Abstract
Ser/Thr/Tyr kinases, which together comprise a major class of regulatory proteins in eukaryotes, were not believed to play an important role in prokaryotes until recently. However, our analysis of 626 prokaryotic genomes reveals that eukaryotic-like protein kinases (ELKs) are found in nearly two-thirds of the sequenced strains. We have identified 2697 ELKs, most of which are encoded by multicellular strains of the phyla Proteobacteria (Myxococcales), Actinobacteria, Cyanobacteria, and Chloroflexi, and 2 Acidobacteria and 1 Planctomycetes. Astonishingly, 7 myxobacterial strains together encode 892 ELKs, with 4 of the strains exhibiting a genomic ELK density similar to that observed in eukaryotes. Most myxobacterial ELKs show a modular organization in which the kinase domain is located at the N terminus. The C-terminal portion of the ELKs is highly diverse and often contains sequences with similarity to characterized domains, most of them involved in signaling mechanisms or in protein–protein interactions. However, many of these architectures are unique to the myxobacteria, an observation that suggests that this group exploits sophisticated and novel signal transduction systems. Phylogenetic reconstruction using the kinase domains revealed many orthologous sequence pairs and a huge number of gene duplications that probably occurred after speciation. Furthermore, studies of the microsynteny in the ELK-encoding regions reveal only low levels of synteny among Myxococcus xanthus, Plesiocystis pacifica, and Sorangium cellulosum. However, extensive similarities between M. xanthus, Stigmatella aurantiaca, and 3 Anaeromyxobacter strains were observed, indicating that they share regulatory pathways involving various ELKs.
Keywords: Anaeromyxobacter, Myxococcus, Plesiocystis, Sorangium, Stigmatella
Signal transduction pathways in the eukaryotes typically are mediated by protein kinases, which transfer the γ-phosphate of ATP to a hydroxyl moiety (Ser, Thr, and/or Tyr) on a protein substrate. This kinase activity resides in a sequence of 250–300 residues known as the catalytic domain, which consists of 12 subdomains differentiated by their respective consensus motifs (1). The solved structure of a prototypical kinase domain (2) revealed that it consists of 2 lobes joined by a hinge segment. Catalysis occurs at the interface of the 2 lobes, where the catalytic residues interact with both ATP and the substrate. The key amino acids for the phosphotransfer reaction are the Lys of subdomain II and the Asp residues of subdomains VIb (D1) and VII (D2). Mutations in any of these residues are predicted to inactivate the proteins.
For the kinases to function properly, their catalytic activity must be tightly controlled. Indeed, several different regulatory mechanisms have been described (3). For example, 1 type of kinases catalyzes an autophosphorylation reaction within the catalytic domain, targeting a region termed the “activation segment” that runs from subdomain VII to VIII. Phosphorylation within this segment changes the conformation of the polypeptide and activates the enzyme (4). Proteins regulated by this mechanism require an Arg just upstream of Asp D1 and so are designated “RD kinases.” Correspondingly, proteins that lack this Arg are known as “non-RD kinases” (5).
Signal transduction pathways dependent on phosphorylation at Ser/Thr/Tyr also occur in the prokaryotes. This reaction also is mediated by a family of protein kinases exhibiting high sequence similarities to their eukaryotic counterparts. The first eukaryotic-like protein kinase (ELK) reported in any prokaryote was found in the myxobacterium Myxococcus xanthus, a δ-Proteobacterium (6). Publication of the M. xanthus DK1622 genome revealed that the strain contains a total of 99 ELKs (7), but this number is dwarfed by that of the myxobacterium Sorangium cellulosum So ce56 (8), which boasts 317, the largest numbers of ELKs reported so far among the prokaryotes.
The M. xanthus genome also encodes a substantial number of typical bacterial 2-component systems (TCSs) (9). The need for a complex regulatory system in this bacterium is evident from consideration of its lifestyle. During growth, M. xanthus cells form mobile, predatory communities known as “swarms,” which feed cooperatively. Under starvation conditions, the cells initiate a multicellular developmental program that culminates in the formation of macroscopic fruiting bodies filled with heat-resistant myxospores. The spores remain dormant until nutrients are available again, at which point they germinate, initiating a new vegetative cycle (10, 11). Thus, the M. xanthus lifestyle requires the cells not only to monitor changes in the environment but also to communicate with each other to coordinate movement and behavior.
Developmental sporulation also depends on the DKxanthene family of natural products (12). The other metabolites produced by the cells (7) are assumed to provide it with a competitive advantage in the soil environment. Therefore, survival of M. xanthus also is intimately linked to the regulation of secondary metabolism.
