Abstract
Hemopexin is an abundant plasma protein that effectively scavenges heme. When infused into rats, hemopexin induces reversible proteinuria, and activated hemopexin is increased in children with minimal change nephrotic syndrome. These observations suggest a role for hemopexin in glomerular disease; in this study, the effects of active hemopexin on human podocytes and glomerular endothelial cells, the two cell types that compose the glomerular filtration barrier, were investigated. Within 30 min of treatment with hemopexin, actin reorganized from stress fibers to cytoplasmic aggregates and membrane ruffles in wild-type podocytes. This did not occur in nephrin-deficient podocytes unless they were transfected with nephrin-expressing plasmids. Furthermore, hemopexin did not affect actin organization in cells that do not express nephrin, specifically human glomerular endothelial cells, fibroblasts, and HEK293 cells. The effects of hemopexin on wild-type podocytes reversed within 4 h and were inhibited by preincubation with human plasma. Treatment with hemopexin activated protein kinase B in both wild-type and nephrin-deficient podocytes but activated RhoA only in wild-type cells. In addition, hemopexin led to a selective increase in the passage of albumin across monolayers of glomerular endothelial cells and to a reduction in glycocalyx. In summary, active hemopexin causes nephrin-dependent remodeling of podocytes and affects permeability of the glomerular filtration barrier by degrading the glycocalyx.
Normal adult kidneys efficiently filter 180 L of plasma per day with minimal loss of protein. The glomerular filtration barrier is the interface at which this process occurs. This barrier is composed of glomerular endothelial cells (GEnC) and podocytes with an intervening basement membrane. In proteinuric states, there is disruption of the filtration barrier, and all three components have been variably implicated. In nephrotic syndrome (NS), there is significant loss of protein into the urine. The contributing role of GEnC, glomerular basement membrane, and podocytes in NS is not well established. Neither is the sequence of events that lead to disruption of the barrier and loss of protein into the urine.
The importance of the podocyte in maintaining the integrity of this barrier emerged with the discovery of genes that when mutated lead to congenital or early-onset steroid-resistant NS.1,2 Subsequently, more mutations have been described in humans and also mouse models, and these mutations result in variable NS phenotypes.3,4 In addition to NS associated with genetic mutations are acquired nephrotic syndromes, and these have been categorized on the basis of the appearance of the glomeruli using light microscopy. One category minimal-change NS (MCNS), has a high incidence in childhood, and its association with viral illnesses has led to theories of immune mechanisms of disease.5 Another category is FSGS, which occurs across the age spectrum and is associated with a circulating factor theory of disease.6 This theory proposes that circulating plasma factors are capable of initiating damage to the glomerular filtration barrier. The evidence for this comes from the described recurrence of FSGS after renal transplantation the maternofetal passage of nephrosis7 and ex vivo glomerular swelling in response to FSGS plasma.8 Despite evidence to support the theory, the identification of specific factors has remained elusive.8
It is conceivable with both MCNS and FSGS that a variety of pathogenic mechanisms operate, and a circulating factor has indeed been implicated in MCNS. Our study of children with MCNS found increased activation of circulating hemopexin (Hx), implicating this molecule in the pathogenesis of the disease.9 Hx is well described as a heme-scavenging protein.10 It is predominantly produced in the liver, and it increases in the acute-phase reaction to inflammation or infection. Plasma-purified and recombinant Hx has been shown to have serine protease activity.11 It has been suggested that various isoforms of Hx exist and that in normal conditions circulating Hx is inactive but under certain circumstances Hx becomes activated as a serine protease. Activated Hx has been shown to have dramatic effects on the glomerular filtration barrier. Kidney sections incubated with Hx have a reduction of the anionic layer and reduced sialoglycoproteins.11 In vivo, activated Hx induced reversible proteinuria in rats and the glomeruli had ultrastructural changes similar to those seen in human MCNS with podocyte foot process effacement.12
Between adjacent podocyte foot processes, the slit diaphragm complex of interacting proteins exists. The integrity of this complex is crucial for normal podocyte function. Nephrin is a key structural component of the slit diaphragm. It is a transmembrane signaling molecule and has been shown to activate protein kinase B (PKB) via phosphatidylinositol-3 kinase.13 More recently, nephrin was shown to interact with Nck adaptor proteins,14,15 and this interaction leads to reorganization of the actin cytoskeleton. In acquired NS, the mechanism that leads to disruption of the slit diaphragm is not understood; however, it is possible that circulating mediators may initiate podocyte-specific damage. Our own data support a novel concept of either missing or inhibitory factors in plasma that are required to maintain normal podocyte signaling via the slit diaphragm proteins.16 The aim of this study was to determine the effects of Hx on the morphology of the cells of the glomerular filtration barrier and to relate this to slit diaphragm signaling and glomerular permeability.
