Abstract
The source of IgA and the mechanism for deposition of IgA in the mesangium remain unknown for primary IgA nephropathy. Because CD19+CD5+ B cells are important producers of IgA and contribute to several autoimmune diseases, they may play an important role in IgA nephropathy. In this study, flow cytometry, quantitative PCR, and confocal microscopy were used to assess the frequency, distribution, Ig production, CD phenotypes, cytokine production, and sensitivity to apoptosis of CD19+CD5+ B cells in the peripheral blood, peritoneal fluid, and kidney biopsies of 36 patients with primary IgA nephropathy. All patients with IgA nephropathy were significantly more likely to have CD19+CD5+ B cells in the peripheral blood, peritoneal fluid, and kidney biopsies than were five control subjects and 10 patients with active systemic lupus erythematosus. The 33 patients who had IgA nephropathy and responded to treatment demonstrated a significant decrease in CD19+CD5+ B cells in the peripheral blood, peritoneal fluid, and kidney (all P < 0.01). In the three patients who had IgA nephropathy and did not respond to treatment, the frequency of CD19+CD5+ B cells did not change. CD19+CD5+ B cells isolated from patients with untreated IgA nephropathy expressed higher levels of IgA, produced more IFN-γ, and were more resistant to CD95L-induced apoptosis than cells isolated from control subjects and patients with lupus; these properties reversed with effective treatment of IgA nephropathy. In conclusion, these results strongly suggest that CD19+CD5+ B cells play a prominent role in the pathogenesis of primary IgA nephropathy.
Primary IgA nephropathy (IgAN), which features polymeric IgA-dominant Ig deposition in the mesangium of kidney, is the most common form of primary glomerulonephritis worldwide.1,2 Although primary IgAN was once considered a benign condition, a high proportion of patients eventually progress to renal failure.2,3 The clinical and pathologic features of the disease are well documented, yet the mechanisms underlying induction of the IgA deposition remain to be defined.2,4
IgA antibodies are important effectors of mucosal protection. The two main subpopulations of B cells, B1 and B2, both are capable of producing IgA.5–9 Differences in their anatomic location, sensitivity to activating signals, and cytokine and receptor expression may determine the relative contribution of B1 versus B2 cells to IgA production at a specific site.5–9 In mice, recent evidences suggest that B1 B cells can be further subdivided into two main subsets as IgMhighIgDlowCD23−CD43+CD5+ (B1a) or IgMhighIgDlowCD23−CD43+CD5− (B1b) B cells. Very little is known concerning functional differences between these B cell subsets. Although CD5+ B1a and CD5− B1b B cells may be functionally distinct, transient expression of CD5 by activated B2 cells has created some controversy over the true origin and characterization of CD5+ B cells.7,10,11 It is generally believed that bona fide CD5+ B1a cells are derived from precursors of the fetal liver and omentum and are most prevalent in the peritoneal and pleural cavities, with few being found in spleen and lymph nodes in normal adult mice.5,6 In humans, however, the functional distinctions between B1a and B1b cells have not been extensively studied, and it is unclear whether conventional B2 cells can express CD5. It is important that there are substantial differences between human and rodent IgA systems5–9; therefore, species specificity may have a large impact on the interpretation of existing reports concerning human and murine B1 and B2 B cell subpopulations.
In mice, the CD19+CD5+ B cells (B1a B cells) have constitutively upregulated expression of some plasma cell–specific genes, including Blimp-1 and XBP-1,12 which may explain the spontaneous and continuous secretion of natural IgM. CD19+CD5+ B cells in the peritoneal cavity and the gut lamina propria serve as an important source of IgA-producing plasma cells in response to various stimuli, contributing significantly to the response against enteric pathogens.13,14 In both mice and humans, CD19+CD5+ B cells are the major source of natural antibodies, which recognize antigens from many common pathogens and are very important for the early response to bacterial and viral infections.7 In humans, CD19+CD5+ B cells are thought to contribute to autoimmune conditions because of their tendency to produce autoreactive antibodies and proinflammatory cytokines including IL-6, as well as their enhanced antigen presentation capabilities.7,15 Expansion of the CD19+CD5+ B cells has been observed in human autoimmunity such as Sjögren syndrome and rheumatoid arthritis.7,15 In systemic lupus erythematosus (SLE), expansion of CD19+CD5+ B cells has been demonstrated only in murine models.7,15 Their elevated numbers, secretion of autoantibodies, and production of a high level of IL-10 has implicated CD19+CD5+ B cells as potential contributors to the development of human autoimmune diseases7,15; however, production of a high level of IL-10 and expression of death-inducing ligands also suggests that CD19+CD5+ B cells may play a regulatory role in human autoimmunity.15,16
Human CD19+CD5+ B cells are important IgA producers, but the importance of this property of CD19+CD5+ B cells in autoimmune diseases is not yet known. For example, the actual role of CD19+CD5+ B cells in IgAN is not completely understood. Although there is a single report that documented elevated numbers of CD19+CD5+ B cells in the tonsils of patients with IgAN and improvement of IgAN after tonsillectomy,17 a systematic study of the numbers of these cells in different anatomic locations and functions of the cells in patients with IgAN has not yet been conducted; however, the existing evidence that this B cell subpopulation is involved in the production of both natural and autoreactive antibodies (including IgA) and evidence of its importance in several autoimmune diseases led us to hypothesize that CD19+CD5+ B cells play a prominent pathogenic role in IgAN, which is characterized by IgA deposition in the kidney. To investigate the precise pathogenic role of CD19+CD5+ B cells in human primary IgAN, we studied the frequency, distribution, and functional properties of CD19+CD5+ B cells in the peripheral blood, peritoneal fluids, and kidney biopsies of patients with primary IgAN. Changes in the CD19+CD5+ B cell compartment were compared before and after treatment with corticosteroids and immunosuppressive drugs.