Growing evidence that ELKs play a role in development, virulence, metabolism, or stress adaptation in many other bacterial genera (13, 14, 15, 16, 17) has prompted efforts to characterize the prokaryotic kinome (18, 19). However, the first analysis in 2005 (18) was limited by the availability of only 125 complete genome sequences, none of which belonged to the myxobacteria, and the second analysis (19) focused on metagenomic sequences from a global ocean sampling and so was unlikely to be representative of all prokaryotes. In this study, we aimed to assess whether typical kinases are widely represented in the prokaryotes and to determine their exact number in each prokaryotic genome. For this study we carried out a comprehensive bioinformatic analysis of the typical ELKs that constitute the kinome of the 7 myxobacterial strains sequenced. We also surveyed the remaining 619 prokaryotic genomes deposited in the database of the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi) before January 15, 2008. This analysis revealed the presence of ELKs in approximately two-thirds of all prokaryotes. Comparison of the largest prokaryotic kinomes with those of several eukaryotes supports a link between ELKs and multicellularity.
Results and Discussion
ELKs in the Myxobacteria.
The genomes of M. xanthus DK1622 (9.14 Mb containing 99 ELKs) and S. cellulosum So ce 56 (13.03 Mb containing 317 ELKs) encode the largest numbers of ELKs reported to date in the prokaryotes (7, 8). Five additional myxobacterial genomes have become available recently, of which 2 are complete [Anaeromyxobacter sp. Fw109–5 (5.3 Mb) and Anaeromyxobacter dehalogenans 2CP-C (5.01 Mb)], and 3 are incomplete [Plesiocystis pacifica SIR-1 (10.59 Mb), Stigmatella aurantiaca DW4/3–1 (10.27 Mb), and Anaeromyxobacter sp. K (5 Mb)]. Consistent with the expectation of a high ELK content, we have found that Anaeromyxobacter sp. K encodes 14 ELKs, A. dehalogenans encodes 18, Anaeromyxobacter sp. Fw109–5 encodes 20, S. aurantiaca encodes 194, and P. pacifica encodes 230. Moreover, these numbers may increase when the incomplete genomes are completed. These data confirm that the presence of a large number of genes encoding ELKs is a general feature of the myxobacteria. Together, the 7 myxobacteria encode at least 892 ELKs.
It presently is unclear why the myxobacterial kinome is so extensive. However, the level of regulation required to control their complex social lifestyles provides an attractive explanation. Four cell types have been identified in the life cycle of M. xanthus (10, 11). During growth, predatory rods must coordinate their efforts to detect prey and secrete inhibitors of microbial growth while adapting to resist the action of biocides produced by the prey. Under conditions of nutrient depletion, the community enters into a developmental cycle in which cells choose 1 of 3 possible fates: (i) programmed cell death (20); (ii) aggregation to form fruiting bodies that then differentiate into myxospores; and (iii) remaining as peripheral rods that surround the fruiting bodies. It is hoped that in the future the role of the kinome in governing these cellular transitions will be confirmed by a global analysis of the M. xanthus phosphoproteome, coupled with a transcriptomic analysis of the ELKs. Encouragingly, initial analysis of the phosphoproteome of S. cellulosum revealed that ≈40% of the proteins are phosphorylated on Ser, Thr, or Tyr residues (8).
Genome Expansion in the Myxobacteria.
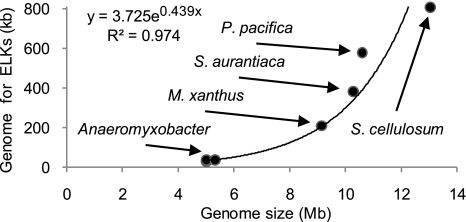
Four of the 7 myxobacterial strains sequenced possess the largest genomes described for any prokaryote, making them excellent models for the study of genome expansion. It recently has been suggested that metabolic genes are acquired by lateral gene transfer, whereas signaling genes arise by duplication and divergence (21). In addition, the number of TCSs in myxobacteria increases linearly with genome size (9), but the number of genes encoding ELKs increases exponentially. The curve obtained when representing the number of ELKs (y) versus the genome size (x) is described by the equation y = 2.271e0.408× with a coefficient of determination (R2) of 0.967. The fit to an exponential function improves when the number of kilobases in each strain devoted to ELKs is plotted against genome size (Fig. 1). The improved fit derives from the increase in the size of the ELKs that corresponds to the increase in genome size [supporting information (SI) Fig. S1]. For example, in the Anaeromyxobacter spp., which have the smallest genomes, no ELK contains >1500 aa. The number of ELKs 1500–2000 residues in size increases between M. xanthus and P. pacifica in accordance with increasing genome size. Only S. cellulosum, with the largest genome, encodes a substantial number of ELKs containing >2000 aa. These data indicate that ELKs have been important contributors to genome expansion in this group of δ-Proteobacteria.
Fig. 1.
Proportion of genome devoted to encoding ELKs in the myxobacteria as a function of genome size. The equation of the exponential curve and the coefficient of determination are indicated.
An intriguing question is why myxobacteria with large genome sizes have increased the number of ELKs more than the number of TCSs (9). In fact, S. cellulosum encodes more ELKs than TCSs (9). A plausible explanation is that the architecture of ELK pathways allows them to be regulated at multiple levels, increasing the overall adaptability of the cells. Thus, evolution of these complex bacteria may have selected cells with more ELKs in preference to using more TCSs.