RESULTS
Hx Caused Rapid, Reversible Reorganization of the Actin Cytoskeleton in Wild-Type Podocytes
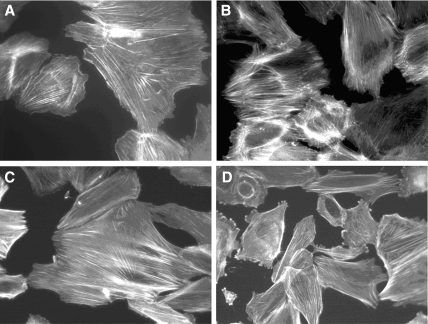
Wild-type (WT) podocytes showed a pattern of actin stress fibers when cultured in normal conditions or in serum-free medium (SFM); however, when treated with Hx, a dramatic change in actin was seen 30 min after exposure (Figure 1). Not all cells were equally affected. Some cells changed dramatically with loss of actin stress fibers, the development of cytoplasmic actin aggregates, and peripheral ruffles. Other cells developed only peripheral ruffling. A proportion of the cells became contracted and more rounded. The cytoplasmic aggregates resembled podosomes, and confocal microscopy confirmed the typical localization at the base of the cell (data not shown). Podosomes are sites of actin polymerization and have been associated with both cell adhesion and matrix degradation.17,18 The actin changes seen in WT podocytes after Hx were not seen in glomerular endothelial cells or in 3T3 fibroblasts (Figure 2), and when WT podocytes were treated with Hx over a time course, recovery of stress fiber formation was seen within 4 h. Furthermore, repeat treatment of WT podocytes with Hx led to a reappearance of the described actin changes. When cells were treated with Hx, there was minimal cell loss and no discernible change in morphology by phase contrast microscopy.
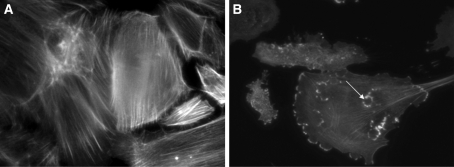
Figure 1.
(A) WT podocytes exhibited actin in stress fibers in normal culture conditions and in SFM. (B) By contrast WT podocytes treated with Hx (0.05 mg/ml for 30 min) showed dramatic reorganization of actin with loss of stress fibers and peripheral ruffles and the development of cytoplasmic aggregates. In some cells, these aggregates resembled podosomes (arrow). Images are representative of n = 6 independent experiments.
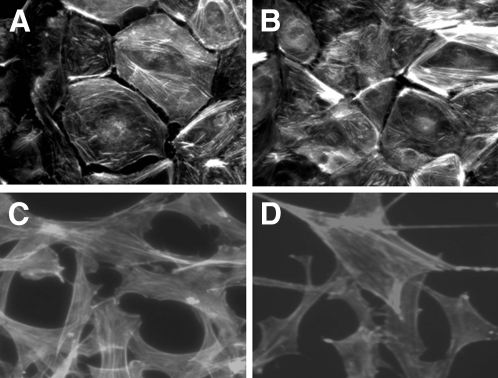
Figure 2.
(A and B) Conditionally immortalized GEnC did not reorganize actin in response to Hx (0.05 to 0.10 mg/ml). (C and D) Similarly, 3T3 fibroblasts demonstrated actin stress fibers when cultured in SFM (C), and this did not change after treatment with Hx (0.05 mg/ml) for a time course up to 4 h (D). Representative images of n = 4 independent experiments.
For further visualization of the actin reorganization seen with Hx treatment, actin–green fluorescence protein (GFP) microinjected into WT podocytes was imaged using real-time confocal microscopy. There were dramatic changes in actin when active Hx was used and cells were imaged over 30 min. In contrast, when cells were treated with heat-inactivated Hx, there was no change in actin over 30 min. Figure 3 shows selected stills from a typical experiment (complete animation of this experiment can be seen as supplemental information online).
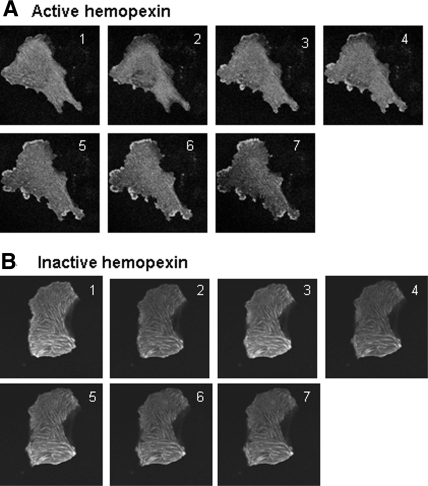
Figure 3.
(A and B) Real-time, laser scanning, confocal microscopy images of WT podocytes microinjected with GFP actin and then treated with active Hx (A) and heat-inactivated Hx (B) (0.02 to 0.05 mg/ml for 30 min). With active Hx, there was a dramatic reorganization of the actin cytoskeleton, with the development of membrane ruffles and cytoplasmic actin aggregates and the loss of actin stress fibers. Representative of n = 3 independent experiments performed in duplicate. Selected stills from a typical experiment are shown (complete animation of this experiment can be seen as supplemental information online).
Hx-Induced Actin Reorganization in the Podocyte Is Dependent on Nephrin
Nephrin-deficient (ND) podocytes demonstrated actin in stress fibers in normal culture conditions and in SFM. When ND podocyte were treated with Hx (0.05 mg/ml for 30 min), there was no change in actin patterning (Figure 4). This suggests that nephrin is required for the actin changes observed in WT podocytes. To test this hypothesis, we performed small interference RNA (siRNA) knockdown in WT podocytes. Cells treated with nephrin siRNA and Hx had a significant reduction in actin reorganization by comparison with cells treated with control siRNA and Hx.