RESULTS
We studied 36 hospitalized patients with primary IgAN, five control subjects, and 10 patients with active SLE18–21 (Supplemental Table s1). All 36 eligible patients with IgAN in this study had new-onset disease (average duration 1.7 mo) with biopsy-proven mesangial IgA deposition and typical clinical features: Moderate hypertension (mean 146/97 mmHg), hematuria, proteinuria (mean 2.4 g/d urine protein excretion), low-level serum albumin (mean 2.9 g/dl), and typical morphologic alterations detected by light and electron microscopy (Supplemental Table s1). None of the patients with IgAN had a diagnosis of Schönlein-Henoch purpura. Ten hospitalized patients with new-onset (average duration 2.3 mo) active SLE were identified using a modified SLE Disease Activity Index (SLEDAI) score.20 None of the patients and control subjects had received treatment with corticosteroids or immunosuppressive therapy before entry into the study. The race of all patients and control subjects was Han as determined and registered by the physicians in this study. None of the patients or control subjects had clinical infectious symptoms when the study samples were taken.
High Frequency of CD19+CD5+ B Cells in Patients with Primary IgAN
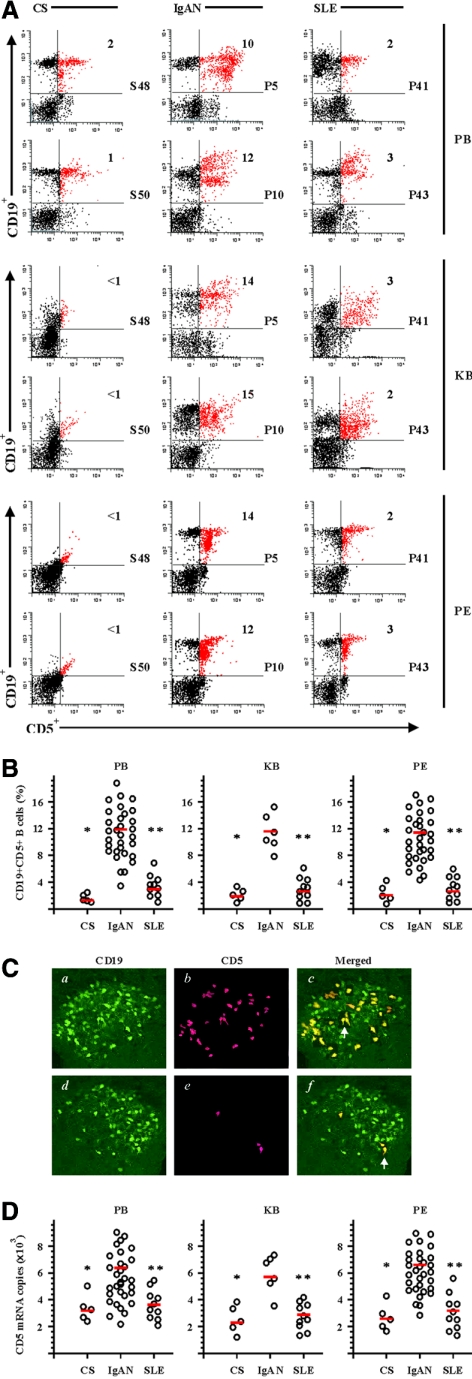
The numbers of CD19+CD5+ B cells in peripheral blood from patients with primary IgAN were significantly increased compared with that in control subjects and the patients with active SLE (Figure 1A). The frequencies of CD19+CD5+ B cells were 1 to 2% in peripheral blood mononuclear cells from control subjects (S48 and S50 are shown), 10 to 12% in the patients with IgAN (P5 and P10 are shown), and 2 to 3% in the patients with SLE (P41 and P43 are shown). The frequency of CD19+CD5+ B cells was <1% in the cell suspensions from kidney biopsies from control subjects, 17 to 18% in the patients with IgAN, and 2 to 3% in the patients with SLE. The peritoneal cavity was known to be one of the predominant places where mature CD19+CD5+ B cells were found.22 The frequency of CD19+CD5+ B cells in peritoneal fluids from control subjects was <1% (S48 and S50 are shown), 12 to 14% in the patients with IgAN (P5 and P10 are shown), and 2 to 3% in the patients with SLE (P41 and P43 are shown). Statistical analysis revealed that the 36 patients with primary IgAN had higher frequencies (all P < 0.01) of CD19+CD5+ B cells in the peripheral blood, peritoneal fluids, and kidney biopsy (12.6 ± 2.7, 11.3 ± 1.8, and 11.8 ± 2.4%) than the five control subjects (1.1 ± 0.7, 1.6 ± 0.8, and 1.8 ± 0.9%) or in 10 patients with active SLE (3.1 ± 0.9, 2.8 ± 0.8, 2.6 ± 1.1%; Figure 1B), respectively. The frequency of CD19+CD5+ B was not significantly different between control subjects and patients with SLE (Figure 1B).
Figure 1.
Increased systemic and local frequency of CD19+CD5+ B cells in patients with IgAN. The cell suspensions were obtained as described in the Concise Methods section. (A) Dual-color flow cytometric analysis of CD19+CD5+ B cells in peripheral blood (PB), cell suspensions from kidney biopsy (KB), and peritoneal fluids (PF) from control subjects (CS), patients with IgAN, and patients with SLE. The cells were freshly isolated and stained with CD19-PE and CD5-FITC. Dot plots were generated after gating on mononuclear cells and show the expression of CD5 relative to CD19. The indicated numbers in the graphs were percentages of CD19+CD5+ B cells. The data shown were from representative patients (S48, S50, P5, P10, P41, and P43) of five (CS), 36 (IgAN), and 10 (SLE) similar experiments performed. For simplification, the isotype Ab controls in the experiments are not shown. (B) CD19+CD5+ percentages for individual patients are shown. Mean values for each group are indicated by red bars. The decreased number of kidney biopsy samples (n = 6) from patients with IgAN was due to limited tissue availability. (C) CD19+, CD5+, and CD19+CD5+ B cell distribution in kidney biopsy from patients with IgAN (a through c) and patients with SLE (d through f). The specimens were stained and photographed under epifluorescence conditions. The arrows indicate typical CD19+CD5+ B cells. The images represent specimens analyzed from six patients with IgAN and six patients with SLE. (D) Q-PCR analysis for CD5 mRNA expression in PB, KB, and PF from CS, patients with IgAN, and patients with SLE. Purified CD19+ B cells were obtained as described in the Concise Methods section. All individual data from each Q-PCR analysis as well as the mean values are shown. *P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and CS; **P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and patients with SLE. Magnification, ×1200.