Conservation of Catalytic and Regulatory Residues in the Myxobacterial ELKs.
Because kinase activity depends on the presence of 3 active site residues, we have analyzed all myxobacterial ELKs for the presence of these amino acids. This analysis revealed that the 3 residues are conserved in all ELKs of Anaeromyxobacter, but some ELKs of the remaining myxobacteria lack 1, 2, or even all 3 catalytic amino acids. These noncanonical ELKs, which represent 3.5% of the total in P. pacifica, 5.3% in S. cellulosum, 11.1% in M. xanthus, and 16% in S. aurantiaca (Fig. S2A), are assumed to be inactive.
We also have classified the myxobacterial ELKs as RD or non-RD. The largest percentages of non-RD kinases were found in the 3 Anaeromyxobacter strains, in which 15% to 29% of proteins lack the regulatory Arg. Non-RD kinases comprise 9% to 13% of ELKs in the remaining myxobacteria (Fig. S2B). In these cases, regulation appears to be achieved through an additional domain that has been added to the C-terminal portion of the ELKs.
Domain Architecture of Myxobacterial ELKs.
Most myxobacterial ELKs (92.5%) contain an additional region at the C-terminal portion of the protein. This peptide might regulate the kinase activity or function as an input/output domain of the ELK signal transduction pathway. This extra region does not exhibit similarities to any defined domain in Pfam in the majority of the ELKs. However, the additional portion of some ELKs comprises 1 or several well defined domains. We have classified the ELKs into 3 different groups, according to the predicted function of the C-terminal region (Table 1 and Table S1). Interestingly, many of these domains are involved in the synthesis or recognition of the second messengers cAMP, cGMP, and cyclic diguanylate. These unusual domains suggest that various signal transduction pathways in myxobacteria are mediated by cyclic nucleotides, although the ability of these ELKs to produce or respond to any type of cyclic nucleotide has not yet been demonstrated directly. Also of note, several ELKs contain a fork-head associated (FHA) domain that is known to interact with phosphothreonine (22). Together, these varying architectures suggest that signal transduction occurs through the integration of several different signals, as suggested by Goldman, et al. (7). It is remarkable that many of these frameworks are unique to the myxobacteria, indicating that myxobacterial ELK signal transduction pathways may be more complex than those of other organisms.
Table 1.
Domain architecture of myxobacterial ELKs
| Domain | Sc | Pp | Sa | Mx | Ad | AF | AK |
|---|---|---|---|---|---|---|---|
| ELKs with 2-component and second messenger signaling domains | |||||||
| Pkinase-GAF-HisKA | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-HisKA-HATPase_c | 6 | 3 | 4 | 1 | 0 | 0 | 0 |
| Pkinase-GAF-PAS-GAF-HisKA-HATPase_c | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-GAF-HisKA-HATPase_c | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-PAS-HisKA-HATpase_c | 2 | 12 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-HisKA-HATPase_c-Response_reg | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-PAS-HWE_HK | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GAF-STAS | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-PAS-PAS | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Response_reg | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-GSPII_E_N-Response_reg | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Pkinase-Response_reg-Guanylate_cyc | 3 | 0 | 3 | 1 | 0 | 0 | 0 |
| Pkinase-GGDEF | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Guanylate_cyc | 6 | 0 | 2 | 1 | 0 | 0 | 0 |
| Pkinase-cNMP_binding | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-PilZ | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pkinase-PilZ-DNAJ | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pkinase-PilZ-TPR | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pkinase-TPR-GAF-Sigma54_activat-HTH_8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| ELKS with Pkinase duplications and protein-protein interaction domains | |||||||
| Pkinase-Pkinase | 4 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Pkinase-WD40 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Pkinase-TPR | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-TPR | 83 | 52 | 13 | 12 | 3 | 3 | 1 |
| Pkinase-WD40 | 3 | 6 | 1 | 0 | 1 | 1 | 1 |
| Pkinase-WD40-DUF323 | 0 | 1 | 0 | 2 | 0 | 0 | 0 |
| Pkinase-FHA | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| FHA-Pkinase | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pkinase-DNAJ-TPR | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| ELKs with other domains | |||||||
| Pkinase-DUF323 | 11 | 0 | 4 | 1 | 0 | 0 | 0 |
| Pkinase-PEGA | 2 | 2 | 4 | 6 | 2 | 2 | 1 |
| Pkinase-Macro | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Globin-Abhydrolase_1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-CCP_MauG | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-SEFIR | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-Fer2-Globin | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pkinase-RHH_1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pkinase-HEAT_PBS | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Pkinase-Usp | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Pkinase-Drf_FH1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
The family numbers in Pfam of the domains listed in this table are indicated in Table S1. Novel organizations are shown in bold. Sc, S. cellulosum; Pp, P. pacifica; Sa, S. aurantiaca; Mx, M. xanthus; Ad, A. dehalogenans; AF, Anaeromyxobacter sp. Fw109–5; AK, Anaeromyxobacter sp. K.
