Abstract
The epithelial sodium channel (ENaC) is critical for sodium and BP homeostasis. ENaC is regulated by Nedd4-2–mediated ubiquitylation, which leads to its internalization; this process can be reversed by deubiquitylation, which is regulated by the aldosterone-induced enzyme Usp2-45. In a second regulatory pathway, ENaC can be activated by luminal serine protease–mediated cleavage of its extracellular loops. Whether these two regulatory processes interact, however, is unknown. Here, in HEK293 cells stably transfected with ENaC, Usp2-45 interacted with ENaC, leading to deubiquitylation of the channel and stimulation of ENaC activity >20-fold. This was accompanied by a modest increase in cell surface expression of ENaC and by proteolytic cleavage of αENaC and γENaC at their extracellular loops. When endocytosis was inhibited with dominant negative dynamin (DynK44R), channel density and γENaC cleavage were increased, but αENaC cleavage and ENaC activity were not augmented. When Usp2-45 was coexpressed with DynK44R, both αENaC cleavage and activity were recovered. In summary, these data suggest that Usp2-45 deubiquitylation of ENaC enhances the proteolytic activation of both αENaC and γENaC, possibly by inducing a conformational change and by interfering with endocytosis, respectively.
The epithelial sodium channel (ENaC), composed of three subunits (α, β, and γ), is important for Na+ homeostasis and BP regulation.1 It is rate limiting in Na+ entry and tightly regulated by diverse mechanisms (including aldosterone). Of interest are two seemingly unrelated regulatory pathways, one involving the ubiquitin system, the other one luminal serine proteases. The first concerns the ubiquitin-protein ligase Nedd4-2 that interacts directly with ENaC, causing ubiquitylation and internalization of the channel.2–10 Ubiquitylation entails the linkage of ubiquitin to lysines on target proteins. This is achieved by an enzymatic cascade, including E1 and E2 enzymes and E3 ubiquitin-protein ligases.11 Deubiquitylation enzymes reverse the ubiquitylation level of target proteins.12 Recently it was shown by Fakitsas et al.6 that aldosterone induces the expression of the deubiquitylation enzyme Usp2-45 in the cortical collecting duct. They demonstrated in Xenopus laevis oocytes that Usp2-45 stimulates amiloride-sensitive Na+ currents and that Usp2-45 deubiquitylates α and γENaC. The other mechanism of ENaC regulation implicates the action of serine proteases and is fundamentally different from the one involving ubiquitin, because it acts either in the lumen of the secretory pathway or extracellularly.13,14 Thereby, the proteases modulate ENaC by cleaving the extracellular loop of either α or γENaC.15–19 Little is known about the regulation of this process, but it seems to involve aldosterone, as evidenced by the observation that in mice or rats kept under low-Na+ diet or treated with aldosterone, γENaC seems to be cleaved as well.20,21 Here we provide evidence that the two regulatory mechanisms are related to each other in that the degree of ubiquitylation controlled by the balance of Nedd4-2 and Usp2-45 regulates the level of cleaved ENaC at the cell surface by a multistep mechanism in which aldosterone, via induction of Usp2-45 protein, stimulates ENaC deubiquitylation, leading to the accumulation of cleaved α and γENaC at the plasma membrane.
RESULTS
HEK293 Cells Stably Expressing ENaC Display Small Amiloride-Sensitive Na+ Currents
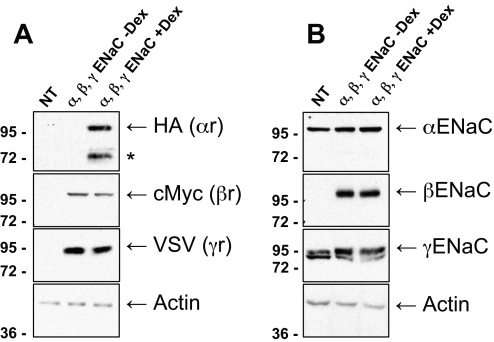
Previously we showed that Usp2-45 increases ENaC activity when coexpressed in Xenopus laevis oocytes.6 Such an increase could be due to a change of intrinsic channel properties, an increase of channel number at the cell surface, or a combination of the two. To study this question, we generated stable HEK293 cell lines expressing all three ENaC subunits. Expression of αENaC, tagged with a triple HA epitope at its C-terminus,22 was under the control of a glucocorticoid-inducible promoter.23 βENaC tagged with myc and γENaC with vesicular stomatitis virus (VSV) tag were expressed from a constitutive cytomegalovirus promoter. Representative Western blots against the tags demonstrated that all three subunits are expressed (αENaC at 100 and 72 kD; βENaC at 100 kD, and γENaC at 95 kD), and the expression of αENaC was under tight control of dexamethasone (Figure 1A). Blotting with ENaC antibodies revealed endogenous, cross-reacting proteins (Figure 1B; α and γENaC); however, we were unable to detect by real-time PCR mRNA encoding α, β, and γENaC in untransfected HEK293 cells. Moreover, these endogenous proteins were not sensitive to deglycosylation of PNGase F, as would be expected for the glycosylated ENaC subunits (Lagnaz and O.S., unpublished observations). We therefore consider it unlikely that there is endogenous ENaC in these cells. A 72-kD fragment of αENaC (Figure 1A, asterisk), detected with the HA antibody (HA tag at the C-terminus) was not seen with the N-terminal αENaC antibody, suggesting that this was a cleavage product comprising the C-terminal region of αENaC, as described previously.15 Having established this cell line, we characterized them by the whole-cell patch-clamping technique, measuring amiloride-sensitive Na+ currents (Figure 2A). Intriguingly, these cells displayed small Na+ current densities (3 ± 1 pA/pF, n = 36; Figure 2A), despite reasonable expression of the ENaC subunits, suggesting that they control the level of functional ENaC expression at the plasma membrane, possibly by taking advantage of the ubiquitin system.
Figure 1.
Expression of α, β, and γENaC in stably transfected HEK293 cells. Cells were generated as described in the Concise Methods section. Untransfected cells (NT) or stable clones (αβγENaC) were induced overnight with 1 μM dexamethasone (when indicated; + or −Dex). Confluent cells were lysed, and 15 μg of total proteins was analyzed by SDS-PAGE/Western blotting and revealed with the indicated antibodies. (A) Analysis with HA (αENaC), myc (βENaC), VSV (γENaC), or actin antibodies. *Cleavage product of αENaC, recognized by the antibodies against the C-terminal HA tag but not the N-terminal αENaC antibodies. (B) Analysis with anti-α, -β, and -γENaC or actin antibodies. α and γENaC antibodies cross-react with bands unrelated to ENaC (see text).
