Abstract
Fluorescence spectroscopy is a convenient tool to examine peptide-membrane interactions at equilibrium (1) owing to the change in emission properties of many fluorophores (including tryptophan) during transfer from an aqueous environment into a lipid bilayer. In some cases (e.g. mechanosensitive channel blocker GsMTx4 described here), however, binding-associated emission changes are too small for reliable determination of the free energy of partitioning, ΔG. To enhance the spectroscopic response to binding we implemented the titration with lipid vesicles in the presence of aqueous ionic quencher iodide, which preferentially quenchers fluorescence of the free peptide in solution. We have verified the accuracy of this new titration protocol using the well-studied peptide melittin.
Keywords: tryptophan, fluorescence quenching, lipid bilayer, ion channels, iodide, membrane partitioning
The mechanosensitive channel blocker GsMTx4 (2), along with a number of other gating modifiers (e.g., VsTx1, hanatoxin, SGTx), belongs to a cysteine-knot family of peptides. These peptides, stabilized by three disulfide bridges, possess a hydrophobic and a hydrophilic face and interact with lipid bilayers, which may be essential for their physiological activity (2–5). We need to understand the thermodynamic and structural properties of these interactions, but progress has been hampered by our inability to measure the small spectroscopic changes associated with membrane binding of peptides like GsMTx4.
The hydrophobic face of GsMTx4 is dominated by two tryptophan residues (6), which are expected to contribute strongly and favorably to the free energy of bilayer partitioning, ΔG (7). Bilayer interaction of tryptophan-containing peptides often results in strong changes in intrinsic fluorescence, which, after appropriate corrections (1), can be used to determine the ΔG of bilayer partitioning using equilibrium titration (8). Surprisingly, we found that the addition of extruded large unilamellar vesicles (LUV) to GsMTx4 leads to marginal changes in tryptophan's emission (Fig. 1A), which normally is associated with lack membrane penetration. (Fluorescence was measured using an SPEX Fluorolog FL3-22 steady-state fluorescence spectrometer (Jobin Yvon, Edison, NJ) equipped with double-grating excitation and emission monochromators. Tryptophan fluorescence was excited at 280 nm. Excitation slits were 1 nm; emission slits were 4 nm. Fluorescence emission spectra were obtained by averaging 5–10 scans collected over a 290–460 nm range using 1 nm steps. The measurements were made in 2×10 mm cuvettes, oriented perpendicular to the excitation beam and maintained at 25°C using a Peltier device from Quantum Northwest (Spokane, WA) with a 0.1°C accuracy.)
Figure 1.
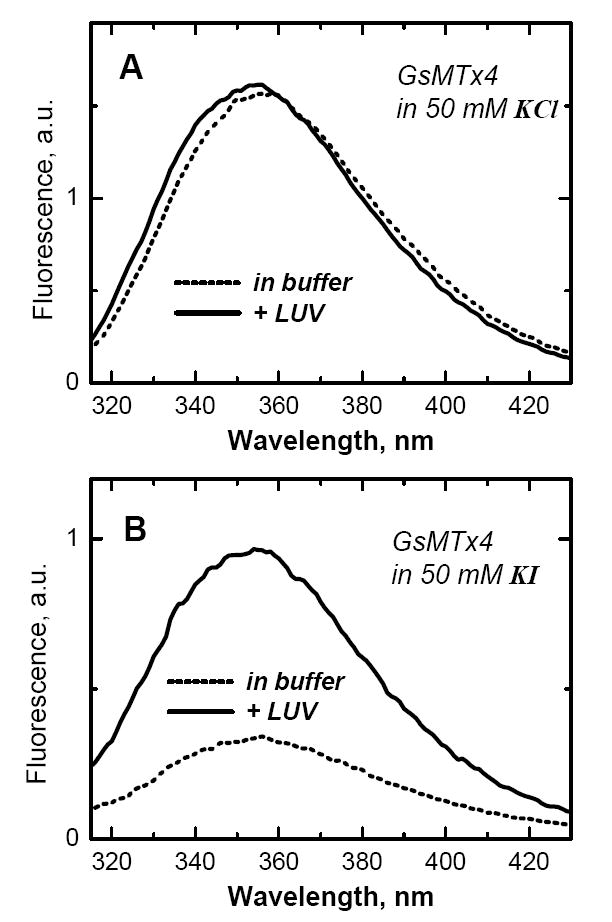
Fluorescence emission spectra of 1 μM GsMTx4 in buffer (dashed lines) and in the presence of 75POPC:25POPG large unilamellar vesicles (LUV) containing 0.8 mM lipid (solid lines). Intensity changes, associated with membrane partitioning, differ substantially depending on the absence (A) or presence (B) of ionic quencher I in the buffer. Because of the preferential quenching of fluorescence of aqueous peptide, the presence of 50 mM KI enhances the signal associated with membrane partitioning.
