Abstract
The efficacy of statins for the prevention of cardiovascular events is well established in the general population but remains unknown in renal transplant recipients. In this study, the association of statin use with patient and graft survival was investigated in a cohort of 2041 first-time recipients of renal allografts between 1990 and 2003. Multivariable Cox regression demonstrated that statin use was independently associated with lower mortality rates. Twelve-year survival rates were 73% for statin users and 64% for nonusers (P = 0.055). The adjusted hazard ratio for all-cause mortality associated with statin use was 0.64 (95% confidence interval 0.48 to 0.86). Graft survival rates during the same time period were 76% for statin users and 70% for nonusers (P = 0.055). The adjusted hazard ratio for graft survival associated with statin use was 0.76 (95% confidence interval 0.55 to 1.04). Results from marginal structural models were virtually identical. In summary, statin use was associated with prolonged patient survival, but no difference in graft survival was detected. Although these results are encouraging, a definitive causal relationship can be determined only from randomized clinical trials.
Cardiovascular disease is the leading cause of death in renal transplant recipients. More than 60% of these patients die of cardiovascular causes compared with 40% of the general population.1–3 Although renal transplant recipients display certain peculiarities concerning their cardiovascular risk profile that discriminate them from nonrenal patients, it is generally believed that the dyslipidemias are equally important risk factors in renal and nonrenal patients.4–10
Several trials could firmly establish the treatment of dyslipidemias with statins as one of the mainstays of cardiovascular pharmacotherapy in the general population11–17; however, in renal patients, this treatment approach is less well established.18 Moreover, most of the large statin trials excluded patients with elevated creatinine and patients who had undergone renal transplantation. Only one randomized clinical trial (RCT), the Assessment of LEscol in Renal Transplantation (ALERT) trial, assessed statin therapy in renal transplant recipients.19 Although the study was underpowered to detect differences in the combined primary end point, the investigators found a significant reduction of cardiac mortality—a secondary end point—in the statin-treated group. Furthermore, the ALERT trial was extended to a median follow-up of 6.7 yr. In this extension trial, the incidence of major adverse cardiac events (MACE)—the primary end point of the ALERT trial—was reduced in statin-treated patients.20 The P value of 0.035 of this primary end point, however, was not adjusted for the second analysis.
Recent guidelines on lipid-lowering therapy in renal transplant recipients issued by the Kidney Disease Outcomes Quality Initiative (K/DOQI) drew most of their conclusions by extrapolating the findings of RCT carried out in the general population to kidney transplant recipients.6 The authors stated that more RCT of lipid-lowering medications in renal transplant recipients would be warranted; however, considering the necessary size of these trials and the competition for participation in other trials such as those assessing new immunosuppressive therapies, their completion seems unlikely in the near future.21
Further insight into the effectiveness of statins in renal transplant recipients will have to be obtained from observational studies and registries. Thus, it was our aim to investigate a possible association between statin therapy and patient and graft survival in the Austrian Dialysis and Transplant Registry. Because of the complete documentation of outcomes, cardiovascular medication, and patients’ comorbidities, this registry is ideally suited for the assessment of drug effectiveness in this target population.
RESULTS
Baseline Comparison
Our study included a total of 2041 renal transplant recipients. Of these patients, 302 were statin users and 1739 were nonusers at baseline. Twenty-five percent of patients received statin treatment within the first 4 yr. Table 1 displays overall and stratified baseline characteristics for the entire population. As is shown, statin-treated patients had a higher cardiovascular disease burden, a higher prevalence of cardiovascular risk factors, and a higher rate of HLA mismatches as compared with patients without statins. In total, 1829 patients were alive and had a functioning graft at day 90 after engraftment and were included in further data analyses.
Table 1.