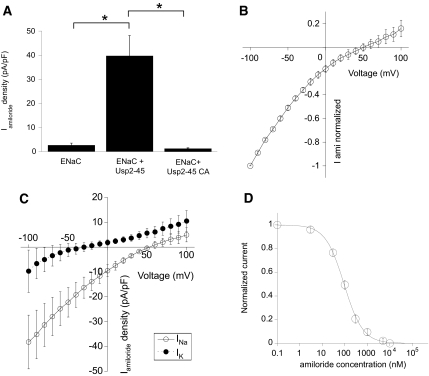
Figure 2.
Usp2-45 induces amiloride-sensitive Na+ currents in with αβγENaC stably transfected HEK293 cells. Cells were transfected with either wild-type (WT) or mutant (CA) Usp2-45 or with an empty plasmid (pcDNA3). After 24 h, cells were diluted by trypsinization and induced with 1 μM dexamethasone for another 24 h. Amiloride-sensitive currents were then measured by whole-cell patch clamp at a holding potential of −60 mV. (A) Amiloride-sensitive current densities (current amplitude normalized to cell capacitance) are shown for the three conditions (n = 25 to 64 per condition). (B through D) The current-voltage relationship for Na+ (B; n = 22), the comparison with K+ (C), and inhibition by amiloride (D; n = 12) are shown for the cells coexpressing ENaC subunits with Usp2-45.
Usp2-45 Stimulates the Expression of Functional ENaC Channels by Deubiquitylating ENaC
We transfected these cells with either wild-type or inactive Usp2-45 (Usp2-45-C67A) and repeated the whole-cell patch-clamp measurements (Figure 2A). Expression of Usp2-45 increased current densities by >20-fold and required the active enzyme. The current-voltage relationship of the induced current exhibited a reversal potential of the current at +55 mV, consistent with a Na+-selective current (Figure 2B). We determined the Na+/K+ permeability ratio of this current to be 151 ± 47 (n = 10) from the shift in the current-voltage relationship, when exchanging the extracellular monovalent cation from Na+ to K+ (Figure 2C). The current was inhibited by amiloride with an IC50 of 94 ± 3 nM (n = 12; Figure 2D). The functional properties identified the current induced by overexpression of Usp2-45 as mediated by ENaC.
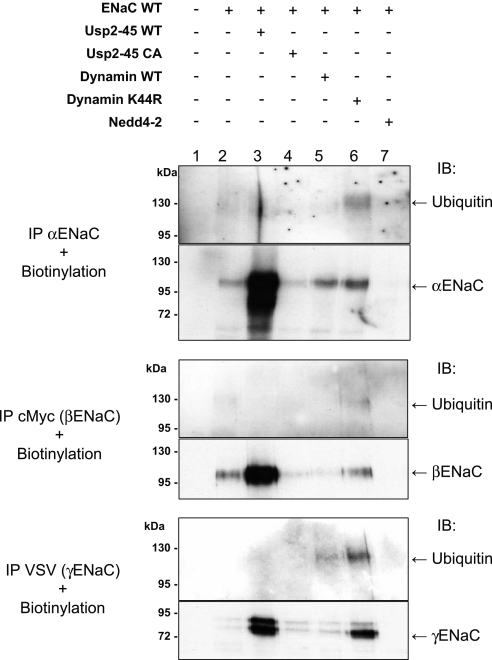
To follow ubiquitylation of ENaC at the cell surface, we biotinylated the HEK293 cells expressing ENaC, recovered the channel subunits by immunoprecipitation, and pulled down the biotinylated fraction of the immunoprecipitated material with streptavidin Sepharose. This material was immunoblotted with either ENaC or ubiquitin antibodies (Figure 3). Biotinylated ENaC subunits were detectable at the expected sizes (Figure 3, bottom). To detect the signal for ubiquitylated ENaC subunits, we coexpressed dominant negative dynamin (DynK44R), an inhibitor of endocytosis (Figure 3, lane 6). Blotting with anti-ubiquitin antibodies in this condition revealed discrete bands for α (125 kD), β (130 kD), and γENaC (120 kD), suggesting that at the cell surface, ENaC subunits exist as ubiquitylated species with a defined number of ubiquitin moieties, in contrast to the total pool of channel subunits in the cell lysate, which is mostly polyubiquitylated (Supplemental Figure S1 and ref. 6). When Usp2-45 was co-transfected (Figure 3, lane 3), the ubiquitylated species disappeared, despite an increase of nonubiquitylated ENaC at the cell surface (Figure 3, lower panels). Co-transfection of Nedd4-2 (Figure 3, lane 7) reduced both ubiquitylated and nonubiquitylated ENaC species, consistent with its role in inducing ENaC internalization.2 Taken together, these data show that ubiquitylation tightly controls ENaC activity and cell surface expression and that Usp2-45 counteracts this control by deubiquitylating ENaC.
Figure 3.
Cell surface ENaC subunits are ubiquitylated, and Usp2-45 interferes with this ubiquitylation. HEK293 cells were transiently transfected with αβγENaC together with Usp2-45, Usp2-45 CA, WT or dominant negative dynamin (DynK44R), or Nedd4-2. After 24 h of transfection, cells were biotinylated and then lysed. Immunoprecipitations were performed using antibodies against the tags of the ENaC subunits (HA for α, myc for β, and VSV for γ). Immunoprecipitated proteins were recovered, and the biotinylated fraction was precipitated with streptavidin Sepharose beads. Proteins were analyzed by SDS-PAGE/Western blotting as indicated.