Histologic sections of kidney biopsy samples were double stained with labeled FITC–anti-human CD19 mAb and PE-labeled anti-human CD5 mAb and analyzed by confocal microscopy. A large number of CD19+CD5+ B cells were found in the glomerular compartment of kidneys from patients with primary IgAN (Figure 1C, a through c), whereas CD19+CD5− B cells were mainly found in the compartment of kidneys from patients with SLE (Figure 1C, d through f). B cells were rarely found in the similar area of kidneys of the control subjects (data not shown). The observation that infiltrating CD19+CD5+ B cells in kidney biopsies from patients with IgAN but not from patients with SLE and lupus nephritis predominately nested in the glomerular compartment suggested that these cells were playing a specific role contributing to glomerular mesangial IgA deposition. The specificity of these findings was also supported by analysis of kidney biopsies from other primary and secondary proteinuric glomerular diseases such as FSGS and diabetic nephropathy, in which almost no infiltrating CD19+CD5+ B cells were found in either glomerular or tubulointerstitial compartments (data not shown). A high level of CD5 mRNA was expressed in purified CD19+ B cells from peripheral blood, peritoneal fluids, and kidney tissues from patients with IgAN in comparison with that in the control subjects and the patients with SLE (Figure 1D), further indicating that a high frequency of CD19+CD5+ B cells appeared in both peripheral blood and the peritoneum, as well as infiltrating and accumulating in kidney tissues in the patients with primary IgAN.
CD19+CD5+ B Cells in Patients with Primary IgAN Are Activated
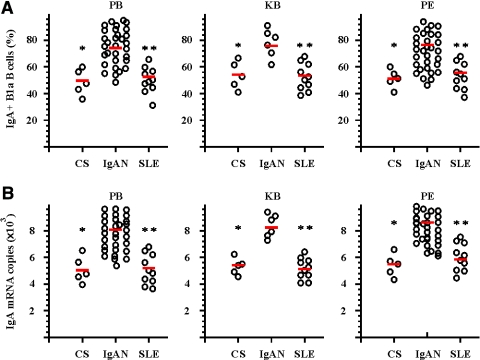
To analyze further the functional state of activation and differentiation of CD5+ B cells in IgAN, we investigated other characteristics of these cells. In comparison with the control subjects and the patients with active SLE, the percentages of surface IgA (sIgA)-positive cells among CD19+CD5+ B cells in peripheral blood and peritoneal fluids from patients with primary IgAN were significantly increased (Figure 2A). The results from quantitative PCR (Q-PCR) confirmed that IgA mRNA transcription was elevated in CD19+CD5+ B cells in the cell suspensions from kidney biopsies from patients with IgAN (Figure 2B). The expression of IgA was low at the mRNA and protein levels in purified or gated CD5− B cells from all three groups of patients and control subjects, respectively (data not shown). The frequencies of sIgA-positive cells among the CD19+CD5+ B cells were not significantly different between control subjects and patients with SLE (Figure 2).
Figure 2.
Elevated IgA expression on CD19+CD5+ B cells from patients with IgAN. Purified CD19+CD5+ B cells were obtained from PB, KB, and PF from CS, patients with IgAN, and patients with SLE as described in the Concise Methods section. (A) Results of triple-color flow cytometric analysis of sIgA expression on gated CD19+CD5+ B cells in PB, KB, and PF from CS, patients with IgAN, and patients with SLE. The cells were freshly isolated and stained with CD19-PE, CD5-FITC, and sIgA-RPE Cy5. The positive cell percentages from each individual patient's samples are shown. The red bars indicate the mean values of each groups. (B) Q-PCR results for IgA mRNA expression in PB, KB, and PF from CS, patients with IgAN, and patients with SLE. Purified CD19+CD5+ B cells were obtained from PB, KB, and PF from CS, patients with IgAN, and patients with SLE as described in the Concise Methods section. Dots indicate mRNA copy number from individual patients, and red bars indicate mean values per group. *P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and CS; **P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and patients with SLE.
The frequencies of surface IgM–positive cells in CD19+CD5+ B cells in peripheral blood and peritoneal fluids from patients with primary IgAN were significantly lower than the percentages found in control subjects and patients with active SLE (Table 1). Surface IgD expression on gated CD19+CD5+ B cells in peripheral blood and peritoneal fluids from patients with primary IgAN were not significantly different from what was found in control subjects and patients with active SLE (Table 1). The frequencies of CD20+ cells within gated CD19+CD5+ B cells were high but not significantly different in any of the three groups (Table 1). The expression of CD20 was comparatively less frequent on CD5− B cells from all three groups (data not shown). In comparison with the control subjects and the patients with active SLE, CD38 and CD43 mRNA expression levels in CD19+CD5+ B cells in peripheral blood and peritoneal fluids from the patients with IgAN were significantly increased (Table 1).
Table 1.