Phylogeny of Myxobacterial ELKs.
To visualize evolutionary relationships among the 892 myxobacterial ELKs and to establish connections between the ELKs from the 7 strains (which represent the 3 suborders of the Myxococcales), we have reconstructed the phylogeny of the kinase domains. For this determination, we have used maximum parsimony, neighbor joining, and Bayesian estimation; the tree resulting from the first method is shown in Fig. S3. The 3 reconstruction approaches gave a similar clustering for most sequences, and therefore we restricted the analysis to phylogenetic patterns that were reproduced by all methods.
This analysis identified a number of orthologous sequence pairs in the genomes of M. xanthus and S. aurantiaca and in the genome of A. dehalogenans relative to the 2 other Anaeromyxobacter genomes (Table S2). In the case of M. xanthus and S. aurantiaca, 65 pairs of sequences originated from a speciation event without further duplications. In the case of A. dehalogenans, we found 14 kinase domains having orthologous pairs in the other Anaeromyxobacter species. However, Anaeromyxobacter Fw109–5 and Anaeromyxobacter sp. K appear to have lost 2 and 4 of these genes after speciation, respectively.
Most of the sequences group according to the species of origin (Fig. S3). Approximately 160 kinase domains from S. cellulosum form groups connected by common ancestors specific to this myxobacterium. Similarly, some 55% of the sequences from P. pacifica are positioned in essentially specific clusters, with the largest cluster containing >100 members. Strikingly, kinase domains of M. xanthus, S. aurantiaca, and Anaeromyxobacter often are combined in the same phylogenetic cluster, demonstrating that they share many common domains that existed before the speciation process. These 3 genera belong to the same suborder of the Myxococcales, the Cystobacterineae, whereas P. pacifica and S. cellulosum are classified in the other 2 suborders, Nannocystineae and Sorangineae, respectively. In general, the strain-specific grouping of sequences is a clear indicator of extensive gene duplications. In some groups, the duplicated genes still are located next to each other in the genome (for example, genes sce5443, sce5444, sce5445, and sce5446 from S. cellulosum). The duplication processes seen in the phylogenetic relationships also mirror to some extent the functional environment of the respective domains. For example, the kinase domain within the gene MXAN_4053 (with the domain structure Pkinase-GAF-HisKA-HATPase_c, defined in Table S1) is located in a cluster that exclusively contains ELKs from various strains with similar modular architectures.
Local Synteny in the ELK Regions.
Our phylogenetic analysis raised the question: does phylogeny correlate with synteny in the ELK regions? To investigate this issue we used the M. xanthus ELKs as the reference, because these kinases are by far the best characterized. We detected synteny among all 7 myxobacteria in only 1 ELK region, corresponding to the well characterized Pkn4 from M. xanthus (23). In M. xanthus, Pkn4 has been demonstrated to phosphorylate 6-phosphofructokinase (Pfk), which is located directly adjacent to the kinase. Surprisingly, Pfk is not encoded in proximity to an ELK in any of the other strains. Furthermore, because this common syntenic block covers a maximum of 3 genes (an ELK, a protein with a TPR domain, and an enhancer-binding protein, all of which are abundant gene types in myxobacteria), the biological significance of this conservation is not clear.
As noted previously (8), despite the absence of global synteny between M. xanthus and S. cellulosum, the 2 strains exhibit a high degree of local synteny. However, the ELK regions do not seem to contribute significantly to this microsynteny. Eighteen of the 25 syntenic blocks detected between these 2 species harbor only 2 genes. In the remaining 7 blocks, the number of orthologs ranges from 3 to 7 (Table S3). A lack of extensive synteny also is evident when M. xanthus and P. pacifica are compared. This observation agrees well with the distant phylogeny among the kinase domains of these 3 species and may be correlated with the classification of the species into different suborders.
In contrast, local synteny is evident between M. xanthus and the other 4 species of the suborder Cystobacterineae (Table S3). We have detected 64 syntenic regions between M. xanthus and S. aurantiaca, 49 of which contain a variable number of orthologs (ranging from 3 to 37). Interestingly, 54 of the conserved syntenic blocks include ELK orthologous pairs discussed in the previous section. The synteny between M. xanthus and the Anaeromyxobacter strains also is broad. The major conservation is found with A. dehalogenans, which covers 14 blocks (7 contain 3–9 orthologs). The synteny diminishes to only 6 and 5 blocks with the strains Fw109–5 and K, respectively, although all cover >3 orthologs. Many of these syntenic blocks also contain the ELK orthologous pairs detected between the Anaeromyxobacter strains, as described earlier. These analyses demonstrate that a good concordance exists between phylogeny, synteny, and taxonomy.