Usp2-45 Controls Cell Surface Expression and Biochemical Properties of ENaC
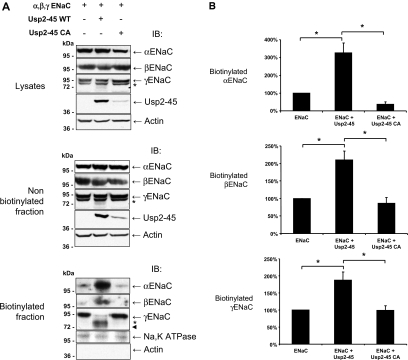
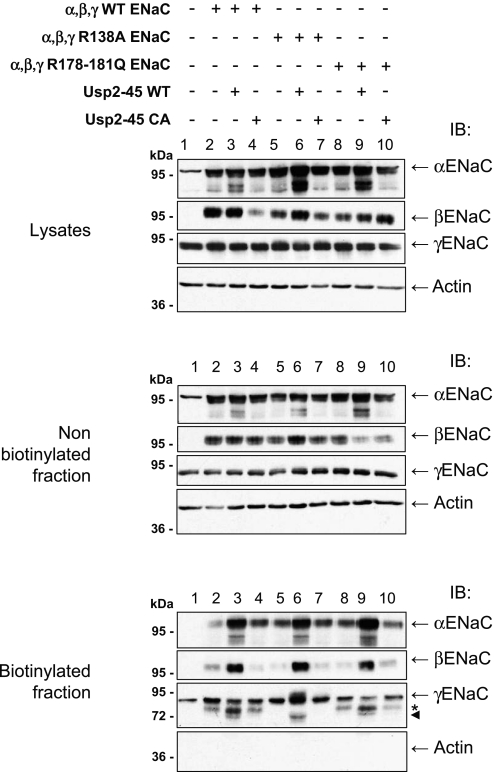
Ubiquitylation of membrane proteins is known to control internalization and endosomal degradation of such proteins.2,24,25 Indeed, the data in Figure 3 tend to suggest that Usp2-45 and Nedd4-2 regulate channel density at the plasma membrane. We made another set of cell surface biotinylation experiments and purified the biotinylated proteins by pulldown with streptavidin Sepharose. This material was analyzed by SDS-PAGE/immunoblotting, using ENaC antibodies. Figure 4A shows that the lysates and nonbiotinylated fraction of α and βENaC subunits were not affected by the expression of Usp2-45 or its mutant. In contrast, we observed weak, faster migrating species for γENaC (with apparent molecular weight between 73 and 77 kD) when Usp2-45 was co-transfected (Figure 4A, middle and top, asterisk). Consistent with the notion that ubiquitylation controls cell surface expression of ENaC, the amount of the ENaC subunits increased significantly at the cell surface when Usp2-45 was coexpressed (Figure 4A, bottom). Quantification of three different experiments indicated roughly a three-fold increase for α and approximately two-fold for β and γENaC (Figure 4B). Importantly, the majority of γENaC changed its apparent molecular weight and migrated as a doublet (Figure 4A, biotinylated fraction), which was estimated on a high-resolution gel to migrate at 77 and 73 kD (Supplemental Figure S2). Similarly, we observed for αENaC that Usp2-45 induced an approximately 100-kD form, a prominent 28-kD protein, and minor 29- and 32-kD products (Supplemental Figure S3, bottom, long exposure, blotted with αENaC antibodies recognizing the N-terminus). Occasionally, we also saw the appearance of fragments at approximately 70 kD. In contrast, blotting with anti-HA (recognizing the C-terminus) revealed a doublet at 66 and 71 kD. We were not able to detect the 100-kD protein with the HA antibody. Similar changes have been described previously for both α and γENaC and attributed to the activity of luminal serine proteases, such as furin, prostasin, or elastase.15,18–20,26,27 To confirm that the observed bands for γENaC represent proteolytic products, we mutated γENaC in either the furin site (rat 135-RKRR-138 to 135-RKRA-138) or the prostasin site 178-RKRK-181 to QQQQ (R178–181Q) described previously.17,26 Cells were transiently transfected with αβENaC, wild-type or mutated γENaC, and active or inactive Usp2-45, and cell surface biotinylation was carried out. Figure 5 (biotinylated fraction) shows that the mutation of the furin (lane 6) but not the prostasin site (lane 9) abolished the main 77-kD fragment, corroborating the idea that this was a furin-dependent cleavage product. Conversely, in this condition, the 73-kD fragment was not affected by either mutation; hence, the nature of this protein fragment remains to be clarified. Our data suggest that Usp2-45 causes a >20-fold increase of amiloride-sensitive Na+ currents that is accompanied by two- to three-fold augmentation of cell surface expression of the ENaC subunits and increased cleavage of both α and γENaC (the latter at the furin site and possibly at another cleavage site yet to be defined).
Figure 4.
Usp2-45 enhances cell surface expression of ENaC and proteolytically cleaved γENaC. HEK293 cells stably expressing αβγENaC were transfected with Usp2-45 WT or Usp2-45-C67A (Usp2-45 CA) constructs. Twenty-four hours after transfection, αENaC was induced with 1 μM dexamethasone. After another 24 h, cells were biotinylated as described in the Concise Methods section. Cells were then lysed, and the biotinylated fraction was recovered with streptavidin Sepharose. Asterisks and arrowhead indicate the 77- and 73-kD fragments for γENaC. (A) Lysates and nonbiotinylated and biotinylated proteins were analyzed by SDS-PAGE/Western blotting as indicated. No actin was detected in the biotinylated fraction. (B) Quantification of αβγENaC in biotinylated fraction. The levels of α, β, and γENaC were detected by Western blot fluorography and quantified on a molecular imager FX. Values were normalized to the control (ENaC alone) and displayed as mean ± SEM (n = 3 experiments) *P < 0.01 versus control as determined by the t test.
Figure 5.
Usp2-45 induces furin-dependent cleavage of γENaC. HEK293 cells were transiently transfected with α and βENaC, together with WT γENaC, or R138A or R178-181Q mutated γENaC and with Usp2-45 (WT or inactive mutant) as indicated. Experiments were carried out as described in Figure 4. Asterisk indicates the 77-kD band; arrowhead indicates the 73-kD fragment.
Deubiquitylating Enzyme Usp2-45 Interacts with ENaC
To understand how Usp2-45 regulates ENaC activity, we tested whether Usp2-45 interacts with the ENaC channel complex. As can be seen in Supplemental Figures S4 and S5, Usp2-45 interacted via its C-terminal region with ENaC. Moreover, this interaction did not involve the PY motifs of ENaC (Supplemental Figure S6). We also show that endogenous Usp2-45 is co-immunoprecipitated with endogenous αENaC in renal epithelial mCCDcl1 cells (Supplemental Figure S7).