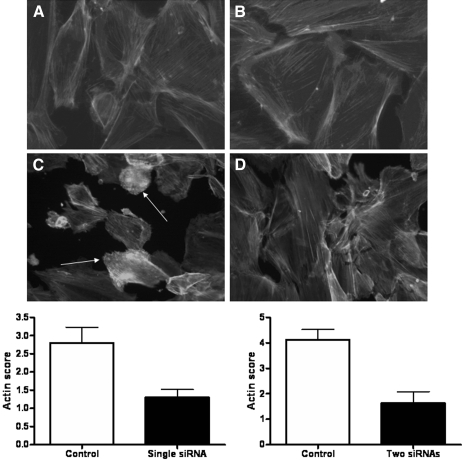
Figure 4.
(A) ND podocytes exhibited actin in stress fibers in normal culture conditions and in SFM. (B) Unlike WT podocytes, ND podocytes did not show actin reorganization after treatment with Hx (0.05 to 0.10 mg/ml for 30 min). Images are representative of n = 4 independent experiments. (C and D) Furthermore, siRNA knockdown of nephrin in WT podocytes followed by treatment with Hx (0.05 to 0.10 mg/ml for 30 min) was associated with a reduction in actin reorganization (D) compared with control siRNA and Hx treatment (C). Arrows indicate actin reorganization. The changes were assessed with an actin scoring system by two independent observers, and there were significant differences (P < 0.05) using both single and two siRNA sequences as shown in the graphs.
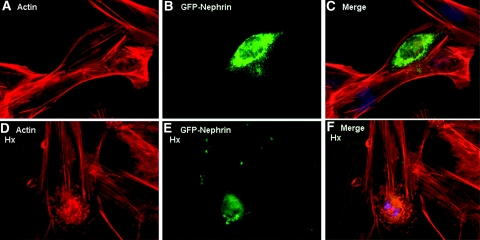
For further validation of these findings, GFP-nephrin–expressing plasmids were transfected into undifferentiated ND podocytes, and cells were then treated with Hx (0.05 to 1.00 mg/ml for 30 min). Only cells expressing nephrin demonstrated actin reorganization (Figure 5); however, when 3T3 fibroblasts and HEK293 cells were transfected with the same GFP-nephrin–expressing plasmids, there was no actin reorganization after Hx treatment in five independent experiments (data not shown). This suggests that other components of the slit diaphragm complex are required for the Hx effects on the actin cytoskeleton.
Figure 5.
ND podocytes were reconstituted with GFP-tagged nephrin by transient transfection. (A through C) Cells expressing GFP-nephrin (B) demonstrated actin stress fibers in SFM (A and merged in C). (D through E) When these cells were treated with Hx, (0.05 to 1.00 mg/ml for 30 min), only the GFP-nephrin–expressing cells (E) demonstrated actin reorganization (D and merged in F). Images are illustrative of n = 3 experiments each performed in triplicate.
Normal Human Plasma and Protease Inhibition Prevented the Effect of Hx
When WT podocytes were preincubated for 12 h with medium containing 10% normal human plasma, there was a stress fiber pattern of actin with some cortical ruffles, as described previously.16 When the cells were then treated with Hx, there was minimal reorganization of actin (Figure 6); in particular, there were no cytoplasmic aggregates. This finding suggests that low concentrations of factors in normal plasma are able to block the effect of Hx on actin reorganization in WT podocytes. Furthermore, low dosages of the serine protease inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) reduced actin reorganization in WT podocytes treated with Hx; however, at dosages >1.0 mM, AEBSF had independent effects on actin reorganization.
Figure 6.
(A) WT podocytes demonstrated actin stress fibers when preincubated with 10% normal human plasma for 12 to 24 h. (B) The addition of Hx (0.05 mg/ml for 30 min) was not associated with dramatic actin reorganization. (C) When WT podocytes were treated with the serine protease inhibitor AEBSF (0.5 mM) and Hx (0.05 mg/ml) for 30 min, there was minimal actin reorganization. At higher concentrations, AEBSF (1.0 mM) had independent effects on the actin cytoskeleton (D). Images are representative of n = 4 independent experiments.
RhoA and PKB Are Rapidly Activated in WT Podocytes after Hx Treatment
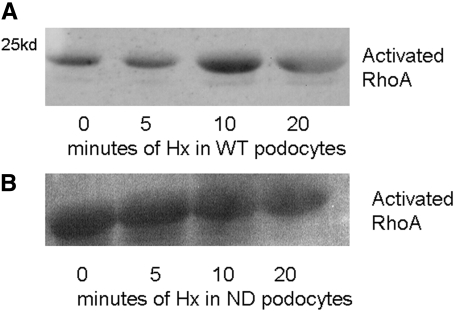
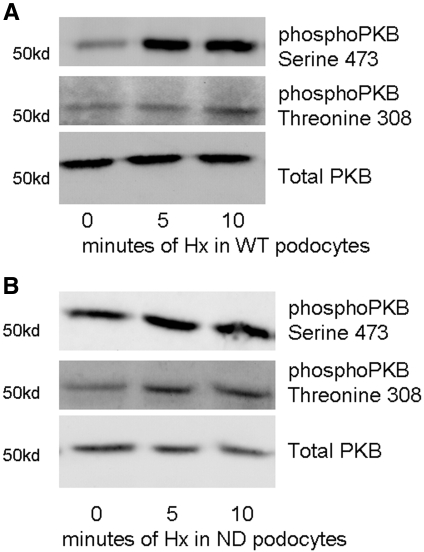
RhoA and PKB signaling pathways are involved in actin reorganization19,20; furthermore, PKB activation is associated with nephrin signaling.13 In WT podocytes, there was activation of RhoA with peak activation at 10 min after Hx treatment. By contrast, ND podocytes had a higher basal activation of RhoA, and this did not increase with Hx treatment (Figure 7). This indicates that nephrin is required to regulate the activation of RhoA in response to Hx. Both of the PKB phosphorylation sites were analyzed: Serine 473 (S473) and threonine 308 (T308). In WT podocytes, there was significant activation of S473 within 5 min and a lesser degree of activation of T308 within 10 min (Figure 8A). In ND podocytes, there was minimal activation of T308 and S473 after Hx treatment (Figure 8B). This suggests that nephrin regulates S473 phosphorylation after Hx treatment.