At least 15 additional M. xanthus regions show significant synteny with the Anaeromyxobacter strains, although, surprisingly, the corresponding ELKs are not present in the 3 species. A notable region in M. xanthus (Fig. 2) is that spanning the pkn3 and relA genes. Synthesis of ppGpp(p) by RelA is the signal that triggers development in myxobacteria (8, 24). The synteny between M. xanthus and S. aurantiaca spans 19 genes (10 shown in Fig. 2). The presence in this region of 2 additional genes encoding proteins with FHA domains surrounding pkn3 in the 2 strains is remarkable. In A. dehalogenans and Anaeromyxobacter sp. K, the synteny in this region is limited to 6 genes, and a protein of unknown function containing a DUF77 domain is observed in place of the ELK gene pkn3. More dramatic differences are observed when M. xanthus is compared with Anaeromyxobacter sp. Fw109–5, because the synteny is restricted to 4 orthologs. In this strain, the 2 genes that encode proteins with FHA domains are lacking also, but the 1 with DUF77 domain is present. These examples support the idea that ELKs have contributed to the expansion of the M. xanthus and S. aurantiaca genomes relative to those of the Anaeromyxobacter strains.
Fig. 2.
Synteny in the ELK region pkn3-relA. Genes shown in the same color in the different strains encode proteins with high homology. The numbers over the first gene in each strain indicate the location of the genes in the genome. Mx, M. xanthus; Sa, S. aurantiaca; Ad, A. dehalogenans; AK, Anaeromyxobacter sp. K; AF, Anaeromyxobacter sp. Fw109–5; + and − indicate that the genes are encoded in the plus or the minus strand, respectively.
ELKs in the Prokaryotes.
We next investigated whether other prokaryotes also exploit ELKs in signaling. For this investigation, we analyzed (including the myxobacteria) 626 genomes, of which 577 belonged to the domain Bacteria and 49 to the Archaea. In total we analyzed ≈ 2250 Mb, encoding >2 million proteins and identified 2697 ELKs. A notable result is that the distribution of ELKs among strains is uneven (Table 2 and Table S4), because 892 ELKs (one-third of the total) are present in the myxobacteria. Moreover, 222 strains (35%) do not encode any ELKs. The number of kinases in ELK-containing genomes (excluding the myxobacteria) ranges from 1 to 75. Interestingly, the strains that encode the most ELKs belong to only 6 phyla within the domain Bacteria (Table 2). This observation indicates that some phylogenetically related strains among different groups have become enriched in signal transduction pathways thought to be typical for eukaryotes. Although these conclusions may have been biased by the number of sequenced strains in each taxon, we believe they are relevant for phyla in which at least 10 genomes have been sequenced (Table 2).
Table 2.
ELKs in sequenced prokaryotic genomes
| Domain | Phylum | Order | Total Genomes | Genomes with |
|
|---|---|---|---|---|---|
| 0 ELKs | >15 ELKs | ||||
| Bacteria | 577 | 194 | 25 | ||
| Acidobacteria | 2 | 0 | 2 | ||
| Acidobacteriales | 1 | 0 | 1 | ||
| Solibacterales | 1 | 0 | 1 | ||
| Actinobacteria | 47 | 0 | 9 | ||
| Actinomycetales | 44 | 0 | 9 | ||
| Aquificae | 1 | 0 | 0 | ||
| Bacteroidetes | 12 | 7 | 0 | ||
| Chlamydiae | 11 | 0 | 0 | ||
| Chlorobi | 5 | 4 | 0 | ||
| Chloroflexi | 7 | 3 | 3 | ||
| Choroflexales | 3 | 0 | 2 | ||
| Herpetosiphonales | 4 | 3 | 1 | ||
| Cyanobacteria | 29 | 13 | 4 | ||
| Nostocales | 2 | 0 | 2 | ||
| Oscillatoriales | 1 | 0 | 1 | ||
| unclassified | 1 | 0 | 1 | ||
| Deinococcus-Thermus | 4 | 0 | 0 | ||
| Firmicutes | 130 | 7 | 0 | ||
| Fusobacteria | 1 | 1 | 0 | ||
| Planctomycetes | 1 | 0 | 1 | ||
| Proteobacteria (α) | 78 | 50 | 0 | ||
| Proteobacteria (β) | 49 | 11 | 0 | ||
| Proteobacteria (γ) | 144 | 67 | 0 | ||
| Proteobacteria (δ) | 21 | 6 | 6 | ||
| Myxococcales | 7 | 0 | 6 | ||
| Proteobacteria (ε) | 19 | 16 | 0 | ||
| Spirochaetes | 9 | 5 | 0 | ||
| Thermotogae | 6 | 6 | 0 | ||
| unclassified | 1 | 1 | 0 | ||
| Archaea | 49 | 28 | 0 | ||
Orders have been included only when there are several genomes sequenced in the phylum and at least 1 of them encodes more than 15 ELKs.