Balance of Usp2-45 and Nedd4-2 Regulates ENaC Cell Surface Expression and the Biochemical Properties of γENaC
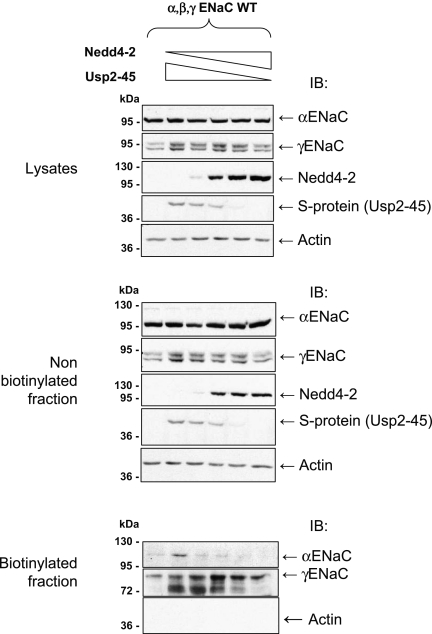
Our previous data suggested that the balance of Usp2-45 and Nedd4-2 activities may control the cell surface pool of active ENaC. We therefore transfected HEK293-αβγENaC cells with varying ratios of Nedd4-2 and Usp2-45 and examined both α and γENaC in the whole-cell lysate, nonbiotinylated and biotinylated fractions (Figure 6). The ENaC subunits were not significantly affected in the total lysate, and in the nonbiotinylated fraction; however, in the biotinylated cell surface fraction, αENaC was less prominent, relative to the control, when Nedd4-2 was expressed, and increased with escalating proportions of Usp2-45. Similarly, the amount of proteolytic fragments of γENaC increased with higher Usp2-45 expression. Hence, Usp2-45 counteracts Nedd4-2–induced ubiquitylation and increases the ENaC pool at the cell surface as well as the cleavage of γENaC.
Figure 6.
The balance between Usp2-45 and Nedd4-2 regulates the cell surface expression of ENaC. Stably transfected HEK293 cells (α, β, and γENaC) were transfected with a total of 20 μg of DNA (comprising different ratios of plasmids encoding Usp2-45 or Nedd4-2: 1, pcDNA3; 2, 1 μg of Usp2-45; 3, 0.75 μg of Usp2-45 + 0.25 μg of Nedd4-2; 4, 0.5 μg of Usp2-45 + 0.5 μg of Nedd4-2; 5, 0.25 μg of Usp2-45 + 0.75 μg of Nedd4-2; 6, 1 μg of Nedd4-2). Whole-cell lysate and nonbiotinylated and biotinylated fractions were recovered as in Figure 4 and analyzed for α and γENaC by SDS-PAGE/Western blotting with the indicated antibodies.
Usp2-45 Controls Cleavage of α and γENaC via Different Modes of Action
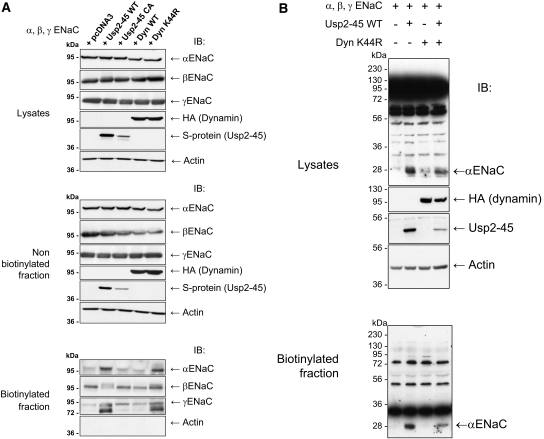
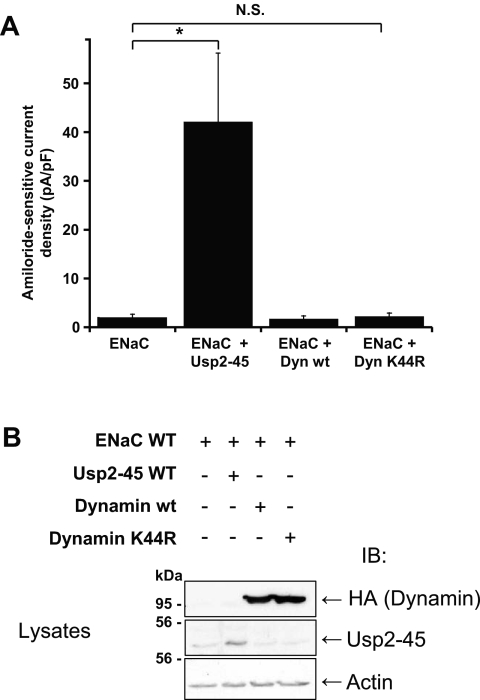
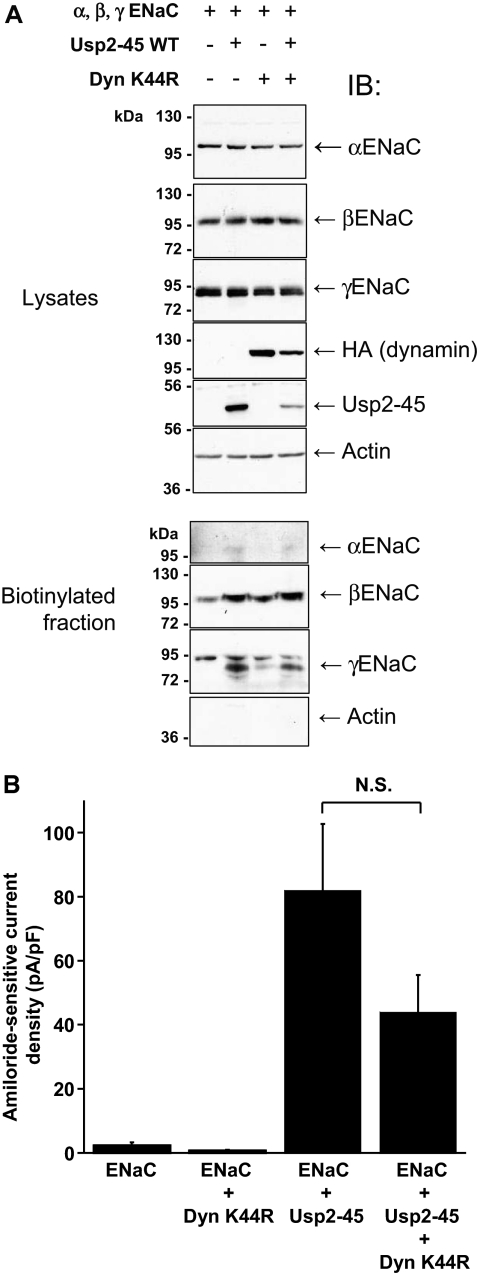
There are at least two ways in which Usp2-45 may regulate cleavage of α and γENaC. Deubiquitylation of ENaC could induce a conformational alteration rendering the proteolytic site accessible, or it may interfere with endocytosis, thereby slowing down the turnover of ENaC and increasing the probability of a cleavage. To test the second model, we interfered with endocytosis by co-transfecting dominant negative dynamin (DynK44R),28 an inhibitor of clathrin-dependent internalization, into the ENaC-expressing cells. Expression of either wild-type or dominant negative dynamin did not affect the total or intracellular pool of the ENaC subunits, similar to Usp2-45 (Figure 7A, lysates and nonbiotinylated fraction). As expected for an inhibitor of endocytosis, DynK44R increased the amount of all three subunits in the biotinylated cell surface pool to the same extent as did Usp2-45. Importantly, it similarly increased the cleavage of γENaC, as indicated by the appearance of the 73- and 77-kD fragment in the biotinylated fraction. In contrast, DynK44R did not induce the appearance of the αENaC 28-kD fragment in the biotinylated fraction (Figure 7B), indicating that there is a different mode of regulation for the cleavage events of α and γENaC. We also noted that with DynK44R or with Usp2-45, a slower migrating species of βENaC was observed, likely as a result of the formation of processed glycans in the mature protein29 (Figure 7A, biotinylated fraction). We wanted to know whether the channels that accumulated as a result of DynK44R were functional and carried out whole-cell patch-clamp analysis (Figure 8A). We found that the currents increased with Usp2-45, as described already, but not with DynK44R, despite proper expression of DynK44R (Figure 8B). This lack of ENaC activity could be due to the accumulation of ubiquitylated channels at the plasma membrane (see Figure 3), which may be silent, because they cannot be activated by αENaC cleavage. If deubiquitylation is crucial, then one would expect that coexpression of Usp2-45 with DynK44R restores αENaC cleavage and activity, as deubiquitylated channels accumulate at the plasma membrane. This prediction was indeed confirmed as shown in Figures 7B and 9. No significant difference between ENaC + Usp2-45 or ENaC + Usp2-45 + DynK44R was observed with respect to αENaC cleavage (Figure 7B), cell surface expression as evidenced by biotinylation (Figure 9A), or amiloride-sensitive currents (Figure 9B). These data therefore suggest that deubiquitylation controls proteolytic activation of ENaC, possibly by inducing a conformational change in the case of αENaC or by interfering with internalization (γENaC).