Figure 7.
Activated RhoA was determined using a GST pulldown assay. (A) WT podocytes had minimal basal activation of RhoA, and this increased after treatment with Hx. (B) ND podocytes had higher basal expression of RhoA, and this did not change after Hx treatment. Images are representative of n = 3 independent experiments.
Figure 8.
Effects of Hx on PKB activation were studied. PKB was significantly activated at S473 within 5 min of Hx treatment in WT podocytes. (A) There was less activation of PKB at T308 in WT podocytes. (B) In ND podocytes, there was minimal activation of PKB beyond basal at T308 and S473 after treatment with Hx. Images are representative of n = 4 independent experiments.
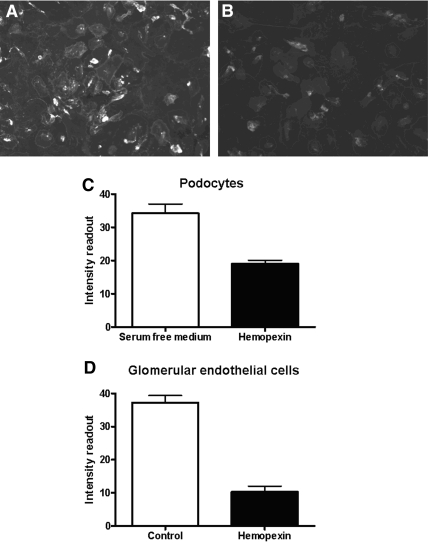
Hx Disrupted Glycocalyx of Podocytes and GEnC
The lectin wheat germ agglutinin (WGA) binds to the sugar moieties of cellular glycocalyx. In SFM, WT podocytes demonstrated expression of glycocalyx, shown by WGA binding (Figure 9A). By contrast, when WT podocytes were treated with Hx (0.05 mg/ml for 30 min), there was a marked reduction in WGA binding (Figure 9, B and C). The same experiment was repeated for glomerular endothelial cells, and, likewise, there was a reduction in WGA binding after Hx treatment (Figure 9D).
Figure 9.
WT podocytes were treated with an antibody to the lectin WGA to detect the sugar moieties of the glycocalyx. (A) Cells in SFM demonstrated abundant WGA binding. (B) By contrast, cells treated with hemopexin (0.05 mg/ml for 30 min) demonstrated a reduction in WGA binding. (C and D) The comparative fluorescence intensity is shown for podocytes (C) and for glomerular endothelial cells (D) for n = 3 to 4 independent experiments showing a significant differences in WGA binding (P = 0.002 and P = 0.003, respectively, by unpaired t test).
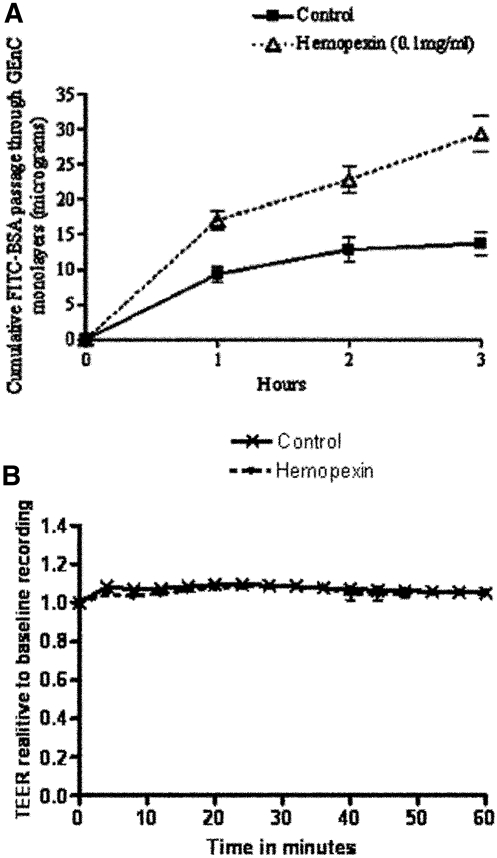
Hx Increased Albumin Clearance across GEnC Monolayers
The effects of Hx treatment on the barrier properties of GEnC were studied in two ways: First by analyzing the changes in transendothelial electrical resistance (TEER), which is a measure of cell integrity and the tightness of monolayers21 and second by estimating the passage of labeled albumin across the monolayers of cells. By comparison with controls, there was no change in TEER with Hx treatment (0.05 to 0.10 mg/ml up to 100 min; Figure 10B); however, using equivalent dosages, there was a significant difference in the albumin clearance across monolayers of cells treated with Hx (P < 0.005; Figure 10A). This observed discordance between the effect of Hx on TEER and albumin clearance of the GEnC monolayers could be explained by changes in glycocalyx and is consistent with our previous observations on the role of GEnC glycocalyx in selective permeability to albumin.22
Figure 10.
