Abstract
The large size and geometric complexity of neuronal dendrites necessitate specialized mechanisms to both deliver postsynaptic cargo over extended distances and regulate dendritic composition on a submicron scale. Despite the fundamental importance of membrane trafficking in dendrite growth, synapse formation, and plasticity, the organelles and cellular rules governing postsynaptic trafficking are only now emerging. We review here what is currently known about dendritic secretory organelles and their role in the development, maintenance and plasticity of postsynaptic compartments.
Keywords: neuron, secretory pathway, spine apparatus, polarized trafficking, dendritic growth, postsynaptic receptors, local protein translation
With surface area up to 10,000 times larger than typical mammalian cells and elaborately branched processes extending up to hundreds of microns from the cell body, neurons are among the largest and most complex metazoan cells. A central question in neuronal cell biology is how evolutionarily conserved pathways for membrane traffic that originally emerged in simpler and smaller cells have become specialized to accommodate these unique dimensions.
While signaling pathways and genetic programs controlling dendrite morphology are increasingly well described (1), the membrane trafficking events underlying dendrite growth and complexity are not yet delineated. Conversely, despite the implicit importance of secretory trafficking in dendrite morphogenesis and synaptic function, and even though the Golgi apparatus (GA) was first described in neurons (2, 3), strikingly little is known about the spatial organization or regulation of the neuronal secretory pathway.
We review here current knowledge of neuronal secretory organelles, emphasizing that, although many general principles described in simpler model cell systems also apply to neurons, neurons have evolved specific mechanisms to govern delivery of lipids and proteins to distal dendrites. After describing the distributed spatial organization of the neuronal secretory pathway, we discuss potential implications for local processing of dendritic membrane and secretory cargo, and describe potential functional consequences for dendrite dynamics and synapse plasticity.
Spatial organization of the neuronal secretory pathway
As in non-neuronal cells, the neuronal endoplasmic reticulum (ER) is distributed throughout the cytoplasm, and extends throughout dendrites and the soma (4-6), functioning both as the site of membrane protein and lipid synthesis (7) and as an intracellular calcium signaling compartment (8). Although smooth ER (SER) dominates in distal dendrites, membrane bound ribosomes and subunits of the translocon that mark the rough ER (RER) are also found distally (4, 9, 10).
Proper folding of newly assembled proteins in the ER and concomitant post-translational modifications, including N-glycosylation and disulfide bound formation, are essential for progression of cargo through the secretory pathway. Molecules that pass quality control concentrate at ER exit sites (ERES), which appear as discrete stationary puncta distributed on the ER membrane throughout the somatodendritic compartment (11, 12). There, cargo leaves the ER in COPII-coated carriers, which then merge with ER-Golgi intermediate compartments (ERGICs) that are also widely distributed throughout dendrites and the soma (5, 6, 13). ER exit is tightly controlled and is a rate-limiting step in the biosynthesis of important neuronal transmembrane proteins such as AMPA-type glutamate receptors (14, 15) and NMDA-type glutamate receptors (16-18), whose surface expression is regulated by ER retention and ER export signals.
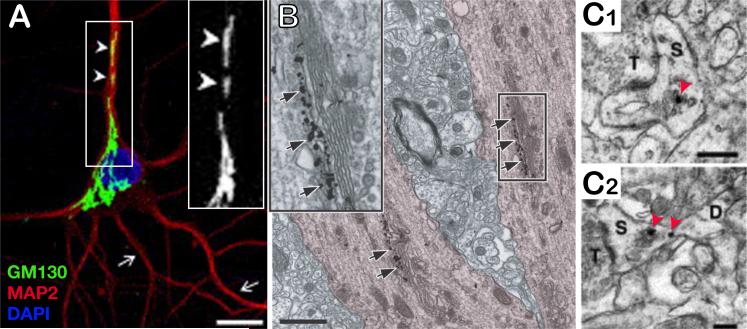
Following ER exit, further modifications occur, including glycosylation and proteolysis, as secretory cargo progresses through the cis, medial and trans compartments of the GA (19). Cargo then reaches the trans Golgi network (TGN) where it is sorted for either transport to the plasma membrane or endosomal compartments (20). In most mammalian cells, the ER, ERES, and ERGIC elements are dispersed throughout the cell, whereas the Golgi network is located in the perinuclear area close to the microtubule-organizing center (MTOC) (21), imposing centripetal and centrifugal directionalities to pre- and post-Golgi trafficking, respectively. In neurons, this arrangement is quite different, as the neuronal GA consists not only of the pericentriolar membrane array found in most cell types, but also includes discrete structures dispersed in dendrites (5, 6, 11, 22-25) termed Golgi outposts (Fig. 1A, 1B). Although immunoreactivity for Golgi markers can be found in presumptive Golgi membranes in dendritic spines (Fig. 1C), Golgi outposts defined by their immunoreactivity for the Golgi-matrix protein GM130 and their characteristic mini-Golgi stack ultrastructure have only been observed in the dendritic shaft (Fig. 1B, 2C), where they are occasionally located in proximity to inhibitory synapses (5, 9, 24). Interestingly not all dendrites contain morphological or molecular markers of the GA (5, 6, 11, 24), indicating dendrite-specific compartmentalization of secretory trafficking.