Figure 7.
Dominant negative dynamin (K44R) effects cell surface expression and cleavage of γENaC but not of αENaC. HEK293 cells stably transfected with the three ENaC subunits were transfected with HA-tagged WT or dominant negative dynamin or WT or mutant Usp2-45. (A) Cells were cell surface–biotinylated and analyzed as described in Figure 4. (B) Usp2-45 but not DynK44R induces a 30-kD cleaved band of αENaC. Proteins were analyzed as described in Supplemental Figure S3. Western blots were done with αENaC, HA (dynamin), and Usp2-45 antibodies.
Figure 8.
Dominant negative dynamin does not induce ENaC currents. Cells were transfected with Usp2-45, WT, or K44R dynamin. (A) Amiloride-sensitive current densities (current amplitude normalized to cell capacitance) are shown for the three conditions (n = 15 to 19 per condition). The t test indicates that WT and dominant negative dynamin are statistically not significantly different from control. (B) Western blots confirming that Usp2-45 and dynamin proteins were expressed.
Figure 9.
Usp2-45 activates channels that accumulate as a result of DynK44R. (A) HEK293 cells transfected with αβγENaC were co-transfected with control plasmid (pcDNA3), Usp2-45, DynK44R, or a combination of the last two. Cells were analyzed as described in Figure 4 and blotted with the indicated antibodies. (B) Amiloride-sensitive current densities as described in Figure 8A (n = 5 to 8 for ENaC, ENaC + Usp2-45, and ENaC + DynK44R; and n = 15 for ENaC).
DISCUSSION
It is well established that ubiquitylation of ENaC controls cell surface expression of the channel and that this regulation is defective in Liddle syndrome, a salt-sensitive form of hypertension2,5,10,24,30–32; however, ubiquitylation of ENaC is a reversible process, involving aldosterone-induced Usp2-45.6 Here, we studied the molecular mechanisms of Usp2-45–dependent regulation and present evidence that Usp2-45 regulates ENaC in a multistep process: (1) It binds to and deubiquitylates ENaC, (2) this deubiquitylation inhibits ENaC internalization and activates ENaC by inducing cleavage of αENaC, and (3) Inhibition of channel endocytosis favors cleavage γENaC.
The presented data reinforce the idea that the ubiquitin system constitutes a powerful system to regulate ENaC activity and consequently protects against excess Na+ influx. Indeed, HEK293 cells stably transfected with αβγENaC subunits expressed significant levels of the three subunits (Figure 1) but displayed low amiloride-sensitive Na+ currents (Figure 2). The co-transfection of Usp2-45 elevated these currents by >20-fold, with properties (Ki amiloride, reversal potential, Na+ over K+ selectivity) corresponding to the ones documented for ENaC channels.33 This was accompanied by the deubiquitylation of the ENaC subunits (Figure 3 and Supplemental Figure S1). Interestingly, the deubiquitylation by Usp2-45 not only increased the number of channels at the plasma membrane but also changed the biochemical properties of α and γENaC at the cell surface by inducing proteolytic cleavage of the two subunits. Comparable observations were made by Knight et al.,27 who showed that Liddle mutations (interfering with Nedd4-2–dependent ubiquitylation) increased cell surface expression, activity, and cleavage of ENaC.
Changes in the apparent molecular weight of the α and γ subunits were reported previously. Masilamani et al.20 showed in vivo that restriction of salt in the diet (to increase aldosterone) or infusion of aldosterone promoted the appearance of a 75-kD γENaC protein in rat kidneys. This was also reported by Ergonul et al.,21 who described that salt restriction caused upregulation of α and β subunits and an increase of a 75-kD γENaC fragment, accompanied by a decrease in the full-length 90-kD γENaC. Evidence was provided by several laboratories that both α and γENaC were cleaved by proteases. It was shown that the cleavage of αENaC occurred at two consensus sites for furin, generating 65- and 30-kD fragments and the one of γENaC at cleavage sites for furin and prostasin, yielding a 75-kD polypeptide15,17,19,21,26,34 (for a detailed review of ENaC regulation by peptidases, see references13,35). We now partly confirm these findings by showing that both α and γENaC are cleaved and, by mutating the furin site on γENaC (R138A), demonstrating that the generation of the 77-kD fragment depends on this site; however, we do not know the nature of the additional 73-kD fragment in γENaC, because mutation of neither the furin nor the prostasin site alters the appearance of this band.
How does Usp2-45 control the cleavage of α or γENaC? Although an isopeptidase, hence a protease, it is unlikely that it directly cleaves ENaC, because the predicted cleavage sites are located on the luminal site of the membrane,15 whereas Usp2-45 is a cytoplasmic enzyme.36 Interestingly, the mode is different for α or γENaC. Whereas both Usp2-45 and dominant negative dynamin induce cleavage of γENaC, suggesting that it is the prolonged stay of ENaC at the cell surface that favors this cleavage event, dominant negative dynamin does not promote cleavage of αENaC. This therefore suggests that deubiquitylation triggers an event that likely involves conformational changes within the channel complex rendering the proteolytic sites on αENaC accessible.