(A) Significant effect of Hx on albumin clearance across GEnC monolayers over time. Each point represents mean ± SEM, n = 5, P < 0.005 by ANOVA. (B) No effect of Hx on TEER (measure of passage of water and solutes) of GEnC monolayers over time. Points represent means ± SEM, n = 4. Test monolayers were treated with Hx (0.05 mg/ml) at time 0.
DISCUSSION
Hx is widely known for its function in heme scavenging; however, the extent of its role in renal disease has not been established. The initial link between MCNS and Hx came with the identification of a vasoactive plasma factor (100KF) with the properties of a serine protease23 that induced MCNS changes in rat glomeruli.24 100KF was later found to be Hx,25 and plasmapurified Hx was found to induce rapid and reversible proteinuria in rats,12 again with glomeruli showing changes similar to those seen in MCNS. Although Hx is abundant in human plasma, circulating at a concentration of approximately 1 g/L in adults,26 it seems that activation of Hx enabling the molecule to act as a serine protease could be the link to pathogenesis in MCNS. This concept is suggested in our study demonstrating that plasma from children with MCNS in relapse had a higher degree of Hx protease activity compared with children with MCNS in remission and with healthy control subjects.9 Whether activation of Hx is a cause or effect in MCNS is uncertain; however, the relevance of this molecule in renal disease is heightened by the finding that Hx can be produced locally by mesangial cells in the glomerulus and that the activity and production of Hx in mesangial cells may be influenced by circulating cytokines such as TNF-α.27,28
This is the first study to consider the effects of Hx on the cells of the glomerular filtration barrier. We report changes observed in the filamentous actin cytoskeleton of cells. GEnC did not have evidence of actin reorganization in response to Hx, and neither did 3T3 fibroblasts or HEK293 cells, which we used as control cells. WT podocytes, however, showed dramatic reorganization of actin with loss of stress fibers and the development of membrane ruffles and cytoplasmic aggregates. The effects of Hx on actin were reversible within 4 h, and this is in keeping with the time course of proteinuria seen after Hx infusion in rats.12 This reversibility could be explained by receptor-binding dynamics or by the physiologic stability of Hx.
Interestingly, ND podocytes did not show actin reorganization after Hx treatment, indicating that the Hx effect on actin was dependent on the expression of nephrin. This finding was further supported by nephrin siRNA knockdown experiments in WT podocytes, where there was a significant reduction in actin reorganization after Hx treatment. Furthermore, reconstitution of nephrin in ND podocytes was associated with changes in the actin cytoskeleton after Hx, and this was comparable to the changes in WT podocytes. These observations firmly establish a link among Hx treatment, nephrin expression, and actin reorganization in podocytes. We therefore hypothesized that nephrin plays a key role in the downstream relay of intracellular signaling leading to actin reorganization in podocytes in MCNS. Because we have also shown that overexpression of nephrin in 3T3 fibroblasts or HEK293 cells was not associated with dramatic actin reorganization after Hx, it is likely that other components of the podocyte slit diaphragm complex are required. This pathway may well involve nephrin tyrosine phosphorylation and interaction with Nck adaptor proteins, because this association has been shown to lead to actin reorganization.14 Because nephrin expression is limited to podocytes in the glomerulus, this could explain the observation of pathology being primarily targeted to this cell in MCNS.
Another interesting observation was that preincubation of podocytes in normal human plasma prevented actin reorganization after Hx treatment. This indicates that factors in normal plasma act to protect podocytes. In MCNS, there may be loss of such factors from the plasma, leaving podocytes exposed to the effects of activated Hx. These plasma factors could be acting directly on podocytes to regulate the expression of receptors or to maintain the integrity of the slit diaphragm complex. Alternatively, these factors could be acting as direct inhibitors of active Hx in a similar manner to other circulating proteases that have circulating inhibitors. In support of this theory, we found that the serine protease inhibitor AEBSF reduced the effect of Hx on the actin cytoskeleton in WT cells.
We have explored the mechanism of Hx's effects on the actin cytoskeleton by studying key cytoskeletal signaling pathways. Whether Hx binds a cell surface receptor is unknown. The heme–Hx complex is known to bind to a specific receptor on hepatocytes (LRP-1), to allow heme transfer29; however, Hx is not known to be a ligand for other receptors. Because it has serine protease activity, Hx may act via the family of protease activated receptors. Interestingly, more than 500 proteins have domains showing homology to the Hx molecule. Among these are the matrix metalloproteinases. These proteins have been implicated in many disease processes, and recent studies investigated the possibility of a matrix metalloproteinase–protease activated receptor 1 signaling axis.30
WT and ND podocytes showed differences in activation of key cytoskeletal signaling pathways after Hx treatment. RhoA is a small GTPase, and, when activated, it is a molecular switch that can lead to actin reorganization. In WT podocytes, there was minimal basal activation of RhoA, and this increased after Hx. In association with this, there was observed actin reorganization. By contrast, ND cells had no change in RhoA activation after Hx. In association with this, ND podocytes had no associated actin reorganization. These findings suggest that nephrin is required to regulate RhoA activation in response to Hx. PKB is central to many intracellular signaling pathways, and it has been associated with actin reorganization31 and nephrin signaling.13 In WT and ND podocytes, there was minimal additional phosphorylation of PKB at T308 after Hx treatment; however, phosphorylation at S473 was upregulated considerably by Hx in WT cells compared with ND cells. This suggests that the phosphorylation of PKB after Hx stimulation occurs at the S473 site via nephrin. The previously reported nephrin-associated activation of PKB13 was also at S473.