Phenotypic and apoptotic analysis of gated CD19+CD5+ B cells from control subjects, patients with IgAN, and patients with SLE at baselinea
| Patient | CD20 (%)b | CD20 mRNAc | CD38 mRNAc | CD43 mRNAc | Surface IgD (%)b | Surface IgM (%)b | Baff mRNAc | Apoptosis to CD95L (%)d |
|---|---|---|---|---|---|---|---|---|
| IgAN | ||||||||
| PB | 89.2 ± 22.1 | 8.1 ± 1.7 | 9.7 ± 1.4f,g | 7.1 ± 1.5f,g | 12.7 ± 4.6 | 31.1 ± 12.6f,g | 10.1 ± 2.6f,g | 7.8 ± 2.4f,g |
| KBe | 92.7 ± 15.2 | 7.3 ± 2.6 | ND | ND | ND | ND | 8.5 ± 1.8f,g | ND |
| PF | 88.7 ± 21.4 | 8.3 ± 1.4 | 8.1 ± 1.1f,g | 8.3 ± 1.9f,g | 18.2 ± 3.1 | 38.7 ± 15.3f,g | 8.8 ± 1.9f | 6.6 ± 3.1f,g |
| SLE | ||||||||
| PB | 86.1 ± 21.1 | 7.8 ± 1.9 | 5.2 ± 1.7 | 4.9 ± 2.1 | 17.1 ± 7.9 | 78.7 ± 17.1 | 3.2 ± 1.7 | 31.1 ± 9.1h |
| KBe | 84.1 ± 21.1 | 9.1 ± 2.1 | ND | ND | ND | ND | 3.1 ± 1.6 | ND |
| PF | 82.3 ± 14.6 | 8.2 ± 2.2 | 5.4 ± 1.6 | 4.2 ± 1.9 | 16.7 ± 6.1 | 82.4 ± 12.7 | 4.1 ± 1.5 | 34.8 ± 13.1h |
| CSe | ||||||||
| PB | 88.2 ± 21.2 | 7.7 ± 2.4 | 4.7 ± 1.2 | 4.9 ± 1.8 | 14.2 ± 3.2 | 85.7 ± 18.4 | 3.2 ± 1.7 | 19.5 ± 6.1 |
| KB | 76.7 ± 13.4 | 8.7 ± 2.4 | ND | ND | ND | ND | 4.1 ± 1.4 | ND |
| PF | 91.2 ± 20.2 | 8.2 ± 2.2 | 4.5 ± 1.3 | 5.1 ± 1.8 | 14.6 ± 4.7 | 73.2 ± 11.6 | 3.6 ± 1.2 | 18.5 ± 5.6 |
KB, kidney biopsy; ND, no determination; PB, peripheral blood; PF, peritoneal fluids.
Measured by flow cytometry.
Measured by Q-PCR in purified CD19+CD5+ B cells from PB and PF or in KB tissues and expressed as (×103) copies in 25 ng of cDNA.
Measured by flow cytometry.
n = 6, partial KB samples measured because of the limited tissue available from kidney biopsy.
P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and control subjects.
P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and patients with SLE.
P < 0.05 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons showed a significant difference between patients with SLE and control subjects.
Interestingly, CD19+CD5+ B cells in peripheral blood and peritoneal fluids from patients with IgAN were significantly resistant to Fas ligand (CD95L)-induced apoptosis compared with the cells from the control subjects; however, the cells from the patients with SLE were significantly more sensitive to CD95L-induced apoptosis compared with the cells from both the control subjects and patients with IgAN. The B cell activating factor (Baff), a member of the family of TNF ligands and an essential factor for B cell development and survival,23 was significantly increased at mRNA level in CD19+CD5+ B cells in peripheral blood, peritoneal fluids, and kidney biopsy tissues from patients with primary IgAN, in comparison with control subjects and patients with active SLE (Table 1).
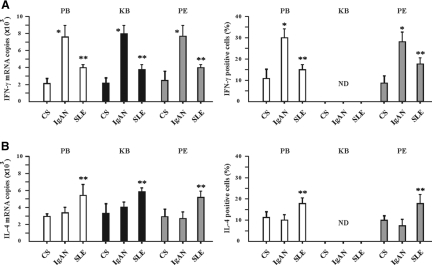
B cells produce cytokines in response to a diverse array of stimuli, including microbial products, antigens, and T cells.24–29 CD19+CD5+ B cells in peripheral blood, peritoneal fluids, and kidney biopsies from the patients with IgAN produced IFN-γ at very high levels compared with patients with SLE and control subjects (Figure 3). CD19+CD5+ B cells from patients with IgAN produced IL-4 at similar levels to control subjects, whereas CD19+CD5+ B cells from patients with SLE produced higher levels of IL-4 (Figure 3).
Figure 3.
Differential IFN-γ and IL-4 cytokine expression by CD19+CD5+ B cells from patients with IgAN and patients with SLE. (A and B) IFN-γ (A) and IL-4 (B) expression by CD19+CD5+ B cells in PB, cell suspensions of KB, and PF from CS, patients with IgAN, and patients with SLE was measured by Q-PCR (left) and intracellular cytokine flow cytometry (right). For Q-PCR analysis, the purified CD19+CD5+ B cells were obtained as described in the Concise Methods section. For intracellular cytokine flow cytometric analysis, the total cells were isolated; stimulated as described in the Concise Methods section; and stained with CD19-PE, CD5-FITC, and the cytokine antibodies conjugated to RPE Cy5 as indicated. ND, no determination. The illustrated data for IFN-γ and IL-4 mRNA and intracellular expression are expressed as means ± SD from CS (n = 5), patients with IgAN (n = 36), and patients with SLE (n = 10). Because of limited tissue availability from kidney biopsies, Q-PCR analysis was performed only on KB from CS (n = 5), patients with IgAN (n = 6), and patients with SLE (n = 10). *P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and CS; **P < 0.05 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and patients with SLE.
CD19+CD5+ B Cells in Patients with Primary IgAN after Treatment
We collected samples from all of the patients with IgAN and with SLE during treatment (Supplemental Table s2). All patients with primary IgAN had received moderate- to high-dosage corticosteroid treatment and at least one immunosuppressive drug, with the exception of one patient, who received corticosteroid plus tonsillectomy. All patients with SLE received corticosteroid treatment alone. The average treatment duration at the end of the study was 22 mo and 18 mo for patients with IgAN and with SLE, respectively. According to clinical findings (clinical symptoms and laboratory examinations), 16 (44%) patients with IgAN were judged to be n clinical and laboratory remission, 17 (47%) patients were evaluated as responsive to treatment (despite persistence of at least one clinical or/and laboratory symptom), and three (8%) patients were evaluated as resistant to treatment (the clinical symptoms and laboratory abnormalities were persistent or even worsening). The responses of individual patients to the treatments are listed in Supplemental Table s2.
