Abstract
The streptococcal transposon Tn916 (Tcr) was used to isolate mutants of Streptococcus mutans altered in glycogen accumulation to investigate whether glycogenlike intracellular polysaccharides (IPS) play an important role in S. mutans-induced caries formation. S. mutans UA130 (serotype c) was transformed with the Escherichia coli plasmid pAM620 (Tn916), and the resultant transposon libraries were screened for glycogen content by iodine staining. A transposon mutant, designated SMS201, demonstrated a glycogen-deficient phenotype on glucose-enriched medium. Quantitative electron microscopy confirmed that IPS concentrations were significantly reduced in SMS201 relative to the wild-type progenitor strain, UA130. Importantly, reversion to wild type correlated at all times with loss of the transposon. Transposon excisants were used to facilitate cloning of the streptococcal gene(s) involved in glycogen biosynthesis and storage. A 2.1-kb chromosomal determinant (glgR) which encodes a putative regulator of S. mutans glycogen accumulation was isolated. A stable deletion mutation (delta glgR) was subsequently generated in E. coli and introduced into S. mutans by allelic exchange. The resultant glycogen synthesis-deficient mutant, SMS203, demonstrated a significantly reduced cariogenic potential (P less than 0.01) on the buccal, sulcal, and proximal surfaces of teeth in germfree rats, relative to animals challenged with the glycogen synthesis-proficient progenitor strain, UA130. These observations confirm previous reports (J. M. Tanzer, M. L. Freedman, F. N. Woodiel, R. L. Eifert, and L. A. Rinehimer, p. 597-616, in H. M. Stiles, W. J. Loesche, and T. L. O'Brien, ed., Proceedings in Microbiology. Aspects of Dental Caries. Special Supplement to Microbiology Abstracts, vol. 3, 1976) which implicate IPS as significant contributors to the S. mutans cariogenic process.
Full text
PDF



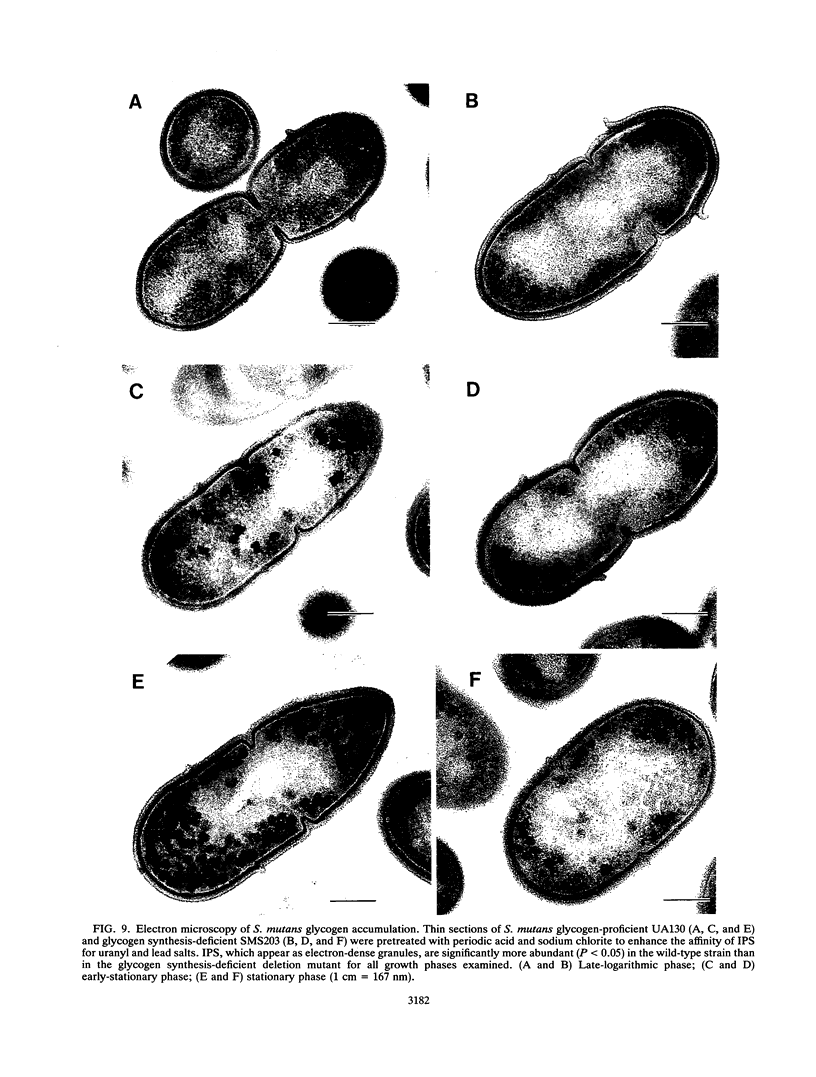
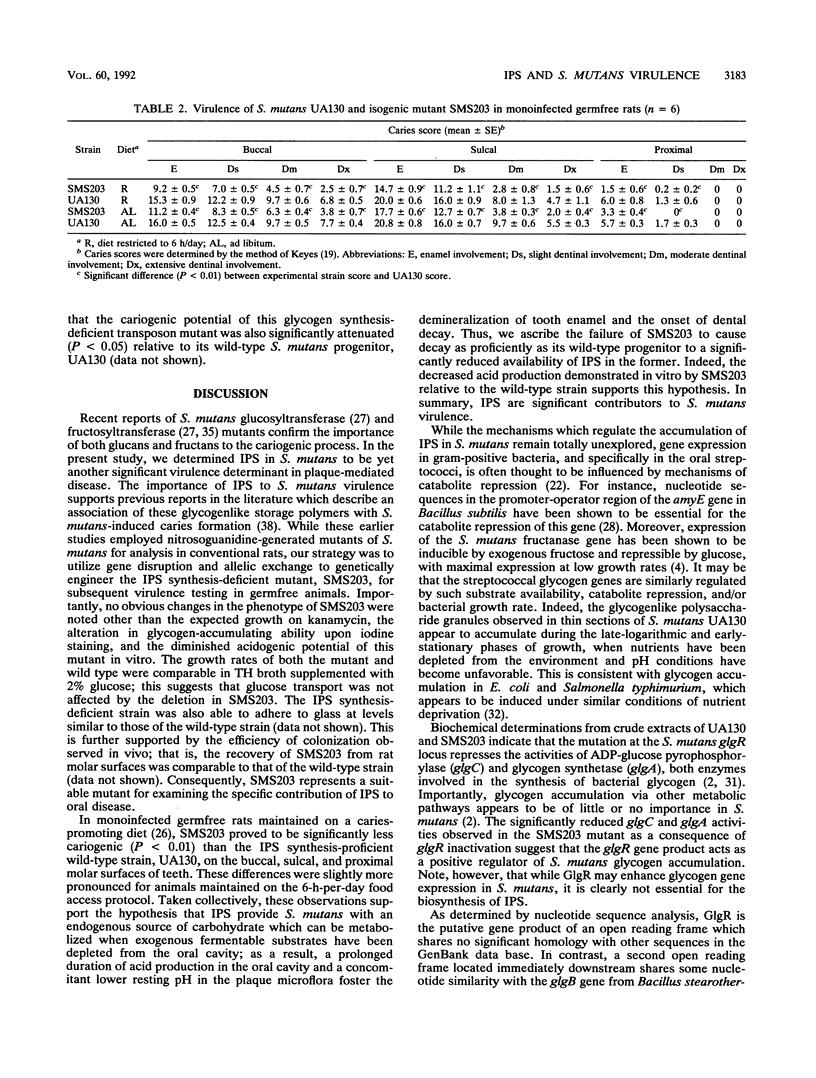