Baseline characteristics of study patients, stratified by statin use at time of transplantationa
| Characteristic | No. of Patients (Statin/No Statin) | Statins | No Statins | P |
|---|---|---|---|---|
| Recipient age (mean [SD]) | 2041 (302/1739) | 54 (11.8) | 47 (15.8) | <0.001 |
| Donor age (mean [SD]) | 1962 (285/1677) | 46.8 (15.3) | 42.7 (16.1) | <0.001 |
| Recipient female gender (n [%]) | 1971 (286/1685) | 127 (44.4) | 664 (39.4) | 0.110 |
| Donor female gender (n [%]) | 1971 (286/1685) | 127 (44.4) | 673 (39.9) | 0.160 |
| Cadaveric organ donor (n [%]) | 2041 (302/1739) | 276 (91.4) | 1576 (90.6) | 0.670 |
| Time on dialysis (yr; median [IQR]) | 2041 (302/1739) | 2.2 (1.2 to 3.3) | 1.7 (0.8 to 3.0) | 0.002 |
| Body weight, kg (mean [SD]) | 1337 (241/1096) | 74 (14.1) | 70 (16.8) | <0.001 |
| Diabetes (%) | 2041 (302/1739) | 98 (32.5) | 285 (16.4) | <0.001 |
| HbA1c level (%; median [IQR]) | 1185 (229/958) | 6.2 (5.7 to 6.9) | 6.1 (5.6 to 6.6) | 0.033 |
| Panel reactive antibodies (%; median [SD]) | 1949 (298/1651) | 4.0 (10.9) | 5.2 (14.1) | 0.150 |
| Sum of HLA mismatches (mean [SD]) | 1819 (291/1528) | 2.7 (1.4) | 2.4 (1.5) | 0.006 |
| Cold ischemic time (h; mean [SD]) | 1768 (258/1510) | 14.5 (7.5) | 18.3 (8.4) | <0.001 |
| Arterial hypertension (n [%]) | 2041 (302/1739) | 292 (96.7) | 1364 (78.4) | <0.001 |
| No. of BP drugs (median [IQR]) | 2041 (302/1739) | 3 (2 to 4) | 2 (0 to 3) | <0.001 |
| SBP (mmHg; mean [SD]) | 1253 (227/1026) | 138.7 (15.9) | 140.2 (39.0) | 0.570 |
| DBP (mmHg; mean [SD]) | 1253 (227/1026) | 82.9 (48.7) | 82.5 (24.0) | 0.840 |
| Cholesterol level, mg/dl (mean [SD]) | 1636 (293/1343) | 205.5 (53.0) | 207.9 (77.9) | 0.610 |
| Coronary heart disease (n [%]) | 1242 (250/992) | 79 (31.6) | 238 (24.0) | 0.014 |
| Other heart disease (n [%]) | 1236 (250/992) | 120 (48.0) | 391 (39.7) | 0.017 |
| Vascular disease (n [%]) | 1159 (244/915) | 79 (32.4) | 254 (27.8) | 0.160 |
DBP, diastolic BP; SBP, systolic BP.
Patient Survival
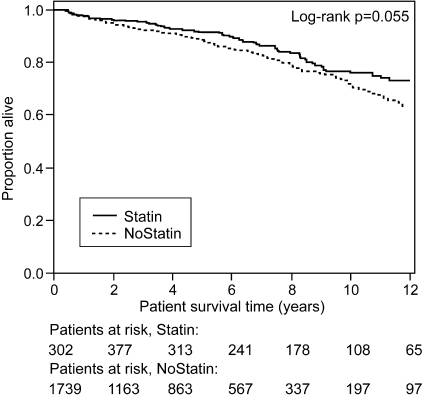
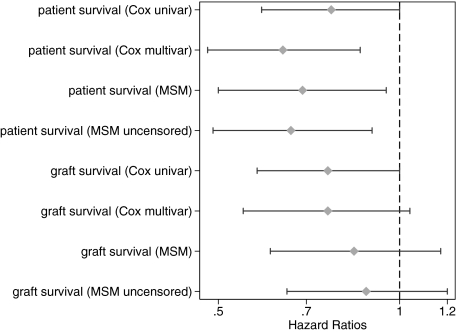
This analysis assessed the influence of statin treatment on all-cause mortality after 90 d of engraftment. Twelve-year survival rates were 73% in the statin group and 64% in the nonstatin group (unadjusted P = 0.055). When patients who died within the first 90 d after engraftment were included in the analysis, 12-yr survival was 72% in the statin group and 61% in the nonstatin group (unadjusted P = 0.009). Of all 692 deaths that occurred after 90 d after transplantation, 181 (26%) were due to cardiac causes. Statin use was not associated with decreased overall mortality rates in the univariate analysis (hazard ratio [HR] 0.77; 95% CI 0.59 to 1.00; P = 0.055; Table 2, Figures 1 and 3); however, when confounding was accounted for in the multivariable model, the association reached statistical significance (HR 0.64; 95% CI 0.48 to 0.86; P = 0.003; Table 2; Figure 3). Similar results were obtained by the marginal structural model (MSM) analysis (HR 0.69; 95% CI 0.5 to 0.95; P = 0.022; Table 2, Figure 3). These results were virtually unchanged when events occurring <90 d after engraftment were included (see uncensored MSM in Figure 3). The HR for cardiovascular death using MSM analysis (adjusting for informative censoring as a result of death of other causes and graft loss) was 0.59 (95% CI 0.34 to 1.01). With the exception of moderate modification of the HR of statin use by hemoglobin and mean arterial pressure (patients with higher hemoglobin or mean arterial pressure (MAP) benefit more from statin treatment), we could not identify any interaction between statin treatment and other covariates of the multivariable model. The inspection of Schoenfeld residuals suggests a stronger association of statin use with outcomes in the first few posttransplantation years, but violation of the proportional hazards assumption could not be statistically confirmed (P = 0.081).