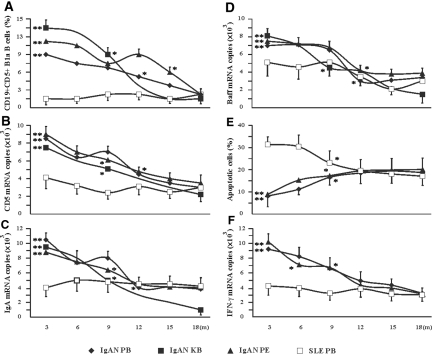
We examined frequency of CD19+CD5+ B cells in peripheral blood, cell suspensions from kidney biopsies, and peritoneal fluids from patients with IgAN at several time intervals after the treatments. At the 18-mo time point, the 33 patients who had IgAN and responded to the treatments had reduced frequencies of CD19+CD5+ B cells (2.1 ± 0.8, 2.4 ± 1.1, and 1.8 ± 0.8%; all P < 0.01 compared with the values of pretreatment) in the peripheral blood, peritoneal fluids, and kidney biopsies, respectively (Figure 4A). Other parameters, such as CD5 mRNA, IgA mRNA, and Baff mRNA expressions, were also significantly decreased in peripheral blood, cell suspensions from kidney biopsies, and peritoneal fluids from these patients with IgAN (Figure 4, B through D). The expression levels of IFN-γ in CD19+CD5+ B cells from patients with IgAN were significantly decreased after the effective treatments (Figure 4F). The cells from patients with IgAN displayed an increased sensitivity to CD95L-induced apoptosis (Figure 4E). The frequency and other cellular properties (CD5, IgA, Baff, and IFN-γ mRNA expressions) were unchanged in CD19+CD5+ B cells from the patients with SLE after treatment with corticosteroids (Figure 4). Interestingly, the sensitivity of CD19+CD5+ B cells from the patients with SLE to CD95L-induced apoptosis was decreased by the effective treatment (Figure 4E).
Figure 4.
Effective treatment resulted in decreased CD5+ B cells and altered CD5+ B cell functional properties in patients with IgAN. All data were obtained from tissues samples of treated patients with IgAN and patients with SLE at the indicated time intervals (m, month). The responses of individual patients to the treatments are listed in Supplemental Table s2. The data for the three patients who had IgAN (P25, P32, and P34) and were resistant to the treatments were not included in these graphs but are shown in Supplemental Table s3. (A) Frequency of CD19+CD5+ B cells in PB, KB, and PF by dual-color flow cytometric analysis. The illustrated data are the percentages of CD19+CD5+ B cells expressed as means ± SD. (B through D and F) Levels of mRNA expression in PB, KB, and PF as measured by Q-PCR analysis of CD5 (B), IgA (C), Baff (D), and IFN-γ (F). All Q-PCR data are expressed as means ± SD. (E) CD95L-induced apoptosis of CD19+CD5+ B cells was measured by triple-color flow cytometry as described in the Concise Methods section. The illustrated data were the percentages of Annexin V–labeled cells in the CD19+/CD5+ gated population and are expressed as means ± SD. To avoid over frequent operative intervention, the patients with IgAN were randomly divided into several subgroups (n = 5 to 6 patients per group). In each subgroup, the patients with IgAN underwent at the beginning of treatment a kidney biopsy that was repeated only once at 3-, 9-, or 18-mo intervals to examine their response to the treatments. Because lacking of samples, the measurements of apoptosis and IFN-γ mRNA in CD19+CD5+ cells in kidney biopsy from patients with IgAN were not conducted. In each subgroup, the patients with IgAN underwent at the beginning of treatment diagnostic paracentesis under ultrasound guidance to collect peritoneal fluid that was repeated only once at 3-, 6-, 9-, 12-, 15-, or 18-mo intervals to examine their response to the treatments. All blood data were from 36 (IgAN) and 10 (SLE) patients. KB and PF were obtained from each different subgroup of patients with IgAN because of ethical concern and limited tissue availability (n = 5 to 6). *P < 0.01 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between different time intervals within the groups of patients with IgAN; **P < 0.05 by the Kruskal-Wallis test, after testing with Dunn method for multiple comparisons, showed a significant difference between patients with IgAN and patients with SLE at the same time intervals.
Three patients with IgAN in this study showed no clinical responses to corticosteroid and immunosuppressive drug therapy (Supplemental able s3). These treatment-resistant patients had similar levels of CD19+CD5+ B cells before and after treatment (Supplemental Table s3). CD5, IgA, Baff, and IFN-γ mRNA expressions as well as sensitivity to CD95L-induced apoptosis of CD5+ B cells in peripheral blood, cell suspensions from kidney biopsies, and peritoneal fluids from these three patients with IgAN were unchanged after the ineffective treatments (Supplemental Table s3). These results strongly supported the hypothesis that CD19+CD5+ B cells played an important role in pathogenesis of primary IgAN.
DISCUSSION
In humans, elevated numbers of CD19+CD5+ B cells have been reported in patients with Sjögren syndrome30 and rheumatoid arthritis.31 In mice, increased numbers of CD19+CD5+ B cells are observed in a number of naturally occurring and genetically manipulated strains that develop lupus-like disease manifestations, although the data are somewhat controversial.32–34 Our results showed that CD19+CD5+ B cells were abundant systemically and locally in patients with primary IgAN but were of relatively low frequency in control subjects and patients with active SLE. The cells from patients with IgAN seemed activated, expressing a high level of IFN-γ and resistance to CD95L-induced apoptosis. A decrease in CD19+CD5+ B cell numbers was shown in the treatment-sensitive patients with IgAN. These results strongly suggest that CD19+CD5+ B cells are involved in the pathogenesis of IgAN but seem not to be associated with human SLE manifestations. Surprising, this study did not find an increased frequency and functions of CD19+CD5+ B cells in patients with active SLE, as has been found in a murine SLE model,7,15 indicating the distinctive behaviors and functions of these B cells in human and mice. Furthermore, our observations of the differences between the patients with IgAN and with SLE led us to investigate further on more precise mechanisms regarding frequency, distribution, Ig production, CD phenotyping, cytokine production, and sensitivity to apoptosis of CD19+CD5+ B cells.