The findings that dominant negative dynamin does not increase ENaC activity are different from published ones, in which DynK44R stimulated ENaC activity.37–39 A possible explanation for this may be that HEK293 cells display much stronger Nedd4-2–dependent inhibition, as evidenced by the observation that Usp2-45 has to be expressed to measure decent ENaC currents (Figure 2), whereas this is not necessary in CHO cells or oocytes.37–39 Our findings confirm reports by others that the accumulation of ENaC at the cell surface and cleavage of γENaC are not sufficient for proper ENaC activation.17,19,40 Most likely, Usp2-45–dependent deubiquitylation of ENaC or another protein involved in ENaC regulation, promoting the cleavage of αENaC, is as important to activate the Na+ channel. Indeed, coexpression of Usp2-45 with DynK44R restores ENaC activity at the cell surface without affecting expression levels or cleavage of γENaC (Figure 9). In addition, a mutant ENaC channel, in which all cytoplasmic lysines (i.e., putative ubiquitylation sites) are mutated to arginine, cannot be further stimulated with Usp2-45 (both functionally and biochemically by cleavage6 (D.R.-D. and O.S., unpublished observations). Such a scenario seems at variance with published data that suggest that ubiquitylation of ENaC controls the cell surface expression and not its intrinsic properties2,41,42; however, these differences may be explained again by the different cellular system used in these studies (Xenopus laevis oocytes). Conversely, ENaC channels with Liddle mutations (mutations of PY motifs and hence missing the binding site for the ubiquitin-protein ligase Nedd4-2) display not only increased channel number at the membrane but also higher intrinsic activity.2,27,43
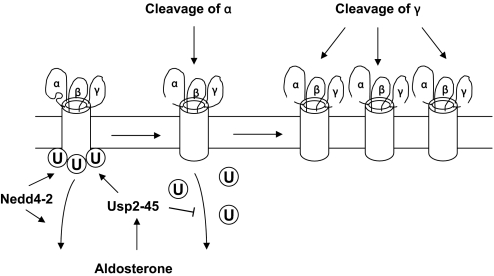
What is the physiologic relevance of these findings? Usp2-45, which is an aldosterone-induced protein,6 may well represent the link that controls the observed aldosterone-induced cleavage of α and γENaC in vivo.20,21 Thereby, aldosterone induces Usp2-45 expression, which binds to ENaC and counteracts Nedd4-2 action by deubiquitylating ENaC. The deubiquitylation of the channel will increase open probability (possibly by promoting a conformational change and rendering accessible an αENaC cleavage site) and enhance channel density, either by interfering with its internalization or by enhancing recycling, thereby favoring γENaC cleavage (Figure 10).
Figure 10.
Proposed mechanism of ENaC regulation by Nedd4-2, Usp2-45, and proteolytic cleavage. Nedd4-2 ubiquitylates ENaC on all three subunits and promotes internalization and lysosomal degradation of ENaC. Aldosterone-induced Usp2-45 interacts with the channel complex and deubiquitylates it, thereby allowing a conformational change in ENaC and rendering accessible the cleavage sites of αENaC. Deubiquitylation also prolongs the stay at the cell surface leading to γENaC cleavage.
CONCISE METHODS
DNA Constructs
All ENaC constructs were based on rat ENaC. Dexamethasone-inducible plkneo-αENaC-(HA)3 was described previously.22,23 Rat βENaC was tagged with a c-myc tag and γENaC with a VSV tag, both at the C-terminus, and cloned into pcDNA3. For binding experiments, a γENaC construct containing a Flag tag on its extracellular loop (as described previously43) and cloned into pCB6 was used. The ENaC PY mutants were previously described6 and subcloned into pcDNA3. The ENaC lysine-to-arginine mutants were generated by PCR as described previously6 and subcloned in pcDNA3.1(−) for α and γ and in pcDNA3 for β. The γENaC furin mutant R138A and γENaC prostasin mutant R178–181Q were generated using site-directed mutagenesis and subcloned in pcDNA3.1(+)Zeo. Usp2-45 wild type and C67A were generated by PCR as described previously and cloned into pcDNA3.6 Usp2-45 N-terminal amino acids 1 through 53 and Usp2-45 C-terminal amino acids 53 through 342 were labeled with an S tag at the N-terminal side and generated by PCR. Usp2-45 N-terminal was subcloned into a pCMV GST vector, and Usp2-45 C-terminal was subcloned as the full length of Usp2-45 in pcDNA3.1(−). The dynamin constructs were previously described28 and provided by S. Schmid (Scripps Research Institute, La Jolla, CA) and subcloned into pcDNA3.
Generation of Stable HEK293 Cells Expressing α, β, and γENaC
HEK293 cells stably expressing rat HA-tagged α, myc-tagged β, and VSV-tagged γENaC were generated. C-terminally triple HA-tagged αENaC in pLKneo was described before22,23 and contains a dexamethasone-inducible promoter and a G418 resistance gene. C-terminally tagged βENaC was cloned into pCEP4 (containing a hygromycin resistance gene; Invitrogen, Paisley, UK), and C-terminally VSV-tagged γENaC was cloned into pcDNA3.1(+)Zeo (Invitrogen), which contains a zeocin resistance gene. Cells were maintained in DMEM, 10% FBS, 5 μM amiloride to prevent cellular Na+ overload, G418 (Chemie Brunschwig AG, Basel, Switzerland; 0.25 mg/ml), hygromycin B (Chemie Brunschwig AG; 0.4 mg/ml), and zeocin (Invitrogen; 0.1 mg/ml). HA-tagged αENaC expression was induced by treating the cells overnight with 1 μM dexamethasone. Two different clones were tested in this study.
Cell Culture and Transfections
HEK293 cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen) and 0.05 U/ml penicillin/streptomycin (Invitrogen). They were incubated at 37°C/5% CO2. Cells were transiently transfected in 10-cm dishes at 60% confluence using the Ca2+ phosphate method.