Table 2.
Survival analysis assessing the effect of statins on all-cause mortality and functional graft survival in an unadjusted and three adjusted models
| Parameter | All-Cause Mortality | Functional Graft Survival |
|---|---|---|
| Events (statin-treated/nontreated) | 59/244 | 49/236 |
| Person-time (yr; statin-treated/nontreated) | 2429/8237 | 2429/8237 |
| HR, unadjusted model | 0.77 (0.59 to 1.00) | 0.76 (0.58 to 1.00) |
| HR, adjusted model | 0.64 (0.48 to 0.86)a | 0.76 (0.55 to 1.04)b |
| HR, clinical experience model | 0.65 (0.47 to 0.89)c | 0.81 (0.59 to 1.12)c |
| HR, MSM | 0.69 (0.50 to 0.95) | 0.84 (0.61 to 1.17) |
Adjusted for age, year of transplantation, MAP, cholesterol, hemoglobin, the presence of diabetes, CHD, peripheral arterial disease, and cardiomyopathy.
Adjusted for age, year of transplantation, the number of BP medications, cholesterol, hemoglobin, immunosuppressive therapy, the presence of diabetes, coronary artery disease, peripheral vascular disease, and cardiomyopathy.
Adjusted for HLA mismatch, induction therapy, cold ischemia time, and donor age in addition to the covariates of the adjusted models.
Figure 1.
Kaplan-Meier curves of patient survival (all-cause mortality) according to statin use treated as a time-dependent variable. Figures on the bottom indicate patients at risk at different times of follow-up.
Figure 3.
Crude and adjusted HR estimates and 95% CI associated with statin use for patient survival (all-cause mortality) and functional graft survival. In our main analyses, events occurring <90 d after engraftment were censored, whereas the models called “MSM uncensored” also included these events.
Functional Graft Survival
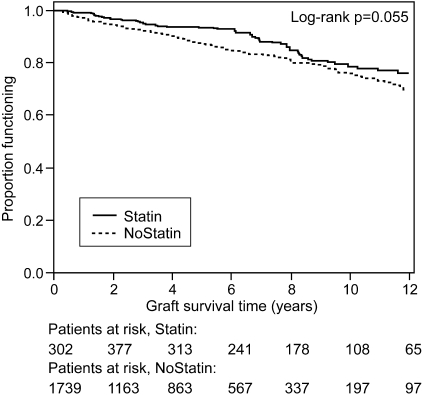
Functional graft survival was defined as graft failure occurring after the 90th day after engraftment with death being right-censored. Twelve-year functional graft survival after 90 d was 76% in the statin-treated group and 70% in the nonstatin group (unadjusted P = 0.055). Statin treatment was not associated with improved functional graft survival in the univariate analysis (HR 0.76; 95% CI 0.58 to 1.00; P = 0.055; Table 2, Figures 2 and 3). Similarly, both multivariable and MSM analyses did not find an association (multivariable model: HR 0.76 [95% CI 0.55 to 1.04; P = 0.086]; MSM: HR 0.84 [95% CI 0.61 to 1.17; P = 0.31]; Table 2, Figure 3). These results were unchanged when events occurring <90 d after engraftment were included (see uncensored MSM in Figure 3). There was no interaction between statin treatment and the covariates of the multivariable model. Time to biopsy-confirmed acute rejection was also not different in an adjusted multivariable analysis (HR 0.91; 95% CI 0.70 to 1.17).