CD19+CD5+ B cells are the main IgA producers in mucosal tissues.13,35,36 The IgA antibodies produced by CD19+CD5+ B cells are usually low affinity, and poly- or autoreactive.37,38 High-affinity, pathogenic autoantibodies have been detected in patients with autoimmune diseases.15,39 As few as 5% of the circulating B cells are CD5+ B cells in normal adults.40 The large number of CD19+CD5+ B cells (approximately 12% of total infiltrating cells) in the kidneys of patients with IgAN is therefore striking (Figure 1). Mechanistically, it seems that a large number of CD19+CD5+ B cells infiltrate into kidneys, secrete IgA and inflammatory cytokines, and cause pathologic lesions in glomerular and tubulointerstitial structures in the kidneys, leading to the clinical manifestations of IgAN. Another important point concerns the normal control samples. The initial peripheral blood samples were taken from the “normal” control subjects within 2 h after trauma, and the (hyper)metabolic responses to injury, including endocrine and systemic, may take place by 6 h after trauma.41 From a pathophysiologic point of view, it is believed that lymphocyte and monocyte counts increase during the secondary phase of the posttraumatic course (after day 4)41; therefore, blood samples taken within 2 h after trauma can be considered as “normal” controls. However, some of the earliest metabolic responses to injury, such as proinflammatory cytokine release that may have an impact on Ig production by B cells, can already be altered by 2 h after trauma. These pathophysiologic changes in controls in this study should be certainly taken into consideration.
Functional differences are also apparent within the CD19+CD5+ B cell compartment of patients with IgAN compared with patients with SLE. The circulating and accumulating CD19+CD5+ B cells of patients with SLE express less sIgA, Baff, and IFN-γ but higher IL-4 than cells from patients with IgAN. Furthermore, CD19+CD5+ B cells of patients with SLE are more sensitive to CD95L-induced apoptosis than the cells from patients with IgAN. The resistance to CD95L-induced apoptosis is higher in patients with IgAN than in normal control subjects. Sensitivity to CD95L-induced apoptosis increases after successful therapy in patients with IgAN. Baff, an essential factor for B cell development and survival,23 is expressed at a significantly higher level in CD19+CD5+ B cells from patients with primary IgAN in comparison with that from control subjects and patients with active SLE. Elevated Baff expression significantly decreased in CD19+CD5+ B cells from the patients with IgAN after successful treatment. Variation in the level of Baff could affect sensitivity to apoptosis or lead to survival of B cells for further proliferation and differentiation; however, precise mechanisms and correlations between Baff expression and sensitivity to CD95L-induced apoptosis in CD19+CD5+ B cells from patients with IgAN require further investigation. The upregulation of Baff by CD19+CD5+ B cells of patients with IgAN suggests that blocking the Baff–Baff receptor axis in patients with IgAN may be an effective treatment strategy.
Under pathophysiologic conditions (primary IgAN but not active SLE), CD19+CD5+ B cells are preactivated and correlate with the development of the pathology of IgAN. A large number of patients with primary IgAN reportedly progress to ESRD.1 As shown in this study, the majority of patients with IgAN were responsive to corticosteroid and immunosuppressive drug treatments, and clinical improvement correlates with reductions in IgA-producing CD19+CD5+ B cells; however, in the minority of patients who do not respond to the treatments, there is evidence to suggest that a poor response to the treatment can be correlated with a lack of effects on the CD19+CD5+ B cell compartment. It is attractive to hypothesize that treatments aimed directly at CD19+CD5+ B cells in patients with IgAN may be an effective means to improve clinical efficacy while reducing the adverse effects of current therapeutic strategies.
CONCISE METHODS
Patients and Control Subjects
Patients were hospitalized at Wuhan University Renmin Hospital between June 1999 and March 2005 and were eligible for the study when they had biopsy-proven primary IgAN or SLE. The diagnosis of IgAN was based on a semiquantitative evaluation of all lesions in the categories of focal (mesangial) and diffuse proliferative glomerulonephritis with unknown cause and confirmed by immunofluorescence studies showing mesangial IgA deposition.18 The SLE diagnosis was based on fulfilling at least four of the American College of Rheumatology 1982 revised criteria for SLE.19 Disease activity was assessed by a modified SLEDAI score.20 The mean SLEDAI of eligible patients in the study was 15.1 (range 3.0 to 28.0). The control subjects (NS) were hospitalized patients with complex blunt abdominal injury as a result of traffic accidents. The initial peripheral blood samples were taken from the post-trauma control subjects as a routine procedure at arrival in the emergency department (within 2 h after trauma). The trauma patients enrolled in this study required emergency exploratory laparotomy and surgical correction of complex abdominal injuries. Upon entering the peritoneal cavity, the peritoneal fluid was anonymously obtained. After establishment of final diagnosis of severe kidney trauma by emergency exploratory laparotomy, control subjects with appropriate surgical indications underwent partial nephrectomy. These patients had no history of autoimmune disease. The characteristics of patients and control subjects included in this study are listed in Supplemental Table s1. In our practice, clinical remission of IgAN was defined as normal kidney function with almost negative proteinuria and hematuria (amount of urine protein excretion <0.3 g/d, urine red blood cells <104/ml) without relapse during a follow-up period of 6 mo. Clinical remission of lupus nephritis was defined either as partial remission (50% reduction in baseline proteinuria to <1.0 g/d and <25% increase in baseline creatinine) or complete remission (proteinuria <0.2 g/d and serum creatinine <105 μmol/L). The study protocol was approved by the institutional review board in Wuhan University in accordance with the current Chinese laws. All patients were given written informed consent according to institutional guidelines. All patients give written consensus.
Kidney Pathologic Evaluation
For histologic grading of kidney biopsies of patients with IgAN, semiquantitative histologic scoring was estimated using a severity index adapted from Radford et al.3 that assigned points (0 to 3) for a number of glomerular, interstitial, and vascular features of the kidney biopsy. The details related to this scoring system were described previously. For histologic grading of kidney biopsies of patients with SLE, glomerular and tubulointerstitial morphologic lesions were graded on a scale of 0 to 3: 0, absence of lesions; 1, lesions involving up to 25% of the component considered; 2, lesions involving 25 to 50%; and 3, lesions involving >50% of the component. The details were described previously.42 All kidney biopsy samples were independently examined by one pathologist, who was not provided with any clinical information about the patients.