The effects observed on the GEnC monolayers offer an additional mechanism for proteinuria. Both podocytes and GEnC demonstrated changes in the expression of glycocalyx, as seen by the reduced lectin binding after Hx treatment. In GEnC, this was associated with an increase in the flux of albumin, without any changes in the morphology of GEnC monolayers. The albumin-restrictive properties of glycocalyx are consistent with our recent publication22 and is also supported by in vivo studies on the intact glomerular filtration barrier.32 It is therefore plausible that in vivo, after the disruption of GEnC glycocalyx, active Hx (80 kD) can gain access to podocytes to have direct action on their glycocalyx and then intracellularly on the actin cytoskeleton
It is important to consider the relevance of these findings in the context of renal disease. The association between activated Hx and relapse in MCNS has been made9; however, the precise circumstances in which Hx is activated are not known. MCNS can be triggered by viral infections, and perhaps the resultant immune activation leads to increased systemic or local mesangial production of active Hx. Alternatively, elements of a disease state such as loss of key inhibitory proteins in MCNS may predispose toward increased circulation of Hx isoforms with protease activity. Once Hx is activated, it can cause disruption of the glomerular filtration barrier. This effect may also be more dramatic in association with a susceptible genotype.
Further investigation is required to determine the mechanisms and signaling pathways by which Hx causes such dramatic actin reorganization in podocytes and also to determine the plasma factors that block this effect. Potential future therapy for MCNS could target the receptors or signaling pathways involved.
CONCISE METHODS
Cell Lines
Several conditionally immortalized human cell lines were used. These were derived by incorporating a temperature-sensitive SV40 gene that enables cells to proliferate at the permissive temperature (33°C) and to differentiate at the nonpermissive temperature (37°C). The characteristics of the WT human podocyte line have been reported.33 The cells have been additionally transfected with a telomerase construct.34 In addition, a podocyte line derived from a patient with Finn Major mutation was used. The characteristics of these podocytes have also been reported previously.16,35 A second WT podocyte line and an additional cell line from a patient with a missense nephrin mutation were used and have been described previously.36 These cells were used for comparison of the Hx effect, and these data are included in Supplemental Figure S1. Conditionally immortalized human glomerular endothelial cells were also used, and the characteristics of these cells were recently reported.37 Finally, murine NIH 3T3 fibroblasts and HEK293 cells were also used.
Cell Culture
Podocytes between passages 10 and 16 were cultured for 14 d at the nonpermissive temperature (37°C) in RPMI 1640 medium with glutamine (R-8758; Sigma, St. Louis, MO) supplemented with 10% FCS (Life Technologies, Grand Island, NY) and insulin transferrin sodium selenite (Sigma I-1184; 1 ml/100 ml). For GEnC culture, polycarbonate supports (0.4-μm pore size, 0.5-cm2 surface area) in tissue-culture inserts (1 cm diameter; Nalge Nunc Int., Rochester, NY) were seeded at 100,000 cells/cm2. Inserts were placed in 24-well plates, and media were changed three times a week. All cells were changed to SFM for 2 h before being stimulated over a time course with plasma-purified Hx diluted in SFM.
Reagents
Plasma-purified Hx was prepared from normal pooled human serum as described previously.25,38 The final solution was made in Tris buffer (pH 7.4). Heat-inactivated Hx was used as an inactive control as described previously.12 Recombinant Hx11 was also used to confirm the same effects on actin modeling in podocytes (data not shown). Normal human plasma was obtained from a patient who was in remission undergoing plasma exchange for cryoglobulinemia and was used at 10% in RPMI. Appropriate ethical approval was obtained for collection and use of this sample. The serine protease inhibitor AEBSF (Sigma 30827) was used at a final concentration of 0.1 to 1.0 mM in RPMI.
Immunofluorescence
Cells on coverslips were treated accordingly, washed with PBS, and then fixed with 2% paraformaldehyde. For F-actin imaging, cells were permeabilized and blocked with 0.1% saponin and 3% BSA in PBS and then incubated with conjugated Texas Red phalloidin (Molecular Probes, Eugene, OR). For lectin binding, the FITC-conjugated WGA antibody (Sigma L4895) was used. Cells were washed and fixed and then incubated with diluted anti-WGA in PBS. After washing, coverslips were mounted and viewed using an inverted fluorescence microscope (AF6000LX; Leica Microsystems, Mannheim, Germany). Images were processed using the Leica Application Suite (version 1.7.0). Each experiment was repeated on at least three independent occasions and imaged separately by two investigators.
Transfection
As described previously,36 the siRNA target sequence used to knock down nephrin was GUCGCUCAUCCUGAACGUA, and an addition sequence was also used: GAUUAAAGGUUGUGAGUCU. A scrambled sequence of GUCGCUCUCACUGAACGUA was used as a control. All sequences were obtained from Dharmacon (Lafayette, CO). A GFP-tagged nephrin construct was generated using full-length nephrin cDNA incorporated in pcDNA3.1 (gift of Dr. K. Tryggvasson, Karolinska Institute, Stockholm, Sweden). The primers for GFP tagged to the extracellular domain of nephrin were as follows: 5′-ACTCAGATCTCGATGGCCCTGGGGACGACGCTC and 3′-TTCGAAGCTTGATTACACCAGATGTCCCCTCAG. These were designed to incorporate the restriction enzymes sites Bgl 2 and Hind III to enable ligation into pEGFP-C2 (Clontech, Takara Bio, Saint-Germain-en-Laye, France). Lipofectamine 2000 (Invitrogen, Paisley, UK) and either siRNA or plasmids were separately diluted in serum-free RPMI, and the two solutions were combined and allowed to stand for 30 min before being added to cells in normal culture conditions. For siRNA, a final concentration of 60 nM was added onto differentiated WT podocytes, and after 48 h, the cells were treated with Hx (0.05 to 0.1 mg/ml for 30 min) and prepared for immunofluorescence. As previously, nephrin knockdown was confirmed by Western blotting (data not shown). For plasmid transfection, cells were treated with Hx (0.05 to 0.1 mg/ml for 30 min) 12 h after transfection and prepared for immunofluorescence.