Figure 1. Spatial organization of the Golgi apparatus in hippocampal neurons.
A. Immunolabeling of the somatodendritic marker MAP2 (red) and the cis-Golgi matrix protein GM130 (green) in a cultured hippocampal neuron. Scale bar, 10 μm. Inset: higher magnification of GM130 labeling illustrating Golgi outposts dispersed in the apical dendrite (arrowheads). Other dendrites lack GM130 positive Golgi outposts (arrows). B. Immunogold labeling for GM130 (arrows) demonstrating the presence of isolated Golgi stacks in the apical dendrite of a pyramidal neuron in vivo. Scale bar, 1 μm. Inset: higher magnification. A and B adapted from (24); reprinted with permission from Elsevier, copyright 2005. C. α-mannosidase II (C1) and giantin (C2) immunogold labeling (red arrowheads) in dendritic spines of CA1 pyramidal neurons in vivo. T, presynaptic terminal; S, spine; D, dendrite Scale bar, 250 nm. Adapted from (9); reprinted with permission from Elsevier, copyright 2001.
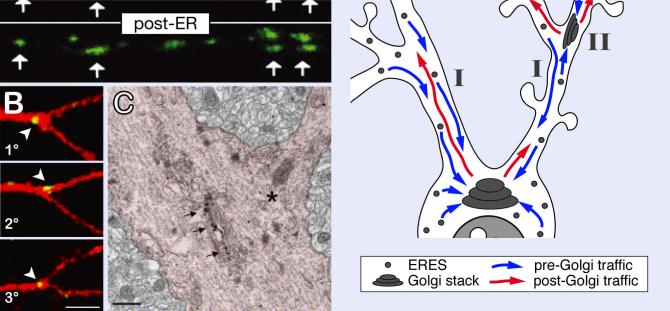
Figure 2. ER-to-Golgi trafficking in neuronal dendrites.
A. VSVG-ts-YFP (red) distribution in a hippocampal neuron dendrite expressing a CFP-tagged Golgi marker (GalT-CFP), before (ER), and 20 min after (post-ER) release of temperature-dependent ER exit blockade (39.5°C to 32°C switch, see text), illustrating the accumulation of post-ER cargo in dendritic Golgi outposts (arrows). Adapted from (11); reprinted with permission from the Society for Neuroscience, copyright 2003. B. VSVG-ts-GFP (green) after a 20°C TGN-exit blockade (see text) in hippocampal neurons expressing a red cell-fill, showing the presence of secretory platforms at primary (1°), secondary (2°), and tertiary (3°) dendritic branch points. C. Electron micrograph of GM130 immunogold labeling (arrows) marking a Golgi outpost located at a dendritic branch point in vivo. B and C adapted from (24); reprinted with permission from Elsevier, copyright 2005. D. Model for dual ER-to-Golgi trafficking in dendrites. While ER and ER exit sites (ERES) are distributed throughout the somatodendritic compartment, only a subset of dendrites contain Golgi outposts. Consequently, dendritic post-ER carriers are transported long distances to the somatic Golgi in dendrites lacking Golgi outposts (I). In dendrites containing Golgi outposts, post-ER carriers either bypass dendritic outposts (I) or deliver their cargo to outposts for local processing (II).
The involvement of dendritic Golgi outposts in post-ER trafficking has been established by imaging the membrane transport of a GFP fusion of the ts045 thermosensitive mutant of the vesicular stomatitis virus glycoprotein (VSVG-ts) in hippocampal neurons (Fig. 2A, 2B)(11). VSVG-ts ER retention and exit are controlled by temperature, allowing for direct visualization of the synchronous progression of cargo through the secretory pathway (26). Upon release from the ER, a fraction of pre-Golgi carriers containing VSVG-ts merge with dendritic Golgi outposts (Fig. 2A), demonstrating that these structures are functional trafficking platforms (11). Golgi outposts also engage in dendritic trafficking of brain-derived neurotrophic factor (BDNF), confirming their role in the processing of relevant neuronal secreted proteins (11).