Cell Suspension Preparation
For making cell suspensions from kidney tissue, the biopsy specimen was finely minced into small pieces with a scalpel and digested for 2 h in RPMI 1640 medium containing 30 U/ml collagenase (Sigma Chemicals, St. Louis, MO) at 37°C, followed by culture with 0.25% trypsin-EDTA solution (Sigma) for 45 min. After passing the digested tissue through a nylon sieve twice, the cells were resuspended in medium with 1% FCS before further experimental procedures. Peritoneal fluid was obtained by aspiration of ascites during diagnostic paracentesis under ultrasound guidance or, in some cases, by peritoneal dialyses with small-volume saline, with patient's written consensus. Some samples were collected from control subjects (undergoing partial nephrectomy as a result of kidney trauma from traffic accidents) during the operations. For making cell suspensions from peritoneal fluid, the samples were centrifuged at 1500 × g for 10 min. The cell suspensions were then subjected to further investigations.
Flow Cytometry
For cell purification, CD19+ or CD19+CD5+ B cells were purified from single-cell suspensions from peripheral blood and peritoneal fluids using a FACSstarPlus cell sorter. For immunophenotyping, the cells were stained with appropriate combinations of fluorochrome-labeled antibodies for 20 min, followed by washing twice in staining buffer as described previously.43 All mAb were purchased from BD Pharmingen (San Diego, CA) unless indicated otherwise. For detection of apoptosis, the cells were stained in staining medium (RPMI 1640, 2% FBS, and 0.1% sodium azide) with 1 μg/ml propidium iodide for 30 min at 4°C, then stained with FITC-conjugated Annexin V with binding buffer (BD Pharmingen). For intracellular cytokine detection, cells were labeled using the Cytofix/Cytoperm method according to manufacturer's protocol (BD Pharmingen).44 Briefly, the cells were stimulated with LPS (isolated from Salmonella typhosa, 25 μg/ml; Sigma) in the presence of 0.2 μl of Golgiplug at 37°C for 12 h. The analyses were performed with flow cytometer (COULTER XL; Coulter Corp., Miami, FL). Flow cytometric data were analyzed using the WinList program (Scripps Research Institute, La Jolla, CA).
Real-Time Q-PCR
All Q-PCR reactions were performed as described previously.45 Briefly, Q-PCR was performed in 96-well MicroAmp microtiter plates (Applied Biosystems, Foster City, CA) using an ABI PRISM 7700 Sequence Detector System (Applied Biosystems). By using SYBR Green PCR Core Reagents Kit, fluorescence signals were generated during each PCR cycle via the 5′ to 3′ endonuclease activity of AmpliTaq Gold. The sequences of the specific primers were as follows: CD5 sense 5′- GTGTGGTCCTCTGGTCTACAAGAAG-3′ and CD5 antisense 5′-GCAGGTCATAGTCACTGT-3′. A mixture of six sense primers for VH family were used: VHL1 5′-CCATGGACTGGACCTGGAGG-3′, VHL2 5′-ATGGACATACTTTGTTCCAGC-3′, VHL3 5′-CCATGGAGTTTGGGCTGAGC-3′, VHL4 5′-ATGAAACACCTG TGGTTCTT-3′, VHL5 5′-ATGGGGTCAACCGCCAT CCT-3′, VHL6 5′-ATGTCTGTC TCCTTCCTCAT-3′. Antisense for IgA Cα 5′-GGGTCAGCTGGGTGCTGCTGG-3′, CD38 sense 5′-ACAAACCCTGCTGCCGGCTCTC-3′, CD38 antisense 5′-GCATCGCGCCAGGACGGTCT-3′, CD43 sense 5′-GTGCTGCGTCCTTATCAGCCGA-3′, CD43 antisense 5′-CTGTTATGGGAACAGCAGGATGACT-3′, Baff sense 5′- GGAGAAGGCAACTCCAGTCAGAAC-3′, Baff antisense 5′-CAA TTCATCCCCAAAGACATGGAC-3′, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense 5′-ACAACAGCCTCAAGATCATCAG-3′, and GAPDH antisense 5′-GGTCCACCACTGACACGTTG-3′.
All unknown cDNA were standardized to contain equal amounts of cDNA by measurement of GAPDH. PCR conditions were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C for amplifications. Potential PCR product contamination was eliminated by digestion with uracil-N-glycosylase. All unknown cDNA were diluted to contain equal amounts of GAPDH cDNA. PCR retain conditions were 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 60 s at 60°C for amplifications. Potential PCR product contamination was digested by uracil-N-glycosylase because dTTP is substituted by dUTP.
Confocal Scanning Microscopy
As described previously,46 slides with kidney biopsy samples were immersed in blocking buffer PBS with 1% BSA for 10 min to avoid nonspecific binding, incubated with either FITC-labeled anti-huCD19 mAb or isotype control mAb at 10 μg/ml, and incubated overnight at 4°C, followed by staining with the PE-labeled CD5 mAb. All samples were then rinsed with PBS containing 0.5% Tween 20 and dried under argon. Confocal microscopic analysis of the samples was performed using a confocal laser scanning microscope system (LSMSIO; Zeiss, Göttingen, Germany). Images of serial cellular section were acquired with the Bio-Rad Comos graphical user-interface (Bio-Rad Laboratories, Philadelphia, PA).
Statistical Analysis
Statistical analysis was performed with InStat software 3.05 (GraphPad, San Diego, CA). Data are means ± SD. Comparisons among the three groups included in the study were performed with the Kruskal-Wallis test, with the use of Dunn method for multiple comparisons. P < 0.05 were considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30730054, 30572119, and 30670937), the Ministry of Science and Technology (2007AA02Z120), Ministry of Education (20060486008), Provincial Department of Science and Technology of Hubei (2007ABC010), China, and a special grant from the Personnel Department of Wuhan University, China. T.J. is a Chang Jiang Scholar supported by Chang Jiang Scholars Program from Ministry of Education, P.R. China, and Li Ka Shing Foundation, Hong Kong, P.R. China.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
Drs. Yuling, Ruijing, Xiang, Yanping, Lang and Li contributed equally to this work.
D.A.F. and S.K.L.'s current affiliation is Division of Rheumatology, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan.