Microinjection
Coverslips of differentiated podocytes were microinjected using an Eppendorf InjectMan NI2 semiautomatic system coupled to a Femtojet. A GFP-actin plasmid was the gift of Dr. A. Varadi (Genomics Research Institute, University of the West of England, Bristol, UK). After microinjection, cells were maintained for 16 to 24 h to allow expression of the protein. Live cells were then imaged using a Leica SP inverted confocal imaging spectrophotometer controlled with TCS-NT software (Leica Microsystems), and active Hx (0.02 to 0.05 mg/ml) or heat-inactivated Hx (0.05 to 0.10 mg/ml) was added to the cells during imaging. Images were collected every 30 s for 30 min. These cells were imaged as described previously with a Leica AF6000LX. Each experiment was performed in replicate and repeated on at least three independent occasions.
RhoA Activation
After Hx stimulation, podocytes were washed with ice-cold PBS and then scraped into ice-cold NP40 extraction buffer (50 mM Tris/HCl [pH 7.5], containing 1 mM EDTA, 120 mM NaCl, 50 mM NaF, 1 mM benzamidine, 1% NP40, 1 μM microcystin, 7.2 mM 2-mercaptoethanol, 5 mM orthovanadate, and protease and phosphatase inhibitor cocktails). Samples were centrifuged at 5000 rpm at 4°C for 10 min. Equal volumes of cell lysates were removed for assessment of total RhoA as a loading control. Remaining, equal volumes of cell lysates were incubated with 30 μg of rhotekin beads (Upstate 14-383, Watford, Hertfordshire, UK). The samples were washed with Tris buffer (50 mM Tris HCl [pH 7.2] containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, and protease and phosphatase inhibitor cocktails) and centrifuged at 5000 rpm. The supernatant was removed, and samples were eluted with 50 μl of Laemmli buffer containing 40 mM dithiothreitol and boiled for 10 min. Finally, samples were spun and loaded for SDS-PAGE, and Western blotting was performed as described next.
Protein Extraction, SDS-PAGE, and Western Blotting
For whole-cell lysates, cells were washed twice with ice-cold PBS and then scraped into ice-cold NP40 extraction buffer (50 mM Tris/HCl [pH 7.5], containing 1 mM EDTA, 120 mM NaCl, 50 mM NaF, 1 mM benzamidine, 1% NP40, 1 μM microcystin, 7.2 mM 2-mercaptoethanol, and 5 mM orthovanadate with protease phosphatase inhibitor cocktails). Cell extracts were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were stored at −80°C. SDS-PAGE using 7.5 to 10% acrylamide gels was performed and protein was transferred onto polyvinylidene difluoride membranes (Millipore, Watford, Hertfordshire, UK). Membranes were blocked with 10% BSA and then incubated with primary antibodies phospho-PKB S473, phospho-PKB T308, and total PKB (Cell Signaling Technology, Hitchin, Hertfordshire, UK; 1:500) and anti-Rho (Upstate; 1:1000). Appropriate species-specific secondary horseradish peroxidase antibodies (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) were used, and luminescence was created using Femto Supersignal luminal (Pierce Biotechnology, Rockford, IL) before imaging in a ChemiDoc-it imaging system (UVP, Upland, CA).
Transendothelial Resistance
TEER was measured using an automated impedance sensing system (ECIS; Applied Biophysics, Troy, NY) as described previously.39 GEnC were seeded at 100,000 cells/cm2 in each well of the eight-well electrode slide (8W10E) supplied by the manufacturer. The base of an individual well has an array of 10 gold film electrodes that connects ECIS electronics to each of the eight wells. The resistance is reported in ohms, and the measurement from each well, at a given time point, is an average of the recordings from 10 electrodes.