Perhaps surprisingly, even in dendrites containing Golgi outposts, many post-ER carriers originating in dendrites bypass Golgi outposts and are trafficked long distances back to the somatic Golgi (11). Thus, dual modes of early secretory trafficking exist in dendrites (Fig. 2D). The major mode of ER-to-Golgi trafficking is directed long distances to the somatic Golgi apparatus and likely represents the exclusive mode of early secretory trafficking in those dendrites lacking Golgi outposts. A second mode of ER-to-Golgi trafficking occurs locally in dendrites containing Golgi outposts, and may be specialized for the processing of specific cargo or the control of dendritic secretion (Fig. 2D).
Generation and maintenance of dendritic Golgi outposts
In general, the size and polarity of the Golgi apparatus reflects the level and direction of secretory trafficking in cells, and both parameters can vary significantly, especially during the cell cycle. The abundance of dendritic Golgi outposts may thus fluctuate in concert with local secretory demand. Although precisely how dendritic Golgi outposts are generated is still unresolved, two scenarios can be postulated based on examples of Golgi dispersal and formation in non-neuronal cells: a local de novo production from the ER as observed in the protozoan Giardia lamblia or in the yeast Pichia pastoris (27), or a fragmentation of the somatic Golgi followed by subsequent dispersal of remnant membranes that then act as templates to rebuild Golgi stacks, as observed during mitosis in most animal cells (28).
The Golgi apparatus also serves as a scaffold for numerous signaling molecules, including kinases and phosphatases that regulate Golgi matrix dispersion and reassembly through the cell cycle (28). Interestingly, several of these kinases and phosphatases are regulated by synaptic activity and are known to influence dendrite morphogenesis and synaptic function. For example, the Ras/ERKMAPK pathway, which triggers Golgi fragmentation during mitosis (29), is stimulated by neuronal activity and is required for activity-dependent dendritic growth (30). It is therefore tempting to speculate that similar signaling pathways regulate Golgi organization in post-mitotic cells, which could control the fragmentation of the neuronal Golgi in response to increased demand for local membrane addition.
Another well-described instance of Golgi fragmentation is during apoptosis (31), where activated caspases cleave several Golgi matrix proteins leading to Golgi membrane dispersal. Recent studies of dendrite pruning in Drosophila pupae have documented non-apoptotic functions of local caspase activation (32, 33). The exact sequence of events linking caspase activity to dendrite pruning is still not fully delineated, but one possibility is that local caspase activation regulates dendritic Golgi outposts that have been described in Drosophila larval sensory neurons (25).
Golgi structure, localization, and function depend on a tight interplay with the cytoskeleton. Whereas cytoplasmic dynein controls the pericentriolar positioning of Golgi stacks, spectrins and F-actin are required to shape Golgi cisternae (28). Additionally, numerous actin regulatory proteins and actin-based motors act synergistically during vesicle fusion and budding at the Golgi. Accordingly, local actin remodeling is likely required for dendritic Golgi outpost maintenance and function, as supported by their dispersal upon disruption of the neuron-specific Golgi-localized RhoA-binding protein Citron-N (34). RhoA is generally not highly enriched on non-neuronal Golgi membranes (35), indicating that it may play an important and specific role in organizing neuronal Golgi. As with non-neuronal Golgi, dynein is required for dendritic Golgi outpost maintenance. Disrupting Drosophila Lava-lamp (Lva) – a protein coupling the dynein/dynactin complex to Golgi membranes – leads to alteration of dendritic Golgi (25), a feature that may be particularly relevant in Drosophila dendrites with their unusual preponderance (>90%) of minus end-out oriented microtubules (36).
Because of the close relationship between microtubule based-transport, post-ER trafficking, and Golgi location, it is intriguing that neuronal microtubule organization is quite different than that of non-neuronal mammalian cells. Rather than having microtubules with their minus ends anchored at the centriole and their plus end radiating towards the cell periphery, proximal dendrites of mammalian neurons display microtubules with both orientations (37). Only axons and very distal dendrites contain microtubules with their plus end pointing exclusively away from the cell body. Whereas ER-derived carriers are transported preferentially toward the centriole in non-neuronal cells, dendritic microtubule organization allows bi-directional transport and eventual fusion with more distal Golgi outposts (11).
Recent studies in fibroblasts have shown that the TGN constitutes a second microtubule organizing center (MTOC), specifically generating arrays of microtubules asymmetrically radiating towards the cell-periphery (38). The nucleation of microtubules at the TGN is likely crucial for the synergistic regulation of cytoskeletal organization and directed secretory flux, allowing asymmetric addition of membranes to the leading edge of migrating cells (39). Whether this also holds for dendritic Golgi outposts remains to be determined, but if so, would provide a potential mechanistic basis and functional context for the peculiar organization of dendritic microtubules.