Figure 2.
Kaplan-Meier curves of functional graft survival (end point: graft failure, death treated as censored) according to statin use treated as a time-dependent variable. Figures on the bottom indicate patients at risk at different times of follow-up.
The HR estimates for actual graft survival, counting death as event, were between the estimates for patient survival and functional graft survival both in the multivariable model (HR 0.68; 95% CI 0.55 to 0.84; P < 0.001) and in the MSM analysis (HR 0.73; 95% CI 0.58 to 0.92; P = 0.007). There was no interaction between statin treatment and any of the covariates of the multivariable model.
DISCUSSION
Our study showed that statin use was associated with reduced mortality rates in a large cohort of kidney transplant recipients; however, graft survival was unaffected by these drugs. Previous trials have consistently demonstrated that statins reduce cardiovascular morbidity and mortality in the general population; however, in renal patients—especially in renal transplant recipients—the beneficial effects of statins are less well established.
Several trials reported that the hyperlipidemias predict cardiovascular disease and mortality after renal transplantation.4,5,19,22 This finding nurtured the idea that lipid-lowering therapy might also be useful in these patients. Further support for this idea came from the observational study by Cosio and coworkers.23 These investigators reported that lipid-lowering therapy with a statin was associated with a 24% reduction in all-cause mortality in 1574 patients after renal transplantation. It has to be acknowledged, however, that these investigators did not apply adequate statistical techniques to account for “confounding by indication” that is likely to occur in this type of study design. It is therefore possible that the reported results could be due to residual confounding rather than to statin treatment.
In 2004, the National Kidney Foundation recommended that kidney transplantation be treated as “coronary heart disease (CHD) risk equivalent” and that LDL should be lowered to <100 mg/dl in these patients6; however, the evidence supporting these guidelines came from only one randomized trial in the field, the ALERT trial, and its extension study.19,20 In addition, this trial produced rather equivocal results insofar as statin treatment reduced cardiac mortality whereas it seemed to leave all-cause mortality and MACE unaffected. The level of evidence of these guidelines was therefore classified as moderate (grade B). The task force stated that additional RCT in the field would be needed to undermine their recommendations; however, bearing in mind that further RCT in the field are missing and that they probably will not become available soon, our study—with approximately the same sample size as ALERT but up to 12 yr of follow-up—provides important endorsement of the task force's guidelines.
In this trial, statins were not associated with prolonged graft survival. A possible renoprotective effect of statins was previously postulated because several observational trials found an interrelation between lipid levels and kidney function in the general population, in patients with chronic kidney disease, and in kidney transplant recipients.9,24–26 Moreover, a meta-analysis of 13 trials found that treatment of hyperlipidemia preserved GFR in patients with chronic kidney failure.27 In addition, a recent reanalysis of the Treating to New Targets (TNT) study revealed that atorvastatin improved GFR in 10,001 patients with CHD. This effect was significantly greater in patients on high-dosage atorvastatin compared with those on low-dosage therapy.28 Chronic allograft nephropathy is the most common cause of late graft failure, it displays many similarities with atherosclerosis, and it has been associated with hypercholesterolemia.9 Alluding to this observation, some investigators therefore suggested that statin treatment could also preserve renal allograft function by reducing the incidence of graft rejection.29 To the surprise of the investigators of the ALERT trial, reducing hypercholesterolemia with a statin had no influence on graft loss or doubling of serum creatinine in their study.19 Similarly, three smaller trials failed to show an association between statin use and acute graft rejection.29–31 In accordance with these studies, the results reported in this study lend further credence to the notion that statins do not affect graft survival in renal transplant recipients.