In contrast, we found a pronounced reduction in quenching by aqueous ions in the presence of LUV, indicating shielding of the tryptophan's by the lipid bilayer. For example, the Stern-Volmer constant for iodide quenching of GsMTx4 is reduced from 12.2 1/M value in buffer to 6.9 1/M and 2.1 1/M by the presence of 0.8 mM POPC LUV and 75POPC:25POPG LUV, respectively. We take advantage of this differential quenching of aqueous and membrane-bound GsMTx4 by iodide to increase the spectroscopic signal upon membrane association. When LUV are added to GsMTx4 in the presence of 50 mM KI, the intensity increases several fold (Fig. 2B). Thus, lipid titration in the presence of aqueous quenchers allows a more reliable detection of membrane binding, and, in principle, a more accurate measurement of ΔG. First, however, we test that substituting KCl for KI in the buffer does not affect the membrane binding of a model peptide with known properties.
Figure 2.
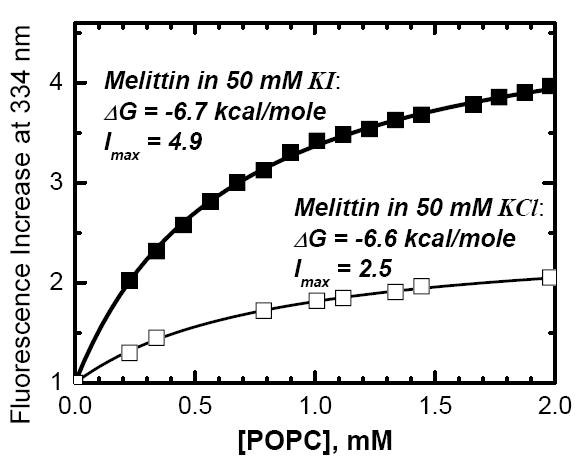
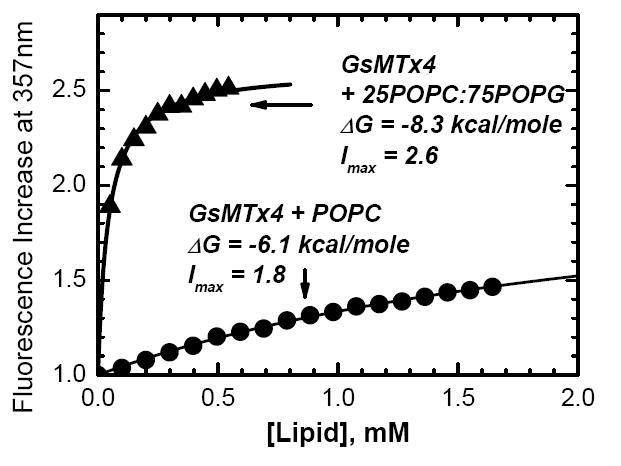
Testing (upper panel) and applying (lower panel) quenching-enhanced titration protocol for determining the free energy of membrane partitioning, ΔG. The well-studied peptide melittin was titrated with POPC LUV in the presence of 50 mM of either KCl (upper panel, open squares) or KI (upper panel, solid squares). Fitting of the titration data with Eqs. 1–2 (curves) demonstrates invariability of ΔG in the two samples, while the fluorescence increase accompanying membrane partitioning (Imax)is markedly increased in the presence of KI. This enhanced signal sensitivity, achieved in the presence of 50 mM KI, allows accurate determination of the ΔG of partitioning of GsMTx4 into membranes of various lipid compositions, such as zwitterionic POPC vesicles (lower panel, circles) and anionic 25POPC:75POPG vesicles (lower panel, triangles).
We chose melittin to test the validity of our new titration protocol because its fluorescence changes markedly upon membrane interaction and its membrane-binding properties have been studied extensively by various methods (1, 8, 9). The results of melittin titration with POPC LUV in two buffer systems, containing 50 mM of KCl or KI, are presented in Fig. 2A. As expected, the increase in fluorescence intensity upon membrane partitioning is higher in the presence of KI, due to higher iodide quenching of an aqueous melittin as compared to a membrane-bound one (10, 11). Fluorescence intensities I, corrected for scattering and dilution, were fitted to (1):
| (1) |
wherein Imax is the fluorescence increase upon complete binding, [L] the lipid concentration, [W] the concentration of water (55.3 M), and Kx is the mole-fraction partition coefficient, [L] and [W] are the molar concentrations of lipid and water (8). The free energies of transfer from water to membrane were calculated from the mole-fraction partition coefficients using
| (2) |
For melittin, a peptide with already pronounced increase in fluorescence upon bilayer partitioning even in the absence of aqueous quenchers, the addition of iodide still increased the spectroscopic response to membrane binding (Imax -1) almost three-fold. At the same time, the free energies calculated for KCl and KI samples from the two curves in Fig. 2A using Eqs. 1–2 practically coincide. Thus, presence of KI does not appear to generally affect the energetics of peptide-membrane interactions.