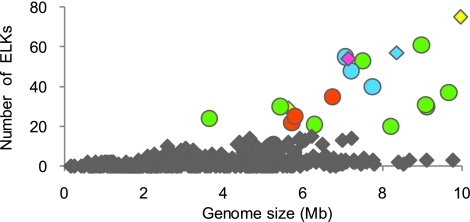
It is reasonable to expect that various ELKs are involved in similar and/or even unrelated processes, as reported for other prokaryotes (13, 14, 15, 16, 17). However, it is intriguing to consider why ELKs are abundant only in specific groups of prokaryotes. If we define a large kinome as a kinome encompassing >15 ELKs, only 25 strains fall into this category. Because of the diversity of bacteria and the dearth of available genotypic and phenotypic information about many sequenced strains (in many cases data are available only on the Web pages of the genome projects for each strain), it is difficult to discern common features among the bacteria exhibiting the largest kinomes. However, our analysis of the 626 sequenced strains points to multicellular behavior as the main evolutionary driver for an extensive kinome (Fig. 3). With only 4 exceptions (1 of which is Anaeromyxobacter sp. K), the strains that are known to exhibit classical multicellular behavior encode >15 ELKs. These strains include the δ-Proteobacteria of the Myxococcales (10, 11), hyphal and mycelial Actinobacteria (25), gliding filamentous Chloroflexi (26), and trichomal Cyanobacteria (27), whose life cycles often encompass differentiation events and several cell fates (Fig. 3). Although this observation also could be biased by the number of sequenced strains, analysis of the incomplete genomes of other multicellular bacteria of these phyla reinforces this view (data not shown). It therefore is tempting to speculate that these multicellular bacteria have evolved to use signal transduction systems involving ELKs to coordinate population behavior instead of the widespread quorum-sensing system used by other communal bacteria (28).
Fig. 3.
ELKs in the prokaryotes as a function of the genome size. All of the complete genomes of prokaryotes deposited at the database of the NCBI have been included, except for the myxobacteria. Genomes encoding <15 ELKs are represented in dark gray. Those containing >15 ELKs are color-coded according to the phylum to which they belong: green, Actinobacteria; blue, Cyanobacteria; yellow, Acidobacteria; pink, Planctomycetes; orange, Chloroflexi. Strains with multicellular behavior are represented by circles.
Four strains with large kinomes—2 Acidobacteria, the Cyanobacteria Acaryochloris, and the Planctomycetes Rhodopirellula—do not show multicellular behavior. This observation indicates that other bacterial lifestyles also may involve the participation of many ELKs. Rhodopirellula is a particularly interesting example, because the cells exhibit internal compartmentalization that resembles that of eukaryotes (29). What benefits the ELKs offer to unicellular bacteria is an open question.
Kinases in Prokaryotes and Eukaryotes.
Kinases are present in all eukaryote genomes, where they regulate intercellular communication and coordinate complex functions (30). Because the number of kinases in eukaryotes increases from unicellular to pluricellular organisms (Table S4), the study of the differences between eukaryote and prokaryote kinomes is interesting. This comparison reveals that the kinome of various myxobacteria is larger than that of the unicellular eukaryotes, Encephalitozoon and fission and budding yeasts. Moreover, S. cellulosum encodes more kinases than the pluricellular eukaryotes Drosophila and Dictyostelium (Table S4). However, this comparison does not take into account relative genome sizes. To take relative genome sizes into account, we calculated the density of kinases (Table S4) in all prokaryotes and several eukaryotes and expressed the density as the number of kinases per 1000 coding sequences (kCDS). With the exception of 3 myxobacteria (Fig. 4), eukaryotes exhibit a higher kinase density than the prokaryotes with large kinomes. Two observations seem to contradict the hypothesis that a large and dense kinome is correlated with a multicellular lifestyle, but both contradictions can be resolved easily. The first observation is that unicellular and pluricellular eukaryotes exhibit similar kinase densities. However, because eukaryotic signal transduction pathways are based mainly on kinases, it is expected that most of them will be involved in cell functions not related to pluricellularity. Simultaneously, the genome size of pluricellular eukaryotes is larger also. As the result of the increment in the number of kinases and genome size, pluricellular and unicellular eukaryotes will possess similar kinase densities. The second contradictory observation is that most multicellular prokaryotes encode fewer kinases than unicellular eukaryotes. However, it should be recalled that TCSs, and not ELKs, are the main mechanism of signal transduction in bacteria. Therefore, most kinases of prokaryotes with large kinomes probably will be devoted to the regulation of the sophisticated mechanisms required for a primitive multicellularity.
Fig. 4.
Density of kinases in prokaryotes that encode >15 ELKs and several representative eukaryotes, expressed as the number of kinases per 1000 CDS (kCDs). Only 1 strain per genus has been included in the graph. White, Eukarya; red, δ-Proteobacteria (myxobacteria); green, Actinobacteria; blue, Cyanobacteria; yellow, Acidobacteria; pink, Planctomycetes; orange, Chloroflexi.