Some caution is warranted when interpreting our results. Because our study patients were not randomly allocated to statin treatment, the possibility of bias from phenomena such as “confounding by indication” must be considered. Confounding by indication refers to a situation in which the patient's risk profile influences the prescription of a given drug. In the case of statins, an unfavorable cardiovascular risk profile could have prompted the treating physicians to prescribe lipid-lowering medication, which would inadvertently bias the statin-treated group. Apparently, this is what happened in our data. Table 1 clearly indicates that statin-treated patients had higher rates of heart disease and worse cardiovascular risk profiles, and these covariates were more completely reported in statin users. They also received more antihypertensive drugs, which may be proxy for better care. We accounted for this potential source of bias by adjusting for this covariate in the models and conducted an additional analysis to assess whether informative missingness of covariates affected our conclusions. This was not the case, as shown in the supplemental data. It is likely that confounding by indication explains why we found no association between statin treatment and mortality in the unadjusted analysis.
In an attempt to remove these sources of bias, we followed two different state-of-the-art modeling strategies. The first strategy used multivariable Cox regression, including, among all available variables, those that are likely to confound the association of statin use and our outcomes. The second strategy used MSM. In both models, a significant association of statin use and patient survival emerged. Marginal structural models were recently proposed as a tool to estimate the causal treatment effect from observational trials. MSM creates a pseudorandomized study population with balanced treatment allocation. In all outcomes, we observed slightly less pronounced effects by MSM than by multivariable modeling. This finding lets us conclude that the causal relationship between statin treatment and these long-term outcomes is weaker than their pure nondirected association.
Notwithstanding the encouraging findings of our trial, it has to be acknowledged that even the most state-of-the-art statistical techniques can adjust only for measured confounders. Unmeasured confounders are not taken into account, and they can be addressed only by truly randomized clinical trials.
This study provides evidence that statin treatment is associated with reduced all-cause mortality in renal transplant recipients; however, the nonrandomized nature of our trial does not provide definitive evidence as to whether the observed associations are truly causal. This question will have to be answered by larger RCT.
CONCISE METHODS
Patient Population and Registries
The study population consisted of 2041 consecutive patients who received their first kidney transplant at the Medical University of Vienna between January 1, 1990, and December 31, 2003, and who were followed-up until December 31, 2005. Selected data from three databases were merged to combine information on recipients, donors, and biopsy results.
Oesterreichisches Dialyse und Transplant Register.
This registry was established by the Austrian Society of Nephrology and mainly provided information on allograft recipients. It has almost complete follow-up; only 17 patients were lost to follow-up since 1990. This database provided baseline information on recipients’ demographics; underlying renal disease; course of renal replacement therapies; panel reactive antibodies (highest and latest); hepatitis B virus, hepatitis C virus, and cytomegalovirus serologies; immunosuppressive regimen; and immediate posttransplantation course. Annual follow-up data included patient and graft status, transplant function, comorbidities (diabetes, cardiovascular, liver, lung, hypertension, malignancies), immunosuppressive therapy, and clinical chemistry values.
EUROTRANSPLANT Registry.
This database contains complete information on organ donor characteristics from transplantations performed in the EUROTRANSPLANT region. It provided information on donor characteristics such as cold ischemia time; HLA mismatches in A, B, and DR; age; gender; cytomegalovirus serology; cause of death; last serum creatinine; and use of vasopressors during the intensive care unit stay. Hepatitis B virus–or hepatitis C virus–positive donors were not accepted.
Vienna Kidney Biopsy Registry.
The registry holds information on all native and transplant kidney biopsies performed at the Medical University of Vienna since 1990. The Banff '93 and '97 criteria were used to define biopsy-confirmed acute rejection (BCAR) and chronic allograft nephropathy.32,33 A total of 3546 biopsies were performed within the study period. A total of 248 biopsies performed before 1994 were reclassified according to the Banff '97 criteria. Biopsy-confirmed acute rejection was classified as Banff borderline and higher grades of cellular rejection. Lesions of native kidney biopsies and of donor kidneys before transplantation were graded according to the World Health Organization classification.34
The aforementioned databases did not provide information on therapies other than immunosuppressive agents. The pertinent information was obtained from health insurance companies. Health insurance is mandatory in Austria, and prescription drugs are available to the public for a small dispensing fee; therefore, information on prescription drug usage can be assumed to be complete.