HEK293 cells stably expressing αβγENaC were cultured in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 0.25 mg/ml geneticin (PAA, Coelbe, Germany), 0.1 mg/ml zeocin (Invitrogen), 0.4 mg/ml hygromycin B (PAA), and 5 μM amiloride (Sigma, St. Louis, MO). They were incubated at 37°C/5%CO2. These cells were transfected in 10-cm dishes at 90% confluence with lipofectamine 2000 (Invitrogen). They were incubated in a medium containing only 10% FBS and transfected with a total of 15 μg of cDNA (for a 10-cm dish) and 3 μg (for a six-well dish). Twenty-four hours after transfection, medium was changed to a selective medium containing 10% FBS, 1% penicillin/streptomycin, 0.25 mg/ml geneticin, 0.1 mg/ml zeocin, 0.4 mg/ml hygromycin B, 10 μM amiloride, and 1 μM dexamethasone (Sigma) for 24 h.
mCCDcl1 cells44 were cultured in complete DMEM/Nutmix F12 with Glutamax-1 1:1 (Invitrogen) in which were added by filtration 5 μg/ml insulin, 5 μg/ml transferrin, 6 × 10−8 M sodium selenate, 5 × 10−8 M dexamethasone, 1 nM triiodothyronine, 10 ng/ml EGF (all from Sigma), 10 mM HEPES (pH 7.4), and 2% FCS (Invitrogen). They were incubated at 37°C/5%CO2.
Cell Surface Biotinylation
Cells were washed 24 h after transfection, twice with cold PBS (1×), then treated for 30 min with 4 ml biotin (0.3 mg/ml; EZ link Sulfo-NHS-SS-Biotin; Pierce, Rockford, IL) per 10-cm dish at 4°C. Cells were washed twice with cold TBS (1×) and lysed in 1-ml/dish lysis buffer (as described in previous section). The cells were solubilized for 3 h on a wheel at 4°C and centrifuged for 30 min at 20,000 × g at 4°C. Supernatants were recovered, and protein concentrations were quantified by the Bradford method (Coo protein dosage kit; Interchim, Montluçon, France). Small parts of the lysates were kept to verify the transfection efficiency. Thirty microliters of streptavidin Sepharose beads was added to 0.7 to 2.0 mg of total proteins and incubated overnight on a wheel at 4°C. A small part of the supernatants was recovered to have the nonbiotinylated fraction. The beads were washed five times with lysis buffer, and dried beads were resuspended in 30 μl of 100 mM dithiothreitol. They were incubated for 1 h at 37°C with shaking. Then they were centrifuged for 2 min at 11,000 × g to recover the supernatant. Sample buffer (5×; 1.47 M sucrose, 10% SDS, 5 mM EDTA, 300 mM Tris [pH 8.8], 0.25% Bromophenol blue, and 130 mM dithiothreitol) was added, and the proteins were run on SDS-PAGE, transferred onto nitrocellulose, and analyzed by Western blotting.
Determination of Ubiquitylation of ENaC Subunits at the Cell Surface
Cells expressing ENaC were biotinylated as described in the previous section. Then they were lysed in 500 μl of lysis buffer as described in the previous section. Cells were vortexed for 15 s and centrifuged for 15 min at 20,000 × g at 4°C. Immunoprecipitation was carried out as described in the previous section, except that proteins were not eluted with sample buffer. Instead, dried beads were incubated with 100 μl of PBS (1×) and 1% SDS and boiled for 15 min at 99°C under agitation. Supernatants were recovered, and 900 μl of PBS (1×) was added together with 30 μl of streptavidin Sepharose beads (GE Healthcare, Buckinghamshire, UK). They were incubated for 1 h on a rotating wheel at 4°C. Beads were then washed two times with 1× PBS/1% Triton X100. Dried beads were resuspended in 30 μl of 2× sample buffer. The samples were run on SDS-PAGE, transferred onto nitrocellulose, and analyzed by Western blot, using antiubiquitin antibodies (FK2; Biomol International, Plymouth Meeting, PA).
Immunoprecipitation from Transfected HEK293 Cells
Cells were dissociated 24 h after transfection in 1 ml of cell dissociation buffer (5% glycerol, 1 mM EDTA, and 1 mM EGTA in PBS). Then the cells were recovered with 10 ml of PBS (1×) in 15 ml of polypropylene tubes. After 2 min of centrifugation at 3000 × g, supernatants were discarded, and the pellets were frozen at −70°C for at least 20 min. They were lysed in 1 ml of lysis buffer (50 mM HEPES, 150 mM NaCl, 1 mM EGTA, 10% glycerol, and 1% Triton X100) containing proteases inhibitors (protease inhibitor cocktail complete; Roche, Basel, Switzerland), 0.2 mM N-ethylmaleimide (Fluka, Buchs, Switzerland), 1 mM dithiothreitol (Sigma), and phosphatases inhibitors (100 mM NaF and 10 mM Di-Na-pyrophosphate) in a 1.5-ml tube. Cells were solubilized for 30 min on a wheel at 4°C. The cells were then centrifuged for 15 min at 20,000 × g. Supernatants were recovered, and protein concentrations were quantified by the Bradford method. Immunoprecipitations were performed with a minimum of 400 μg of total proteins. 1/250 of antibody (HA [Santa Cruz Biotechnology, Santa Cruz, CA] for αENaC, c-Myc [Sigma] for βENaC, flag M2-Agarose [Sigma] for γENaC, biotinylated S-Protein [Novagen, Madison, WI] for Usp2-45, or anti-Usp2 for endogenous Usp2) was added to the lysate and incubated for 2.5 h on a rotating wheel at 4°C. Then 20 μl of Protein G Sepharose (GE Healthcare; for HA, c-myc), Protein A Sepharose (GE Healthcare; for Usp2-45), or streptavidin Sepharose (GE Healthcare; for S-protein) was added to the mix protein-antibody and incubated in the same conditions during 1.25 h. Four washes were done with 1 ml of wash buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EGTA, 10% glycerol, 0.2% Triton X100, and 1.5 mM MgCl2), the centrifugations were done at 3000 × g. Dried beads were resuspended in 40 μl of sample buffer (2×). Then the samples were run on SDS-PAGE, transferred onto nitrocellulose, and analyzed by Western blot.
Antibodies/Western Blotting
Anti αENaC antibodies were raised against the N-terminus and the β and γ antibodies against the C-terminus, as described previously,45 and used at a dilution of 1:500. Tagged αENaC was also detected with anti-HA antibodies (1:500; Santa Cruz Biotechnology), βENaC with anti c-myc (1:500; Sigma), and γENaC either with anti–Flag-M2 antibody (Sigma; in transient transfection for binding studies) or with anti-VSV antibody (1:500; Sigma; in all of the other experiments). Anti–Usp2-45 antibodies were generated against GST-Usp2-45 (amino acids 1 through 49) and used at a dilution of 1:500. Tagged Usp2-45 was also detected with commercial S-protein horseradish peroxidase (1:10,000; Novagen). Anti–Nedd4-2 (Abcam, Cambridge, UK) was diluted 1:500 for immunoblots. Anti-actin (Sigma) was used diluted 2000×. Secondary antibodies were coupled with horseradish peroxidase (GE Healthcare), and Western blots were revealed with ECL (GE Healthcare or Pierce, Rockford, IL).