Conclusions and Future Perspectives
The analysis of ELKs in prokaryotic genomes has provided insight into signal transduction in the prokaryotes. Signal transduction pathways based on ELKs are not present in one-third of the sequenced genomes, whereas bacteria encoding the largest kinomes exhibit multicellular behavior. Although bacterial multicellularity clearly relies on other factors in addition to signal transduction, our data provide a strong argument that large kinomes have evolved preferentially to support this lifestyle. In fact, the observation that the small number of 7 myxobacteria encodes 892 ELKs may be explained by the extraordinarily complex lifestyle of this bacterial group. Finally, ELKs encoded by strains of various phyla exhibit a complex modular organization (see Table 1; many domain architectures also are present in prokaryotes other than the myxobacteria). This finding indicates a high degree of complexity and interconnectedness within the signaling pathways in these microbes.
In the future, experimental elucidation of the elements of each pathway and the determination of the activating factors will be of significant interest and will be important to deepen our understanding of the occurrence and function of numerous kinases in multicellular bacteria. Furthermore, it will be intriguing to explore the accompanying processes that have evolved to use signal transduction pathways based on multiple ELKs in unicellular prokaryotes. Certainly, elucidating the detailed function of ELKs will be a major challenge in understanding bacterial multicellular behavior and adaptation.
Materials and Methods
Identification of ELKs.
Genes encoding ELKs in the prokaryotes were identified by BLASTP analysis of all complete genome sequences deposited in the database of the NCBI, and of 3 incomplete genomes belonging to the myxobacteria (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi) using the Pfam matrix for protein kinases (Pkinase; PF00069) (31). All sequences obtained with an E-value ≤ 1 were back-searched against Pfam and Prosite (http://expasy.org/prosite/) to verify unequivocally which proteins matched to the kinase domain.
Characterization of ELKs.
Myxobacterial ELKs have been aligned with the Pfam matrix for protein kinases to identify the catalytic residues Lys and Asp (D1 and D2) of subdomains II, Vib, and VII, respectively, and the regulatory Arg of subdomain VIb. When the residues were not identified in the alignment, the ELK sequence was analyzed manually to confirm their absence. The domain architecture of the ELKs was determined using Pfam.
Phylogeny of Myxobacterial ELKs.
Amino acid sequences of all myxobacterial kinase domains were aligned using ClustalX (32) and edited manually. Large deletions and insertions and sequence characters that could be not aligned with confidence were excluded. For maximum parsimony analysis, the heuristic search option of the software PAUP*4.0b10 was used with branch swapping by tree-bisection-reconnection and alignment gaps treated as missing data. From all best trees a strict consensus tree was calculated. Reconstruction with the neighbor joining method was performed by means of the Seqboot, Protdist, Neighbor, and Consens modules of the PHYLIP package version 3.65 (33). The JTT model for amino acid replacement was used in combination with a gamma distribution having 4 categories. Bayesian estimation was performed using the MrBayes software version 3 (34). The calculations also used the JTT model and a gamma function with 4 categories.
Synteny Determination in the ELK Regions.
Initially, 2 upstream and 2 downstream predicted proteins from each M. xanthus ELK were aligned with the other predicted myxobacterial proteomes using the BLASTP program (E-value <1e-4). We then manually analyzed the gene organization of positive matches in the 7 genomes at NCBI database. In cases of conservation, we extended the BLASTP searches to other genes within the same region. A “syntenic block” was defined as containing at least 2 consecutive genes exhibiting similarity, 1 of which encoded an ELK.
Supplementary Material
Acknowledgments.
We thank K. Weissman for critical reading of this manuscript and all investigators who have made genome sequences available before publication. The work in the laboratory of J.M.D. has been funded by Ministerio de Educación y Ciencia Grants BFU2003–02038 and BFU2006–00972/BMC and a Ministerio de Educación y Ciencia fellowship (to A.C.G.) and Junta de Andalucía Grant CVI1377. Research in the laboratory of R.M. was funded by the Bundesministerium für Bildung und Forschung and the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806851105/DCSupplemental.