Patient Comorbidities and Immunosuppressive Therapies
Patients were classified as hypertensive when the mean arterial BP was >107 mmHg or when the patient received at least one antihypertensive drug for at least 50% of the time at risk. CHD was judged to be present when patients had received a diagnosis of CHD through angiography or radioisotopic techniques, when they had a history of myocardial infarction, or when they experienced symptoms of angina pectoris. Noncoronary vascular disease was defined as cerebrovascular or peripheral vascular disease. Immunosuppressive regimens were classified into four groups: (1) Standard immunosuppression (a triple therapy consisting of corticosteroids, mycophenolate mofetil, and a calcineurin inhibitor); (2) triple therapy with corticosteroids, azathioprine, and cyclosporine; (3) all corticosteroid-free regimens; and (4) calcineurin inhibitor–free immunosuppression or other. Induction therapies with either a polyclonal antibody or an IL-2 antibody were not analyzed separately.
Outcomes
Patient survival was classified as time from kidney transplantation until death or end of follow-up. Patients who survived less than 90 days were censored. Functional graft survival was defined as time from kidney transplantation to dialysis, retransplantation, or end of follow-up with death being censored. Grafts lost before 90 d from transplantation were also treated as censored.
Data Analysis
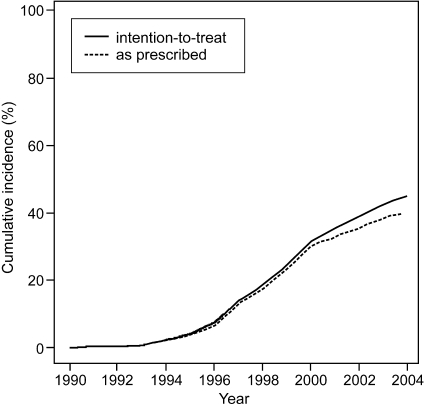
For the description of characteristics of patients at time of transplantation, we used frequencies and percentages for categorical variables, median, 25th and 75th percentiles for skewed continuous variables, and mean and SD for normally distributed continuous variables. These characteristics were compared between statin users and nonusers using χ2 tests, Wilcoxon rank-sum tests and t test, respectively. In all analyses of patient survival, actual graft survival, and functional graft survival, statin use was entered as a time-dependent variable and was defined according to the intention-to-treat principle (i.e., once statin treatment was started in a patient, that patient was counted as a statin user from time of treatment onset until the end of follow-up, irrespective of discontinuation of treatment; Figure 4). Extended Kaplan-Meier plots were used to compare the survival between statin users and nonusers.35
Figure 4.
Percentage of statin users over time. Solid line, percentage of statin users according to intention-to-treat principle (once statins were started, patient remained in statin user group); dashed line, percentage of statin users as prescribed.
The association of statin use with patient and graft survival was further quantified by HR estimates and 95% CI resulting from Cox regression analysis.36 In addition to a univariate analysis of statin use, we applied two different approaches to address confounding by indication and to obtain adjusted HR estimates. In each of the approaches, we considered static and time-dependent variables as potential confounders. Static confounders were time on dialysis before transplantation, age at transplantation, and year of transplantation. Time-dependent variables were number of prescribed BP-lowering drugs, diabetes status, coronary or other heart disease, cerebrovascular disease, peripheral vascular disease, type of immunosuppressive therapy, median hemoglobin level per calendar year, median of the MAP by calendar year, and median cholesterol level per calendar year. All variables that were treated as time dependent in the Cox analysis were also treated as time dependent in the analysis using MSM; however, changes that occurred after the initiation of statin treatment were not considered because these changes could be a consequence of statin treatment and lead to “overadjustment” of the model.
As a first approach to obtain confounder-adjusted HR estimates, we performed multivariable Cox regression analysis. Selection of confounders for use in multivariable models was based on an adaptation of the Purposeful Selection algorithm for Cox regression.37 This algorithm was proposed as improvement to P value–based stepwise selection procedures. The algorithm selects confounders that would change the log hazard of the variable(s) of interest by >15% after the confounder is excluded from the model; therefore, we can safely assume that among all potential confounders considered, those selected for the final multivariable models were sufficient to adjust the HR of statin use. We assessed presence of effect modification by evaluating the significance of interaction terms of statin use with any other variables in the models.