Secretory platforms for local delivery of postsynaptic receptors to the cell surface
Although recycling endosomes constitute the major reservoir for AMPA receptors and membranes mobilized during long-term potentiation (LTP) and LTP-induced spine enlargement (40, 41), the secretory pathway must ultimately be involved in synapse maintenance and plasticity, since it constitutes the obligatory biosynthetic pathway for neurotransmitter receptors. Indeed, acute disruption of the Golgi in hippocampal slices with brefeldin A impairs NMDA-induced potentiation and AMPA receptor insertion (42). However, the precise itinerary followed by newly assembled receptors to reach the plasma membrane is still not clearly defined, due largely to our yet embryonic knowledge of post-Golgi trafficking in dendrites.
Dendritic secretory platforms have been visualized using a variety of approaches. Temperature dependent blockade of post-TGN trafficking (43) induces accumulation of newly ER-released VSVG-ts in compartments located at dendritic branch points (Fig. 2B, 2C), including not only Golgi outposts but also distinct structures that are likely post-Golgi elements (11, 24). These secretory organelles engage in ongoing trafficking after release of TGN-exit blockade and appear ideally positioned to regulate the identity and quantity of cargo being trafficked to distinct dendritic branches or to nearby synapses. Supporting this model, recent data in Drosophila larval sensory neurons indicate that Golgi outposts locally provide secretory material to developing dendritic branches, thereby contributing to dendritic complexity (25).
Presumptive TGN-derived carriers, reminiscent of VSVGts-containing dendritic compartments, have been observed using fluorescent lipid probes such as FM1−43 or ceramides (44, 45). Dendritic FM1−43-containing carriers are rapidly unloaded in response to Ca2+ influx (44) and are recruited to newly formed axo-dendritic contacts sites by the neural cell adhesion molecule (NCAM) (45), indicating that they could be directed by intercellular signaling to deliver early postsynaptic components to cellular contacts marked as future synaptic sites. Moreover, Ca2+-triggered exocytosis of post-Golgi vesicles in dendrites requires Ca2+/calmodulin-dependent protein kinase type II (CaMKII) (46), supporting a common signaling pathway with LTP, and suggesting that post-Golgi compartments might also participate in activity-dependent exocytosis.
Sub-synaptic secretory compartments have been described at vertebrate NMJs, in Torpedo electrocytes, and in larval Drosophila neuromuscular synapses, where they serve as trafficking platforms for postsynaptic receptors. Whereas in vertebrates these compartments include RER, GA, and TGN membranes directly polarized towards the postsynapse (47), those observed in fly muscles are morphologically distinct and consist primarily of an ER-like reticulum (48). Quite similarly in mammals, the dendritic SER often extends into the neck of mature dendritic spines (Fig.3A), providing a conduit between the dendritic shaft and the spine head (4), where it occasionally forms a specialized derivative composed of stacked cisternae termed the spine apparatus (Fig. 3B, 3C) (49). Although clearly recognizable by its ultrastructural morphology, the precise compartmental identity of the spine apparatus has yet to be determined. Intriguingly, whereas continuity with the shaft SER lumen and immunoreactivity for proteins such as ERGIC53/p58 and Rab1 point towards an ER origin, stacked cisternae and immunoreactivity for markers such as giantin, α-mannosidase II (Fig. 1C) and Rab6 (9) suggest an affiliation of the spine apparatus with Golgi membranes. Although the spine apparatus contains AMPA and NMDA receptors (50, 51) and is required for specific forms of synaptic plasticity (52, 53), the precise nature of its involvement in postsynaptic receptor trafficking remains elusive.
Figure 3. The dendritic ER and the spine apparatus.
A. Three-dimensional reconstruction of serial electron micrographs showing the distribution of smooth ER (SER, dark grey) in a short segment of a CA1 hippocampal neuron dendrite in vivo. Large flat compartments (arrowheads) are linked by thin extensions (thin arrows). Note the extension of SER membranes within the head of a mature spine (crossed arrow). Adapted from (68); reprinted with permission from the Society for Neuroscience, copyright 2002. B. Electron micrograph of the spine apparatus (SA) showing the lamination of cisternae (thick arrows) between regions of high electron density (wavy arrows). Adapted from (4); reprinted with permission from the Society for Neuroscience, copyright 1997. Scale bar, 0.5 μm. C. Schematic representation of a large dendritic spine illustrating vesicles at the tip of the SA, the presence of glutamate receptors in the SA, and the presence of ribosomes (r) and polysomes (p) at the base of the spine.