References
- 1.Hanks SK, Hunter T. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 2.Akamine PH, et al. Dynamic features of cAMP-dependent protein kinase revealed by apoenzyme crystal structure. J Mol Biol. 2003;327:159–171. doi: 10.1016/s0022-2836(02)01446-8. [DOI] [PubMed] [Google Scholar]
- 3.Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: Structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 4.Kornev AP, Haste NM, Taylor SS, Ten Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci USA. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LN, Lewis RJ. Structural basis for control by phosphorylation. Chem Rev. 2001;101:2209–2242. doi: 10.1021/cr000225s. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz-Dorado J, Inouye S, Inouye M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell. 1991;67:995–1006. doi: 10.1016/0092-8674(91)90372-6. [DOI] [PubMed] [Google Scholar]
- 7.Goldman BS, et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci USA. 2006;103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneiker S, et al. Complete genome sequence of the myxobacterium Sorangium cellulosum. Nat Biotechnol. 2007;25:1281–1289. doi: 10.1038/nbt1354. [DOI] [PubMed] [Google Scholar]
- 9.Whitworth DE, Cock PJA. Two-component signal transduction systems of the myxobacteria. In: Whithworth DE, editor. Myxobacteria, Multicellularity and Differentiation. Washington DC: ASM Press; 2008. pp. 169–189. [Google Scholar]
- 10.Diodati ME, Gill RE, Plamann L, Singer M. Initiation and early development events. In: Whithworth DE, editor. Myxobacteria, Multicellularity and Differentiation. Washington DC: ASM Press; 2008. pp. 43–76. [Google Scholar]
- 11.Søgaard-Andersen L. Contact-dependent signaling in Myxococcus xanthus: The function of the C-signal in fruiting-body morphogenesis. In: Whithworth DE, editor. Myxobacteria, Multicellularity and Differentiation. Washington DC: ASM Press; 2008. pp. 77–91. [Google Scholar]
- 12.Meiser P, Bode HB, Müller R. The unique DKxanthene secondary metabolite family from the myxobacterium. Myxococcus xanthus is required for developmental sporulation. Proc Natl Acad Sci USA. 2006;103:19128–19133. doi: 10.1073/pnas.0606039103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye S, Nariya H, Muñoz-Dorado J. Protein Ser/Thr kinases and phosphatases in Myxococcus xanthus. In: Whithworth DE, editor. Myxobacteria, Multicellularity and Differentiation. Washington DC: ASM; 2008. pp. 191–210. [Google Scholar]
- 14.Zhang CC, Gonzalez L, Phalip V. Survey, analysis and genetic organization of genes encoding eukaryotic-like signaling proteins on a cyanobacterial genome. Nucleic Acids Res. 1998;26:3619–3625. doi: 10.1093/nar/26.16.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Av-Gay Y, Everett M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 2000;8:238–244. doi: 10.1016/s0966-842x(00)01734-0. [DOI] [PubMed] [Google Scholar]
- 16.Petøíčková K, Petøíček M. Eukaryotic-type protein kinases in Streptomyces coelicolor: Variations on a common theme. Microbiology. 2003;149:1609–1621. doi: 10.1099/mic.0.26275-0. [DOI] [PubMed] [Google Scholar]
- 17.Wehenkel A, et al. Mycobacterial Ser/Thr protein kinases and phosphatases: Physiological roles and therapeutic potential. Biochim Biophys Acta. 2008;1784:193–202. doi: 10.1016/j.bbapap.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Krupa A, Srinivasan N. Diversity in domain architectures of Ser/Thr kinases and their homologues in prokaryotes. BMC Genomics. 2005;6:129. doi: 10.1186/1471-2164-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannan N, Taylor SS, Zhai Y, Venter C, Manning G. Structural and functional diversity of the microbial kinome. PLoS Biol. 2007;5:467–478. doi: 10.1371/journal.pbio.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nariya H, Inouye M. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell. 2008;132:55–66. doi: 10.1016/j.cell.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Goldman B, Bhat S, Shimkets L. Genome evolution and the emergence of fruiting body development in Myxococcus xanthus. PLoS ONE. 2007;12:e1329. doi: 10.1371/journal.pone.0001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallen M, Chaudhuri R, Khan A. Bacterial FHA domains: Neglected players in the phospho-threonine signalling game? Trends Microbiol. 2002;10:556–563. doi: 10.1016/s0966-842x(02)02476-9. [DOI] [PubMed] [Google Scholar]
- 23.Nariya H, Inouye S. Activation of 6-phosphofructokinase via phosphorylation by Pkn4, a protein Ser/Thr kinase of Myxococcus xanthus. Mol Microbiol. 2002;46:1353–1366. doi: 10.1046/j.1365-2958.2002.03251.x. [DOI] [PubMed] [Google Scholar]
- 24.Knauber T, et al. Mutation in the rel gene of Sorangium cellulosum affects morphological and physiological differentiation. Mol Microbiol. 2008;69:254–266. doi: 10.1111/j.1365-2958.2008.06285.x. [DOI] [PubMed] [Google Scholar]
- 25.Ventura M, et al. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatt CG, Bryant DA, Ward DM. Comparative genomics provides evidence for the 3-hydroxypropionate autotrophic pathway in filamentous anoxygenic phototrophic bacteria and in hot spring microbial mats. Environ Microbiol. 2007;9:2067–2078. doi: 10.1111/j.1462-2920.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang CC, Laurent S, Sakr S, Peng L, Bédu S. Heterocyst differentiation and pattern formation in cyanobacteria: A chorus of signals. Mol Microbiol. 2006;59:367–375. doi: 10.1111/j.1365-2958.2005.04979.x. [DOI] [PubMed] [Google Scholar]
- 28.Kolter R, Greenberg EP. The superficial life of microbes. Nature. 2006;444:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 29.Fuerst JA. Intracellular compartmentation in Planctomycetes. Annu Rev Microbiol. 2005;59:299–328. doi: 10.1146/annurev.micro.59.030804.121258. [DOI] [PubMed] [Google Scholar]
- 30.Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- 31.Finn RD, et al. Pfam: Web tools and services. Nucleic Acids Res. 2006;34:D247–D251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felsenstein J. Phylogeny inference package version 3.6. Cladistics. 1989;5:164–166. [Google Scholar]
- 34.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.