The assumption of linearity of the effects of continuous-scaled covariates (hemoglobin, cholesterol level, MAP, age, and year of transplantation) was assessed by fitting another multivariable Cox model that included continuous-scaled covariates using restricted cubic splines with four knots placed at their fifth, 35th, 65th, and 95th percentiles. Because the nonlinear treatment of these variables did not lead to a marked change in the adjusted HR of statin use in any of our outcomes, we chose to report only on the final models for which all continuous-scaled variables were treated as linear. Scaled Schoenfeld residuals were computed and plotted against time to evaluate potential violations of the proportional hazards assumption.38
The second approach to obtain confounder-adjusted estimates applied the recently proposed technique of MSM.39 This approach first uses logistic regression to estimate the probabilities of statin treatment initiation in any 3-mo interval after transplantation, depending on the patients’ covariates up to the beginning of each interval. The reciprocal values of these probabilities are then used as weights (inverse probability of treatment weights) in a Cox regression analysis. At any time after transplantation, the sum of weights of patients on statins equals the sum of weights of patients not on statins, irrespective of their covariates. Thus, MSM retrospectively simulates random allocation of drug therapy. Unlike an RCT, MSM can control only for confounders that were actually measured; the so-called assumption of no unmeasured confounders remains untestable. More details on this method can be found in the literature.39–41
Before all analyses, we applied multiple imputation, generating 20 completed versions of the data set. All analyses were performed on each completed data set, and results were combined using SAS/PROC MIANALYZE. Results from multiple imputation were cross-checked against a complete-case-only analysis, and the sensitivity of the results to the assumption of randomly missing data was assessed. The gain in precision using multiple imputation was substantial, particularly for variables such as statin use that were completely documented. We used SAS 9.1 (SAS Institute, Cary, NC) for all statistical computations. P < 0.05 was considered as indicating significance.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was supported by grants from the Austrian Science Fund (FWF P-18325) and the Austrian Academy of Science (OELZELT EST370/04) to R.O.
Published online ahead of print. Publication date available at www.jasn.org.
F.W. and G.H. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/ and http://www.meduniwien.ac.at/nephrogene.
See related editorial, “Statin Use Prolongs Patient Survival after Renal Transplantation,” on pages 2037–2040.
REFERENCES
- 1.Division for Heart Disease and Stroke Prevention: Addressing the Nation's Leading Killers. Available at: http://www.cdc.gov/nccdphp/publications/AAG/dhdsp.htm. Accessed January 10, 2008
- 2.Lindholm A, Albrechtsen D, Frödin L, Tufveson G, Persson N, Lundgren G: Ischemic heart disease: Major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation 60: 451–457, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Cardiovascular Special Studies. Available at: http://www.usrds.org/2006/pdf/09_cv_06.pdf. Accessed January 10, 2008
- 4.Aker S, Ivens K, Grabensee B, Heering P: Cardiovascular risk factors and diseases after renal transplantation. Int Urol Nephrol 30: 777–788, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Kasiske B, Chakkera H, Roel J: Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 11: 1735–1743, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Kasiske B, Cosio F, Beto J, Bolton K, Chavers B, Grimm R, Levin A, Masri B, Parekh R, Wanner C, Wheeler D, Wilson P: Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: A report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant 4[Suppl 7]: 13–53, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Massy ZA, Mamzer-Bruneel MF, Chevalier A, Millet P, Helenon O, Chadefaux-Vekemans B, Legendre C, Bader C, Drueke T, Lacour B, Kreis H: Carotid atherosclerosis in renal transplant recipients. Nephrol Dial Transplant 13: 1792–1798, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Ong CS, Pollock CA, Caterson RJ, Mahony JF, Waugh DA, Ibels LS: Hyperlipidemia in renal transplant recipients: Natural history and response to treatment. Medicine (Baltimore) 73: 215–223, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Roodnat JI, Mulder PG, Zietse R, Rischen-Vos J, van Riemsdijk IC, IJzermans JN, Weimar W: Cholesterol as an independent predictor of outcome after renal transplantation. Transplantation 69: 1704–1710, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Bumgardner GL, Wilson GA, Tso PL, Henry ML, Elkhammas EA, Davies EA, Qiu W, Ferguson RM: Impact of serum lipids on long-term graft and patient survival after renal transplantation. Transplantation 60: 1418–1421, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389, 1994 [PubMed] [Google Scholar]
