (23). However, none of those factors has been directly associated with the form of synaptic scaling that requires local translation of GluR1 in neuronal dendrites (5, 6). Our recent findings (8) revealed that RA, whose synthesis can be regulated by neuronal activity, activates GluR1 synthesis under activity blockade and mediates synaptic scaling. Moreover, we demonstrated a critical involvement of RARα in homeostatic plasticity. In the current study, we further explored the dynamic localization of RARα in homeostatic plasticity at the ultrastructural level.
We made four major observations that shed light on the mechanism by which RA mediates synaptic scaling: (i) RA induced the local translation in neuronal dendrites of a GluR1-UTR reporter mRNA (Fig. 1); (ii) RA or activity-blockade induced rapid GluR1 synthesis in neuronal RNA granules (Fig. 4), and the dendritic GluR1 synthesis activated by RA is independent of mTOR signaling (Fig. 2); (iii) The classical nuclear receptor RARα is present in dendritic RNA granules, and its concentration in these granules is rapidly enhanced upon activity blockade or RA treatment (Fig. 5); (iv) RARα is found in RNA granules that are undergoing active GluR1 synthesis (Fig. 6); and (v) The time courses with which RARα and GluR1 appear in RNA granules upon RA treatment differ dramatically, with the former preceding the latter by >5 min.
Translational regulation in neurons is important for the expression and maintenance of long-term synaptic plasticity. The seminal discovery of polyribosomes in neuronal dendrites (16) pioneered the investigation of the functional significance and mechanism of regulated local protein synthesis in neuronal dendrites (19). In addition, the organelles that constitute the secretory pathway, such as the endoplasmic reticulum (ER), the Golgi apparatus, and the trans-Golgi network, are present in dendrites (24); thus, not only cytoplasmic proteins but also integral membrane proteins (i.e., receptors and channels) can be synthesized locally in dendrites. Supporting this notion, mRNAs encoding synaptic proteins, including those that are integral membrane proteins, are sorted and trafficked into dendrites to be translated locally there (25), a process made possible through packaging mRNAs into RNA granules. In addition to mRNAs, RNA granules also contain various ribosomal subunits, translation factors, decay enzymes, helicases, scaffold proteins, and RNA-binding proteins, which are involved in the localization, stability, and translation of their RNA cargo (14). In the present study, we characterized neuronal RNA granules with ImmunoGold labeling and EM and found that local protein translation can indeed occur rapidly in RNA granules upon translation activation by activity blockade or direct RA treatment. In agreement with earlier observations, we have observed that translation activation recruits eIF4E into neuronal RNA granules (15, 17). Upon RA treatment, we observed the appearance of newly synthesized GluR1 receptors as early as 10 min after the treatment. This rapid enhancement of GluR1 mRNA translation can be explained by an increase in the efficiency of translation initiation and/or by removal of inhibition during elongation (26). The exact mechanism of RA/RARα-regulated translation activation remains to be determined. Recent studies indicate that some well characterized transcription factors, such as p53 tumor suppressor protein, may play a dual role as RNA-binding proteins (27). This raises the intriguing possibility that, in a translational context, RARα may bind directly to mRNAs. Moreover, it is unclear whether RA-regulated protein synthesis is GluR1-specific or is a general mechanism for regulation of diverse local protein synthesis-dependent processes. Addressing these and related questions will be of great interest for understanding the entire range of RA signaling in the brain.
Experimental Procedures
Detailed experimental procedures are described in supporting information (SI) Experimental Procedures.
Cell Cultures.
Primary hippocampal cultures were prepared from the brains of rats at embryonic day 22 and maintained in serum-free Neurobasal medium supplemented with B-27 and Glutamax (GIBCO-BRL) for 2 weeks in vitro (28). Hippocampal slice cultures were prepared from 7- to 8-day-old rat pups (29) and maintained in Neurobasal-A medium supplemented with horse serum (HyClone), insulin (Sigma), and Glutamax. For details, see SI Experimental Procedures.
ImmunoGold EM.
Cultured hippocampal neurons were fixed for ImmunoGold EM for 1 h by using 1% paraformaldehyde, 2.5% glutaraldehyde, 0.1% tannic acid, and 0.1% picric acid in PBS, followed by 1% tannic acid incubation on ice for 30 min and 1% uranyl acetate incubation at room temperature for 2 h. After dehydration in ascending concentrations of ethanol, fixed cells were infiltrated and embedded in LRwhite (Ted Pella). See SI Experimental Procedures for details on ImmunoGold labeling.
Supplementary Material
Acknowledgments.
We thank Qi Feng for technical assistance and members of the L.C. laboratory for discussion and comments on the manuscript. The work was supported by the Mabel and Arnold Beckman Foundation, the David and Lucile Packard Foundation, the W. M. Keck Foundation, and the National Institutes of Health (L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804801105/DCSupplemental.
References
- 1.Davis GW. Homeostatic control of neural activity: From phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–23. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 2.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–37. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 5.Ju W, et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–53. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 6.Sutton MA, et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–99. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–83. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 8.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008 doi: 10.1016/j.neuron.2008.08.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–21. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 10.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA. 2003;100:7135–40. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zetterstrom RH, et al. Role of retinoids in the CNS: Differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11:407–16. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 12.Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 2008;22:236–45. doi: 10.1096/fj.07-8739com. [DOI] [PubMed] [Google Scholar]
- 14.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–8. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krichevsky AM, Kosik KS. Neuronal RNA granules: A link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–96. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- 16.Steward O, Falk PM. Protein-synthetic machinery at postsynaptic sites during synaptogenesis: A quantitative study of the association between polyribosomes and developing synapses. J Neurosci. 1986;6:412–23. doi: 10.1523/JNEUROSCI.06-02-00412.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart FM, Edelman GM, Vanderklish PW. BDNF induces translocation of initiation factor 4E to mRNA granules: Evidence for a role of synaptic microfilaments and integrins. Proc Natl Acad Sci USA. 2003;100:14403–8. doi: 10.1073/pnas.2436349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grooms SY, et al. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–51. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. α- and β-CaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–14. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 21.Guzowski JF, et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–16. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 23.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–62. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramham CR, Wells DG. Dendritic mRNA: Transport, translation and function. Nat Rev Neurosci. 2007;8:776–89. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 26.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–61. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Cassiday LA, Maher LJ., III Having it both ways: Transcription factors that bind DNA and RNA. Nucleic Acids Res. 2002;30:4118–26. doi: 10.1093/nar/gkf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam CI, Chen L. Postsynaptic assembly induced by neurexin–neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA. 2005;102:6137–42. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–7. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.