Satellite secretory systems and local translation of membrane proteins
The presence of secretory compartments in dendrites is particularly interesting with respect to local protein translation in dendrites, for it suggests a local capacity to process and transport integral membrane proteins and secreted factors to the plasma membrane independent of the cell body. An expanding list of mRNAs have been shown to be actively transported to dendrites, including mRNAs encoding postsynaptic receptors (e.g. subunits for glycine receptors and AMPA receptors) and secreted proteins (e.g. BDNF) (54). Local protein translation in dendrites is now well established for cytoplasmic proteins and is critical for the maintenance of activity-dependent changes of synaptic properties (55). However, whereas local synthesis of a cytoplasmic protein only requires mRNA, ribosomes, tRNA, and metabolites, local synthesis and membrane transport of secreted proteins requires the entire complement of secretory organelles.
Although dendritic translation of mRNAs encoding membrane proteins is still not as clearly delineated as that of cytoplasmic proteins, the dendritic localization of RER, ERES, ERGIC and Golgi membranes suggests that “satellite” secretory systems exist in some dendrites. Indeed, enzymatic activities associated with the secretory pathway, such as glycosylation of newly synthesized proteins, persist in ∼30% of hippocampal dendrites after mechanical isolation from the soma (6). The ability of isolated dendrites to synthesize overexpressed integral membrane proteins (e.g., AMPA receptor subunits) and deliver them to the plasma membrane has been reported (56, 57). However, it remains unknown whether the presence of secretory outposts in dendrites directly influences local synthesis and trafficking of integral membrane proteins.
Intriguingly, mRNAs encoding dendritically translated integral membrane proteins, such as AMPA receptor subunits, are detected in most dendrites of cultured hippocampal neurons (58), raising fundamental questions about the regulation of mRNA transport in dendrites. As discussed earlier, only a fraction of dendrites contain Golgi outposts within a given neuron, raising the question of why local translation would occur in dendrites without local secretory platforms. As illustrated by the spine apparatus, immunoreactivity for Golgi structural proteins and Golgi enzymes can be localized to non-conventional Golgi structures in dendrites (9). More systematic studies of the distribution of Golgi proteins at the ultrastructural level will be required to determine whether secretory outposts in distal dendrites and canonical Golgi outposts contain distinct casts of structural proteins and whether other, yet unidentified, dendritic organelles might perform functions of the Golgi apparatus.
Another important consideration in local membrane protein synthesis is that the dendritic ER likely constitutes a dynamic continuous internal membrane network as observed in non-neuronal cells (59). Nascent polypeptides for membrane proteins or secreted proteins may diffuse in the dendritic ER, although the extent of such diffusion and its impact on the spatial confinement of local synthesis have not been investigated. It is very likely that the time spent in the ER affects the spatial domain over which new membrane proteins are produced from a local ER-bound ribosome synthetic source. Local secretory processing in Drosophila oocytes provides a compelling illustration of this concept. In this cell type (as is typical for Drosophila), the Golgi apparatus consists of multiple mini-stacks or tubulo-vesicular aggregates located in close proximity to ERES, which function as discrete secretory units dispersed throughout the cytoplasm (60). Although the ER is also a continuous network in Drosophila oocytes, targeting the mRNA encoding secreted proteins such as Gurken to specific subdomains of the cell periphery allows cognate polypeptides to be selectively processed by peripheral secretory units. This targeting is required for subsequent polarized secretion of synthesized proteins at the appropriate location, provided that ER exit is efficient and sufficiently rapid (60). If ER export of Gurken is delayed, polarized secretion is lost despite polarization of the mRNA, due to diffusion of nascent Gurken polypetides throughout the ER. In vertebrate neurons, postsynaptic receptors translated in dendrites display ER residency times ranging from minutes to hours (14). Such strikingly different ER dwell times may allow polypeptides to explore smaller or larger areas of the neuronal ER, potentially sharpening or relaxing the spatial domain of local secretion. Conversely, deciphering how local structural complexity of the dendritic ER (4) (Fig. 3A) affects the diffusion of ER retained cargo and their subsequent post-ER trafficking in dendrites will be an important step towards a more integrated view of local protein synthesis in dendrites.
Polarized secretory trafficking and asymmetric dendrite growth
The secretory pathway represents the primary site of lipid biosynthesis and membrane addition, and thus contributes to polarized cell growth (27). The immediate requirement for secretory trafficking in dendritic growth and maintenance has been established by various approaches. Disrupting Golgi function with brefeldin A or by overexpressing inhibitory mutants of protein kinase D (PKD) or Arf1 to block post- and intra-Golgi trafficking, respectively, prevents dendritic growth (24). Perhaps more surprisingly these manipulations cause a dramatic simplification of dendrite morphology in mature neurons within hours (Fig.4) (24). Intriguingly, axons are unaffected by the same conditions in young and mature neurons, indicating different trafficking requirements for the maintenance of distinct neuronal domains (24).
Figure 4. Polarized secretory trafficking and asymmetric dendritic growth in hippocampal pyramidal neurons.