- 12.West of Scotland Coronary Prevention Study: Identification of high-risk groups and comparison with other cardiovascular intervention trials. Lancet 348: 1339–1342, 1996 [PubMed] [Google Scholar]
- 13.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339: 1349–1357, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Armitage J, Parish S, Sleigh P, Peto R: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet 361: 2005–2016, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Downs J, Clearfield M, Weis S, Whitney E, Shapiro D, Beere P, Langendorfer A, Stein E, Kruyer W, Gotto A: Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615–1622, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Sacks F, Pfeffer M, Moye L, Rouleau J, Rutherford J, Cole T, Brown L, Warnica J, Arnold J, Wun C, Davis B, Braunwald E: The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335: 1001–1009, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Shepherd J, Cobbe S, Ford I, Isles C, Lorimer A, MacFarlane P, McKillop J, Packard C: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333: 1301–1307, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Holdaas H, Fellström B, Jardine A, Holme I, Nyberg G, Fauchald P, Grönhagen-Riska C, Madsen S, Neumayer H, Cole E, Maes B, Ambühl P, Olsson A, Hartmann A, Solbu D, Pedersen T: Effect of fluvastatin on cardiac outcomes in renal transplant recipients: A multicentre, randomised, placebo-controlled trial. Lancet 361: 2024–2031, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Holdaas H, Fellström B, Cole E, Nyberg G, Olsson A, Pedersen T, Madsen S, Grönhagen-Riska C, Neumayer H, Maes B, Ambühl P, Hartmann A, Staffler B, Jardine A: Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: The ALERT extension study. Am J Transplant 5: 2929–2936, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Holdaas H, Fellström B, Jardine A: Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: Lessons to be learnt from the assessment of Lescol in renal transplantation (ALERT) trial. Am J Transplant 5: 1574–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ: Cardiovascular disease after renal transplantation. J Am Soc Nephrol 7: 158–165, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Cosio FG, Pesavento TE, Pelletier RP, Henry M, Ferguson RM, Kim S, Lemeshow S: Patient survival after renal transplantation: the effects of statins. Am J Kidney Dis 40: 638–643, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Massy ZA, Nguyen Khoa T, Lacour B, Descamps-Latscha B, Man NK, Jungers P: Dyslipidaemia and the progression of renal disease in chronic renal failure patients. Nephrol Dial Transplant 14: 2392–2397, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R: Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med 158: 998–1004, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Fried LF, Orchard TJ, Kasiske BL: Effect of lipid reduction on the progression of renal disease: A meta-analysis. Kidney Int 59: 260–269, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK: Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: The Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2: 1131–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kasiske BL, Heim-Duthoy KL, Singer GG, Watschinger B, Germain MJ, Bastani B: The effects of lipid-lowering agents on acute renal allograft rejection. Transplantation 72: 223–227, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Holdaas H, Jardine AG, Wheeler DC, Brekke IB, Conlon PJ, Fellstrom B, Hammad A, Holme I, Isoniemi H, Moore R, Rowe PA, Sweny P, Talbot DA, Wadstrom J, Ostraat O: Effect of fluvastatin on acute renal allograft rejection: A randomized multicenter trial. Kidney Int 60: 1990–1997, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Sahu K, Sharma R, Gupta A, Gulati S, Agarwal D, Kumar A, Bhandari M: Effect of lovastatin, an HMG CoA reductase inhibitor, on acute renal allograft rejection. Clin Transplant 15: 173–175, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF: International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int 44: 411–422, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Churg J, Sobin LH: Benign nephrosclerosis. In: Renal Disease: Classification and Atlas of Glomerular Diseases, Vol 1, edited by Churg J, Tokyo, Igaku-Shoin, 1982, pp 211–224
- 35.Snapinn B: Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 59: 301, 2005 [Google Scholar]
- 36.Cox DR: Regression models and life-tables. J R Stat Soc 34: 187–220, 1972 [Google Scholar]
- 37.Bursac ZG, Williams D, Hosmer D: A Purposeful Selection of Variables Macro for Logistic Regression. Proceedings of the SAS Global Forum 2007 Conference [Paper 173]. Cary, NC: SAS Institute Inc., 2007
- 38.Grambsch PM, Therneau TM: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526, 1994 [Google Scholar]
- 39.Robins JM, Hernan MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Bodnar LM, Davidian M, Siega-Riz AM, Tsiatis AA: Marginal structural models for analyzing causal effects of time-dependent treatments: An application in perinatal epidemiology. Am J Epidemiol 159: 926–934, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Hernan MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.