Electrophysiologic Measurements and Analysis
Electrophysiologic measurements were taken at 24 to 48 h after induction of the αENaC subunit. Macroscopic amiloride-sensitive currents, defined as the difference between ionic currents obtained in the presence and absence of 10 μM amiloride in the bath, were recorded using the patch-clamp technique in the whole-cell configuration at room temperature. All macroscopic currents shown are amiloride-sensitive currents as defined above in this paragraph. We used an EPC-10 amplifier (HEKA Electronics, Lambrecht, Germany) and Pulse and PulseFit software for data acquisition and analysis. The sampling interval was 5 ms for all measurements except for current-voltage relationships, for which it was 100 μs. Filtering was set to 5 kHz. For rapid changes of extracellular solutions, we used a micromanifold that brings eight tubes into one outlet tube (MPRE8; Cell MicroControls, Norfolk, VA). The solution flow was controlled by computer-driven solenoid valves. The standard extracellular solution contained 140 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM MES, 10 mM HEPES, and 10 mM glucose, and pH was adjusted to 7.4 with NaOH. For selectivity measurements, we used solutions containing 10 mM MES, 10 mM HEPES, and 10 mM glucose with either 140 mM NaCl or 140 mM KCl. pH was adjusted to 7.4 with Tris-base. Pipettes were pulled from Borosilicate glass (World Precision Instruments, Sarasota, FL). Pipettes had a resistance of 1 to 3 MΩ when filled with the pipette solution. The pipette solution contained 90 mM K gluconate, 10 mM KCl, 10 mM NaCl, 1 mM MgCl2, 60 mM HEPES, and 10 mM EGTA (pH 7.3 adjusted with KOH). All chemicals were obtained from Sigma or Fluka. Except for current-voltage curves, whole-cell currents were measured at −60 mV.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation to O.S. (3100A0-103779/1), F.V. (31-108021/1), and S.K. (3100A0-105262); the Roche Research Foundation in Basel (to O.S.); the Novartis Research Foundation (O.S.); and the Fondation Muschamp in Lausanne (O.S.).
We thank Drs. Bernard Rossier, Jean-Daniel Horisberger, Dmitri Firsov, Hugues Abriel, Michael Harris, and Phil Shaw and the members of the Staub Laboratory for commenting on the manuscript. We thank Dr. J. Loffing for γENaC antibodies and D. Lagnaz for preparing Usp2-45 antibodies.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Abriel H, Loffing J, Rebhun JF, Pratt JH, Horisberger J-D, Rotin D, Staub O: Defective regulation of the epithelial Na+ channel (ENaC) by Nedd4 in Liddle's syndrome. J Clin Invest 103: 667–673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O: A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J 15: 204–214, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Snyder PM, Steines JC, Olson DR: Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem 279: 5042–5046, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Harvey KF, Dinudom A, Cook DI, Kumar S: The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J Biol Chem 276: 8597–8601, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F: Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kamynina E, Staub O: Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Am J Physiol 283: F377–F387, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Malik B, Price SR, Mitch WE, Yue Q, Eaton DC: Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway. Am J Physiol Renal Physiol 290: F1285–F1294, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Staub O, Verrey F: Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol 16: 3167–3174, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Zhou R, Patel SV, Snyder PM: Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Glickman MH, Ciechanover A: The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev 82: 373–428, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R: A genomic and functional inventory of deubiquitinating enzymes. Cell 123: 773–786, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Planes C, Caughey GH: Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol 78: 23–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallet V, Chraibi A, Gaeggeler H-P, Horisberger J-D, Rossier BC: An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR: Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC: Synergistic activation of ENaC by three membrane-bound channel- activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191–201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR: Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC: A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282: 58–64, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC: Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: Role of furin-mediated endogenous proteolysis. J Biol Chem 283: 7455–7463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masilamani S, Kim G-H, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ergonul Z, Frindt G, Palmer LG: Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D: WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380, 1996 [PMC free article] [PubMed] [Google Scholar]
- 23.Hirt RP, Poulain-Godefroy O, Billotte J, Kraehenbuhl JP, Fasel N: Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene 111: 199–206, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D: The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8: 1246–1264, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Staub O, Rotin D: Role of ubiquitylation in cellular membrane transport. Physiol Rev 86: 669–707, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR: Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Knight KK, Olson DR, Zhou R, Snyder PM: Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci U S A 103: 2805–2808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damke H, Baba T, Warnock DE, Schmid SL: Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR: Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kamynina E, Tauxe C, Staub O: Differential characteristics of two human Nedd4 proteins with respect to epithelial Na+ channel regulation. Am J Physiol 281: F469–F477, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Henry PC, Kanelis V, O'Brien MC, Kim B, Gautschi I, Forman-Kay J, Schild L, Rotin D: Affinity and specificity of interactions between Nedd4 isoforms and the epithelial Na+ channel. J Biol Chem 278: 20019–20028, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP: Liddle's syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Palmer LG, Frindt G: Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A 83: 2767–2770, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR: The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kleyman TR, Myerburg MM, Hughey RP: Regulation of ENaCs by proteases: An increasingly complex story. Kidney Int 70: 1391–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Gousseva N, Baker RT: Gene structure, alternate splicing, tissue distribution, cellular localization, and developmental expression pattern of mouse deubiquitinating enzyme isoforms Usp2-45 and Usp2–69. Gene Expr 11: 163–179, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimkets RA, Lifton RP, Canessa CM: The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272: 25537–25541, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Pochynyuk O, Staruschenko A, Bugaj V, Lagrange L, Stockand JD: Quantifying RhoA facilitated trafficking of the epithelial Na+ channel toward the plasma membrane with total internal reflection fluorescence-fluorescence recovery after photobleaching. J Biol Chem 282: 14576–14585, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Staruschenko A, Pochynyuk O, Stockand JD: Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem 280: 39161–39167, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, Palmer LG: Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1395, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM: Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle's syndrome. J Biol Chem 273: 30012–30017, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D: Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firsov D, Schild L, Gautschi I, Mérillat A-M, Schneeberger E, Rossier BC: Cell surface expression of the epithelial Na+ channel and a mutant causing Liddle syndrome: A quantitative approach. Proc Natl Acad Sci U S A 93: 15370–15375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC: Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Duc C, Farman N, Canessa CM, Bonvalet J-P, Rossier BC: Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: Localization by in situ hybridization and immunocytochemistry. J Cell Biol 127: 1907–1921, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.