A. Polarization of the somatic Golgi in pyramidal neurons (left) versus GABAergic inhibitory interneurons (right). Shown is a pseudocolored map of GM130 fluorescence intensity (Golgi-IR) as a function of radial orientation relative to the axis formed by the longest dendrite (0°, up). Insets: fluorescence fractions in each quadrant. Note that the somatic Golgi is polarized towards the apical dendrite in neurons displaying polarized dendritic trees. B. Polarization of the somatic Golgi (red) correlates with asymmetric dendrite growth to produce a single apical dendrite (1), imposing a bias to post-Golgi trafficking (blue arrows). Alteration of pre- and post-Golgi trafficking either by overexpressing dominant negative (dn) mutants of PKD or Arf1, by Sar1 RNAi knockdown, or using BFA prevents dendritic growth (1)(2). Axons are not affected under these conditions (not illustrated). Dispersion of the somatic Golgi by overexpression of GRASP65 abolishes the asymmetric growth of the apical dendrite without affecting total dendritic growth (3). Adapted from (24); reprinted with permission from Elsevier, copyright 2005.
Recently, a mutagenesis screen in peripheral neurons of Drosophila larvae has led to the identification of several mutants that selectively disrupt growth or maintenance of class IV da neuron dendrites, but not axons, in a cell autonomous manner (25). Tellingly, over one third of these Dar mutants (three complementation groups out of eight) were due to mutations in genes encoding proteins functioning at the ER-Golgi interface and required for anterograde secretory trafficking, namely Rab1, Sar1 and Sec23. The dendritic alterations seen in these mutants correlate with disrupted Golgi membranes, and, together with selective effects of post-Golgi trafficking on dendrite growth seen in mammalian neurons (24), indicate an evolutionarily conserved cellular mechanism controlling dendrite versus axon outgrowth. Indeed, RNAi knockdown of Sar1 in hippocampal cultures selectively impairs secretory cargo trafficking in dendrites but not in axons (Fig. 4)(25).
Perhaps the clearest indication of how the specialized spatial organization of the neuronal secretory pathway influences neuronal development and plasticity came from studies of dendritic morphogenesis. Although the site of membrane addition to growing dendrites is uncertain, the spatial organization of the neuronal secretory pathway provides some important clues. Somatic Golgi is oriented toward the apical dendrite in hippocampal pyramidal cells in vivo, and toward the longest and most complex dendrite of hippocampal neurons in culture, imposing a spatial bias to the post-Golgi flux originating from the somatic Golgi (Fig. 4)(24). Time-lapse imaging showed that cultured hippocampal pyramidal neurons develop progressive dendritic asymmetry in the absence of spatially organized extrinsic cues, unraveling striking differences with GABAergic neurons in terms of Golgi orientation and dendritic geometry (61). In both cell types, a strict correlation exists between dendritic morphology and somatic Golgi orientation, but only pyramidal neurons display a polarization of the somatic Golgi towards a single dendrite, which becomes the apical dendrite (Fig. 4A) (24, 61). This program for polarized dendrite growth raises questions about whether polarization of the somatic Golgi determines asymmetric dendrite growth, or results from the emergence of an apical dendrite. Arguing for the former, Golgi polarity precedes dendrite polarity (24). Moreover, disrupting somatic Golgi orientation by overexpressing GRASP65 – a matrix protein required for cisternal stacking – prevents the specification of the apical dendrite without diminishing the rate and extent of total dendrite growth (Fig. 4B) (24). Thus, polarized post-Golgi trafficking sustains asymmetric dendritic growth and determines the generation of an apical dendrite.
On a smaller and more local scale, the preferential positioning of Golgi outposts (and related secretory platforms) at branch points (Fig. 2B, 2C, 2D) (24, 25) raises the possibility that these organelles regulate the amount of cargo being trafficked to each branch. Supporting this possibility, altering Golgi outpost maintenance and localization by disrupting the dynein/dynactin adaptor Lava-lamp (Lva) in larval Drosophila sensory neurons results in reduced and aberrant dendrite branching, demonstrating a direct correlation between Golgi outpost abundance and dendritic complexity (25). Interestingly, time-lapse imaging in the same study documented that Golgi outpost stability and escape predict developing dendritic branch extension or retraction, respectively. Focal laser disruption of cellular domains containing Golgi outposts resulted in cessation of nearby branch dynamics, supporting a causal relationship between Golgi outposts and dendritic branch dynamics (25). Notably, such local branch point disruption prevents not only branch extension but also branch retraction (25), implicating the existence of undefined functions of dendritic Golgi outposts or associated structures in dendritic branch dynamics.