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birkhed D., Tanzer J. M. Glycogen synthesis pathway in Streptococcus mutans strain NCTC 10449S and its glycogen synthesis-defective mutant 805. Arch Oral Biol. 1979;24(1):67–73. doi: 10.1016/0003-9969(79)90177-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Schilling K., Bowen W. H., Yasbin R. E. Expression, purification, and characterization of an exo-beta-D-fructosidase of Streptococcus mutans. J Bacteriol. 1987 Oct;169(10):4507–4517. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud F., Trieu-Cuot P., Carlier C., Courvalin P. Nucleotide sequence of the kanamycin resistance determinant of the pneumococcal transposon Tn1545: evolutionary relationships and transcriptional analysis of aphA-3 genes. Mol Gen Genet. 1987 May;207(2-3):509–513. doi: 10.1007/BF00331623. [DOI] [PubMed] [Google Scholar]
- Charlton G., Fitzgerald D. B., Keyes P. H. Hydrogen ion activity in dental plaques of hamsters during metabolism of sucrose, glucose and fructose. Arch Oral Biol. 1971 Jun;16(6):655–661. doi: 10.1016/0003-9969(71)90069-0. [DOI] [PubMed] [Google Scholar]
- Charlton G., Fitzgerald R. J., Keyes P. H. Determination of saliva and dental plaque pH in hamsters with glass micro-electrodes. Arch Oral Biol. 1971 Jun;16(6):649–654. doi: 10.1016/0003-9969(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. R., Mattingly S. J., Higgins M. L., Shockman G. D. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974 Sep;10(3):597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYES P. H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958 Nov-Dec;37(6):1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- Kiel J. A., Boels J. M., Beldman G., Venema G. Molecular cloning and nucleotide sequence of the glycogen branching enzyme gene (glgB) from Bacillus stearothermophilus and expression in Escherichia coli and Bacillus subtilis. Mol Gen Genet. 1991 Nov;230(1-2):136–144. doi: 10.1007/BF00290661. [DOI] [PubMed] [Google Scholar]