Measurement of Transendothelial Protein Passage
Transendothelial permeability to macromolecules was assessed by measuring passage of FITC-labeled BSA (Sigma) across the monolayer essentially as described previously.39 Medium in both wells and inserts, containing GEnC monolayers, was replaced with SFM. After 1 h, the medium in the insert was replaced with 500 μl of SFM containing 0.5 mg/ml FITC-labeled BSA; that in the well was replaced with 500 μl of SFM containing 0.5 mg/ml unlabeled BSA (Sigma). At 1, 2, and 3 h, 100-μl aliquots were removed and replaced with 100 μl of SFM containing unlabeled BSA (0.5 mg/ml). The fluorescence of the aliquots was measured as already described, and the amount of FITC-BSA passing through the monolayer was calculated by reference to a set of standard dilutions. Clearance of albumin was defined as the abluminal chamber activity expressed as luminal chamber volume that was cleared of albumin tracer across the monolayers per unit time. The clearance volume was calculated by dividing the sum of abluminal volume at each time point (constant, 500 μl) and the increase in tracer concentration by luminal tracer concentration at each time point.40
Statistical Analysis
For fluorescence intensity and actin scoring, the unpaired t test was used. For TEER and albumin passage, ANOVA was performed and the groups were compared with a post hoc Bonferroni multiple comparison test. The GraphPad Prism 4 (GraphPad, San Diego, CA) program was used for analysis. P < 0.05 was deemed significant. The SEM is shown for all experiments.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
R.L. was funded by the Wellcome Trust for this work, A.S. is funded by Kidney Research UK, and R.J.C. is funded by the Medical Research Council. We acknowledge the Medical Research Council for providing an Infrastructure Award and Joint Research Equipment Initiative Grant to establish the School of Medical Sciences Cell Imaging Facility, which was used for cell imaging. Finally, we thank North Bristol NHS Trust for funding that supported this work.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Patari-Sampo A, Ihalmo P, Holthofer H: Molecular basis of the glomerular filtration: Nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med 38: 483–492, 2006 [DOI] [PubMed] [Google Scholar]
- 5.van den Berg JG, Weening JJ: Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond) 107: 125–136, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Marszal J, Saleem MA: The bioactivity of plasma factors in focal segmental glomerulosclerosis. Nephron Exp Nephrol 104: e1–e5, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kemper MJ, Wolf G, Muller-Wiefel DE: Transmission of glomerular permeability factor from a mother to her child. N Engl J Med 344: 386–387, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F: Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 334: 878–883, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bakker WW, van Dael CM, Pierik LJ, van Wijk JA, Nauta J, Borghuis T, Kapojos JJ: Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr Nephrol 20: 1410–1415, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Delanghe JR, Langlois MR: Hemopexin: A review of biological aspects and the role in laboratory medicine. Clin Chim Acta 312: 13–23, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bakker WW, Borghuis T, Harmsen MC, van den Berg A, Kema IP, Niezen KE, Kapojos JJ: Protease activity of plasma hemopexin. Kidney Int 68: 603–610, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cheung PK, Klok PA, Baller JF, Bakker WW: Induction of experimental proteinuria in vivo following infusion of human plasma hemopexin. Kidney Int 57: 1512–1520, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coward RJ, Foster RR, Patton D, Ni L, Lennon R, Bates DO, Harper SJ, Mathieson PW, Saleem MA: Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol 16: 629–637, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Spinardi L, Marchisio PC: Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol 85: 191–194, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lener T, Burgstaller G, Crimaldi L, Lach S, Gimona M: Matrix-degrading podosomes in smooth muscle cells. Eur J Cell Biol 85: 183–189, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Stambolic V, Woodgett JR: Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol 16: 461–466, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ridley AJ: Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16: 522–529, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF: Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol 276: C812–C820, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW: Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol 18: 2885–2893, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bakker WW, Baller JF, van Luijk WH: A kallikrein-like molecule and plasma vasoactivity in minimal change disease: Increased turnover in relapse versus remission. Contrib Nephrol 67: 31–36, 1988 [PubMed] [Google Scholar]
- 24.Cheung PK, Klok PA, Bakker WW: Minimal change-like glomerular alterations induced by a human plasma factor. Nephron 74: 586–593, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Cheung PK, Stulp B, Immenschuh S, Borghuis T, Baller JF, Bakker WW: Is 100KF an isoform of hemopexin? Immunochemical characterization of the vasoactive plasma factor 100KF. J Am Soc Nephrol 10: 1700–1708, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Kanakoudi F, Drossou V, Tzimouli V, Diamanti E, Konstantinidis T, Germenis A, Kremenopoulos G: Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem 41: 605–608, 1995 [PubMed] [Google Scholar]
- 27.Kapojos JJ, van den Berg A, van Goor H, te Loo MW, Poelstra K, Borghuis T, Bakker WW: Production of hemopexin by TNF-alpha stimulated human mesangial cells. Kidney Int 63: 1681–1686, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kapojos JJ, Poelstra K, Borghuis T, Banas B, Bakker WW: Regulation of plasma hemopexin activity by stimulated endothelial or mesangial cells. Nephron Physiol 96: P1–P10, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Piccard H, Van den Steen PE, Opdenakker G: Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leukoc Biol 2006 [DOI] [PubMed]
- 30.Goerge T, Barg A, Schnaeker EM, Poppelmann B, Shpacovitch V, Rattenholl A, Maaser C, Luger TA, Steinhoff M, Schneider SW: Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res 66: 7766–7774, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Stambolic V, Woodgett JR: Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol 16: 461–466 2006 [DOI] [PubMed] [Google Scholar]
- 32.Jeansson M, Haraldsson B: Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol 290: F111–F116, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 34.O'Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR, Davies DC, Alsop AE, Neville AM, Jat PS: Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci U S A 98: 646–651, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA: The human glomerular podocyte is a novel target for insulin action. Diabetes 54: 3095–3102, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O'Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW: Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Hrkal Z, Kuzelova K, Muller-Eberhard U, Stern R: Hyaluronan-binding properties of human serum hemopexin. FEBS Lett 383: 72–74, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Satchell SC, Anderson KL, Mathieson PW: Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol 15: 566–574, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Cooper JA, Del Vecchio PJ, Minnear FL, Burhop KE, Selig WM, Garcia JG, Malik AB: Measurement of albumin permeability across endothelial monolayers in vitro. J Appl Physiol 62: 1076–1083, 1987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.