REFERENCES
- 1.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Levy M, Berger J: Worldwide perspective of IgA nephropathy. Am J Kidney Dis 12: 340–347, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Radford MG Jr, Donadio JV Jr, Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Emancipator SN: IgA nephropathy: Morphologic expression and pathogenesis. Am J Kidney Dis 23: 451–462, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez E, Dorshkind K: New perspectives in B-1 B cell development and function. Trends Immunol 27: 428–433, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa K, Hardy RR: Development and function of B-1 cells. Curr Opin Immunol 12: 346–354, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Su I-h, Tarakhovsky A: B-1 cells: Orthodox or conformist? Curr Opin Immunol 12: 191–194, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Berland R, Wortis HH: Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 20: 253–300, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Haas KM, Poe JC, Steeber DA, Tedder TF: B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23: 7–18, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Schoenbeck S, McKenzie DT, Kagnoff MF: Interleukin 5 is a differentiation factor for IgA B cells. Eur J Immunol 19: 965–969, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Kim PH, Eckmann L, Lee WJ, Han W, Kagnoff MF: Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-beta 1. J Immunol 160: 1198–1203, 1998 [PubMed] [Google Scholar]
- 12.Tumang JR, Frances R, Yeo SG, Rothstein TL: Cutting edge: Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol 174: 3173–3177, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Macpherson A, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM: A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288: 2222–2226, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kunisawa J, Kurashima Y, Gohda M, Higuchi M, Ishikawa I, Miura F, Ogahara I, Kiyono H: Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 109: 3749–3756, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Duan B, Morel L: Role of B-1a cells in autoimmunity. Autoimmun Rev 5: 403–408, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe N, Ikuta K, Nisitani S, Chiba T, Honjo T: Activation and differentiation of autoreactive B-1 cells by interleukin 10 induce autoimmune hemolytic anemia in Fas-deficient antierythrocyte immunoglobulin transgenic mice. J Exp Med 196: 141–146, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodama S, Suzuki M, Arita M, Mogi G: Increase in tonsillar germinal centre B-1 cell numbers in IgA nephropathy (IgAN) patients and reduced susceptibility to Fas-mediated apoptosis. Clin Exp Immunol 123: 301–308, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donadio JV, Bergstralh EJ, Offord KP, Holley KE, Spencer DC, Mayo Nephrology Collaborative Group: Clinical and histopathologic associations with impaired renal function in IgA nephropathy. Clin Nephrol 41: 65–71, 1994 [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–1277, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH: Derivation of the SLEDAI: A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35: 630–640, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Kotzin BL: Systemic lupus erythematosus. Cell 85: 303–306, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Martin F: Kearney JF: B1 cells: Similarities and differences with other B cell subsets. Curr Opin Immunol 13: 195–201, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Rolink AG, Melchers F: BAFFled B cells survive and thrive: Roles of BAFF in B-cell development. Curr Opin Immunol 14: 266–275, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK: Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16: 219–230, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Mauri C, Gray D, Mushtaq N, Londei M: Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197: 489–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE: Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1: 475–482, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM: B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lund FE, Garvy BA, Randall TD, Harris DP: Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun 8: 25–54, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Yuling H, Yanping J, Xinti T, Yaofang Y, Feng Y, Ruijin X, Li W, Lang C, Jingyi L, Zhiqing T, Jingping O, Bing X, Li Q, Chang AE, Sun Z, Youxin J, Jinquan T: CCL19 and CXCL13 synergistically regulate interaction between B-ALL CD23+CD5+ B cells and CD8+ T cells. J Immunol 179: 2880–2888, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Dauphinee M, Tovar Z, Talal N: B cells expressing CD5 are increased in Sjogren's syndrome. Arthritis Rheum 31: 642–647, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Youinou P, Mackenzie L, Katsikis P, Merdrignac G, Isenberg DA, Tuaillon N, Lamour A, Le Goff P, Jouquan J, Drogou A, et al.: The relationship between CD5-expressing B lymphocytes and serologic abnormalities in rheumatoid arthritis patients and their relatives. Arthritis Rheum 33: 339–348, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T: Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature 357: 77–80, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ: Spontaneous murine lupus-like syndromes: Clinical and immunopathological manifestations in several strains. J Exp Med 148: 1198–1215, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reap EA, Sobel ES, Cohen PL, Eisenberg RA: Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J Exp Med 177: 69–78, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka K, Fujihashi K, Sekine S, Fukuiwa T, Kobayashi R, Suzuki H, Nagata H, Takatsu K, Shizukuishi S, McGhee JR, Fujihashi K: Nasal cholera toxin elicits IL-5 and IL-5 receptor alpha-chain expressing B-1a B cells for innate mucosal IgA antibody responses. J Immunol 178: 6058–6065, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Fagarasan S, Honjo T: T-Independent immune response: New aspects of B cell biology. Science 290: 89–92, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Hardy RR: B-1 B cells: Development, selection, natural autoantibody and leukemia. Curr Opin Immunol 18: 547–555, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Tumang JR, Hastings WD, Bai C, Rothstein TL: Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur J Immunol 34: 2158–2167, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Fischer M, Klein U, Kuppers R: Molecular single-cell analysis reveals that CD5-positive peripheral blood B cells in healthy humans are characterized by rearranged Vκ genes lacking somatic mutation. J Clin Invest 100: 1667–1676, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youinou P, Jamin C, Lydyard PM: CD5 expression in human B-cell populations. Immunol Today 20: 312–316, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Keel M, Trentz O: Pathophysiology of polytrauma. Injury 36: 691–709, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Hill GS, Delahousse M, Nochy D, Tomkiewicz E, Rémy P, Mignon F, Méry JP: A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int 58: 1160–1173, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Chunsong H, Yuling H, Li W, Jie X, Gang Z, Qiuping Z, Qingping G, Kejian Z, Li Q, Chang AE, Youxin J, Jinquan T: CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J Immunol 177: 6713–6722, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Chalmers IM, Janossy G, Contreras M, Navarrete C: Intracellular cytokine profile of cord and adult blood lymphocytes. Blood 92: 11–18, 1998 [PubMed] [Google Scholar]
- 45.Jinquan T, Quan S, Jacobi HH, Jing C, Millner A, Jensen B, Madsen HO, Ryder LP, Svejgaard A, Malling HJ, Skov PS, Poulsen LK: CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: Chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood 96: 1230–1238, 2000 [PubMed] [Google Scholar]
- 46.Mueller A, Kelly E, Strange PG: Pathways for internalization and recycling of the chemokine receptor CCR5. Blood 99: 785–791, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.