- Kiel J. A., Elgersma H. S., Beldman G., Vossen J. P., Venema G. Cloning and expression of the branching enzyme gene (glgB) from the cyanobacterium Synechococcus sp. PCC7942 in Escherichia coli. Gene. 1989 May 15;78(1):9–17. doi: 10.1016/0378-1119(89)90309-0. [DOI] [PubMed] [Google Scholar]
- Lane M. A., Bayles K. W., Yasbin R. E. Identification and initial characterization of glucose-repressible promoters of Streptococcus mutans. Gene. 1991 Apr;100:225–229. doi: 10.1016/0378-1119(91)90371-h. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975 Jul;12(1):69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro C., Michalek S. M., Macrina F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991 Jul;59(7):2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Park Y. K., Henkin T. M., Won M., Weickert M. J., Gaskell J. A., Chambliss G. H. Catabolite repression-resistant mutations of the Bacillus subtilis alpha-amylase promoter affect transcription levels and are in an operator-like sequence. J Mol Biol. 1987 Dec 20;198(4):609–618. doi: 10.1016/0022-2836(87)90204-x. [DOI] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., WAGNER M., GORDON H. A., LUCKEY T. D. Use of the germfree animal technic in the study of experimental dental caries. I. Basic observations on rats reared free of all microorganisms. J Dent Res. 1954 Apr;33(2):147–174. doi: 10.1177/00220345540330020201. [DOI] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schroeder V. A., Michalek S. M., Macrina F. L. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun. 1989 Nov;57(11):3560–3569. doi: 10.1128/iai.57.11.3560-3569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Jones J. M., Senghas E., Gawron-Burke C., Clewell D. B. Generation of Tn5 insertions in streptococcal conjugative transposon Tn916. Appl Environ Microbiol. 1987 May;53(5):1069–1072. doi: 10.1128/aem.53.5.1069-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]