Although clear differences exist in the secretory mechanisms accounting for dendrite versus axon maintenance (24, 25), the spatial relationship between Golgi membranes and axon specification remains controversial. A study in dissociated neurons showed that the centrosome and associated organelles cluster at sites opposite the plane of final mitotic division where the first neurite emerges and becomes the axon (62). However, at slightly later developmental stages after initial neurite outgrowth but before either polarized growth or the appearance of spatially restricted axonal markers, the somatic Golgi shows no polarization towards the axon (24). Post-Golgi trafficking might be redirected from axons to dendrites at different developmental stages, during migration, or in different in vivo contexts. In newborn cortical neurons in vivo, the centrosome, and thus likely secretory organelles, is oriented toward the leading process diametrically opposed to the axon (63). It will be interesting to test whether the sequential protrusion of the axon and that of the leading process (64) involves a 180° rotation of the centriole and secretory organelles around the nucleus.
Perspectives
Many questions remain regarding the generation and regulation of dendritic secretory outposts. Are outposts locally generated from the dendritic ER or produced by a fragmentation of the somatic Golgi? To what extent does the spatial organization of dendritic secretory organelles differ among neuronal types, and how is it developmentally regulated? Although miniature Golgi stacks have been observed in dendrites by EM, dendritic Golgi and secretory outpost ultrastructure must be studied more systematically. Recent progress in cryo-EM and 3D-reconstruction will likely foster a more detailed description of these structures, providing new venues for functional studies.
Although compelling progress has been made in dissecting mechanisms that control receptor insertion and removal at synapses, our understanding of postsynaptic membrane dynamics is limited by a lack of knowledge about the fundamental organization of dendritic endomembranes. Within a given neuron, mechanisms supporting ongoing synaptic turnover and plasticity seem to vary with synapse location. For example, membrane trafficking and lateral diffusion in the plasma membrane may differentially contribute to receptor turnover at synapses localized near the soma versus those localized to distal dendrites (65). In pyramidal neurons, Golgi outposts are present primarily in apical dendrites (24), suggesting distinct trafficking mechanisms in apical versus basolateral dendrites. Indeed, synapses onto these dendritic compartments contain different subclasses of NMDA receptors (66) and perhaps other molecules. The heterogeneous spatial restriction of dendritic organelles such as Golgi outposts, post-Golgi compartments, or various classes of endosomes (40) might thus contribute to spatial variations in synapse composition.
Finally, from a clinical perspective, fragmentation of the neuronal Golgi is seen in numerous neurodegenerative diseases (31) and might be functionally linked to defects in membrane and secreted protein trafficking that occur in these pathological contexts. The role of postsynaptic trafficking in Alzheimer's disease (AD) is particularly interesting. The processing of the amyloid precursor protein (APP) during β-amyloid (Aβ) production occurs during trafficking through the early secretory pathway and/or endocytic compartments (67), but the exact modalities of APP production in neurons are still not fully delineated. A better understanding of the core dendritic secretory machinery will be critical to determine the pathogenesis of AD and other neurodegenerative diseases.
Acknowledgments
We thank Ian Davison, Juliet Hernandez, Matthew Kennedy, and Jason Yi, for their critical reading of the manuscript. We apologize to those whose work we could not cite due to space limitations. Work in the laboratory of MDE is supported by grants from the NIH. MDE is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Jan YN, Jan LY. The control of dendrite development. Neuron. 2003;40(2):229–242. doi: 10.1016/s0896-6273(03)00631-7. [DOI] [PubMed] [Google Scholar]
- 2.Golgi C. On the structure of nerve cells. 1898. Journal of microscopy. 1989;155(Pt 1):3–7. doi: 10.1111/j.1365-2818.1989.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar MG, Palade GE. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8(1):2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17(1):190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardiol A, Racca C, Triller A. Dendritic and postsynaptic protein synthetic machinery. J Neurosci. 1999;19(1):168–179. doi: 10.1523/JNEUROSCI.19-01-00168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16(19):5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgese N, Francolini M, Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr Opin Cell Biol. 2006;18(4):358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose CR, Konnerth A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron. 2001;31(4):519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 9.Pierce JP, Mayer T, McCarthy JB. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr Biol. 2001;11(5):351–355. doi: 10.1016/s0960-9822(01)00077-x. [DOI] [PubMed] [Google Scholar]
- 10.Pierce JP, van Leyen K, McCarthy JB. Trans-location machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nat Neurosci. 2000;3(4):311–313. doi: 10.1038/73868. [DOI] [PubMed] [Google Scholar]
- 11.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aridor M, Guzik AK, Bielli A, Fish KN. Endoplasmic reticulum export site formation and function in dendrites. J Neurosci. 2004;24(15):3770–3776. doi: 10.1523/JNEUROSCI.4775-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell. 1995;6(10):1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34(5):759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 15.Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17(3):289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28(3):887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 17.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21(9):3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40(3):581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 19.de Graffenried CL, Bertozzi CR. The roles of enzyme localisation and complex formation in glycan assembly within the Golgi apparatus. Curr Opin Cell Biol. 2004;16(4):356–363. doi: 10.1016/j.ceb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 21.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 22.De Camilli P, Moretti M, Donini SD, Walter U, Lohmann SM. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. J Cell Biol. 1986;103(1):189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowenstein PR, Morrison EE, Bain D, Shering AF, Banting G, Douglas P, Castro MG. Polarized distribution of the trans-Golgi network marker TGN38 during the in vitro development of neocortical neurons: effects of nocodazole and brefeldin A. Eur J Neurosci. 1994;6(9):1453–1465. doi: 10.1111/j.1460-9568.1994.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 24.Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48(5):757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130(4):717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergmann JE. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods in cell biology. 1989;32:85–110. doi: 10.1016/s0091-679x(08)61168-1. [DOI] [PubMed] [Google Scholar]