Next we apply the quenching-enhanced titration protocol to GsMTx4, which is found to bind strongly both zwitterionic and anionic membranes (Fig. 2B). The partitioning free energy is more favorable for POPG-containing vesicles than for pure POPC vesicles, but the difference is small for a peptide of valency of +5, indicating lack of additivity of hydrophobic and electrostatic interactions (12). The increase in fluorescence upon complete binding is also noticeably higher for anionic LUV (Imax=2.6 for 25POPC:75POPG, Imax =1.8 for POPC). This is consistent with the expected higher level of protection from quenching with anionic quencher, due to its repulsion by anionic lipids.
The determinants that dictate the strength of membrane interactions of GsMTx4, will be further investigated with the help of point mutations. Such studies and the studies of other cysteine-knot peptides are needed to enhance our understanding of membrane interactions of various channel blockers. The high sensitivity of the quenching-enhanced titration protocol introduced by this paper will ensure the high accuracy and reliability of future thermodynamic measurements.
Acknowledgments
We are grateful to Dr. F. Sachs for the gift of GsMTx4 and for helpful discussions.
ABBREVIATIONS
- GsMTx4
peptide with the following sequence: GCLEFWWKCNPNDDKCCRPKLKCSKLFKLCNFSF-amide.
- LUV
extruded Large Unilamellar Vesicles of 100 nm diameter.
- POPC
palmitoyl-oleoyl-phosphatidylcholine.
- POPG
palmitoyl-oleoyl-phosphatidylglycerol.
- 75POPC:25POPG and 25POPC:75POPG LUV
vesicles containing corresponding molar fractions of the two lipids
Footnotes
† This research was supported by NIH grant GM-069783 (A.S.L.). P.A.G. was supported by NIH HL-054887 and the Oshei Foundation grants to Frederick Sachs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 2.Suchyna TM, Tape SE, Koeppe RE, II, Anderson OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–240. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 4.Wiggins P, Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys J. 2005;88:880–902. doi: 10.1529/biophysj.104.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung HJ, Lee JY, Kim SH, Eu YJ, Shin SY, Milescu M, Swartz KJ, Kim JI. Solution structure and lipid membrane partitioning of VSTx1, an inhibitor of the KvAP potassium channel. Biochemistry. 2005;44:6015–6023. doi: 10.1021/bi0477034. [DOI] [PubMed] [Google Scholar]
- 6.Oswald RE, Suchyna TM, McFeeters R, Gottlieb P, Sachs F. Solution structure of peptide toxins that block mechanosensitive ion channels. J Biol Chem. 2002;277:34443–50. doi: 10.1074/jbc.M202715200. [DOI] [PubMed] [Google Scholar]
- 7.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nature Struct Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 8.White SH, Wimley WC, Ladokhin AS, Hristova K. Protein folding in membranes: Determining the energetics of peptide-bilayer interactions. Methods Enzymol. 1998;295:62–87. doi: 10.1016/s0076-6879(98)95035-2. [DOI] [PubMed] [Google Scholar]
- 9.Ladokhin AS. Analysis of protein and peptide penetration into membranes by depth-dependent fluorescence quenching: Theoretical considerations. Biophys J. 1999;76:946–955. doi: 10.1016/S0006-3495(99)77258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladokhin AS, Holloway PW, Kostrzhevska EG. Distribution analysis of membrane penetration of proteins by depth-dependent fluorescence quenching. J Fluorescence. 1993;3:195–197. doi: 10.1007/BF00862742. [DOI] [PubMed] [Google Scholar]
- 11.Ladokhin AS, Holloway PW. Fluorescence quenching study of melittin-membrane interactions. Ukrainian Biochemical Journal. 1995;67:34–40. [PubMed] [Google Scholar]
- 12.Ladokhin AS, White SH. Protein chemistry at membrane interfaces: Non-additivity of electrostatic and hydrophobic interactions. J Mol Biol. 2001;309:543–552. doi: 10.1006/jmbi.2001.4684. [DOI] [PubMed] [Google Scholar]