- 27.Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6(7):585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- 28.Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol. 2004;16(4):364–372. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Shaul YD, Seger R. ERK1c regulates Golgi fragmentation during mitosis. J Cell Biol. 2006;172(6):885–897. doi: 10.1083/jcb.200509063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50(6):897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Gonatas NK, Stieber A, Gonatas JO. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. Journal of the neurological sciences. 2006;246(1−2):21–30. doi: 10.1016/j.jns.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51(3):283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9(10):1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 34.Camera P, da Silva JS, Griffiths G, Giuffrida MG, Ferrara L, Schubert V, Imarisio S, Silengo L, Dotti CG, Di Cunto F. Citron-N is a neuronal Rho-associated protein involved in Golgi organization through actin cytoskeleton regulation. Nat Cell Biol. 2003;5(12):1071–1078. doi: 10.1038/ncb1064. [DOI] [PubMed] [Google Scholar]
- 35.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural development. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippo-campal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85(21):8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR, 3rd, Maiato H, Khodjakov A, Akhmanova A, Kaverina I. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Developmental cell. 2007;12(6):917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106(4):489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 40.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 41.Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52(5):817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broutman G, Baudry M. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J Neurosci. 2001;21(1):27–34. doi: 10.1523/JNEUROSCI.21-01-00027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matlin KS, Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- 44.Maletic-Savatic M, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part I: trans-Golgi network-derived organelles undergo regulated exocytosis. J Neurosci. 1998;18(17):6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sytnyk V, Leshchyns'ka I, Delling M, Dityateva G, Dityatev A, Schachner M. Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol. 2002;159(4):649–661. doi: 10.1083/jcb.200205098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maletic-Savatic M, Koothan T, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: mediation by calcium/calmodulin-dependent protein kinase II. J Neurosci. 1998;18(17):6814–6821. doi: 10.1523/JNEUROSCI.18-17-06814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchand S, Cartaud J. Targeted trafficking of neurotransmitter receptors to synaptic sites. Mol Neurobiol. 2002;26(1):117–135. doi: 10.1385/MN:26:1:117. [DOI] [PubMed] [Google Scholar]
- 48.Gorczyca D, Ashley J, Speese S, Gherbesi N, Thomas U, Gundelfinger E, Gramates LS, Budnik V. Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin. J Neurosci. 2007;27(5):1033–1044. doi: 10.1523/JNEUROSCI.3160-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 50.Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21(3):545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 51.Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20(7):2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28(1):233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 53.Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100(18):10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40(2):347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- 55.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Kacharmina JE, Job C, Crino P, Eberwine J. Stimulation of glutamate receptor protein synthesis and membrane insertion within isolated neuronal dendrites. Proc Natl Acad Sci U S A. 2000;97(21):11545–11550. doi: 10.1073/pnas.97.21.11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7(3):244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 58.Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26(32):8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siggia ED, Lippincott-Schwartz J, Bekiranov S. Diffusion in inhomogeneous media: theory and simulations applied to whole cell photobleach recovery. Biophys J. 2000;79(4):1761–1770. doi: 10.1016/S0006-3495(00)76428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herpers B, Rabouille C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulumgolgi units involved in gurken transport in Drosophila oocytes. Mol Biol Cell. 2004;15(12):5306–5317. doi: 10.1091/mbc.E04-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horton AC, Yi JJ, Ehlers MD. Cell type-specific dendritic polarity in the absence of spatially organized external cues. Brain cell biology. 2006;35(1):29–38. doi: 10.1007/s11068-006-9003-y. [DOI] [PubMed] [Google Scholar]
- 62.de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436(7051):704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- 63.Higginbotham HR, Gleeson JG. The centro-some in neuronal development. Trends Neurosci. 2007;30(6):276–283. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippo-campal circuitry. Science. 2003;300(5621):990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- 67.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 68.Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22(6):2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]