Abstract
MicroRNAs (miRNAs) regulate gene expression by binding the 3′ untranslated region of mRNAs. To define their role in glomerular function, miRNA biogenesis was disrupted in mouse podocytes using a conditional Dicer allele. Mutant mice developed proteinuria by 3 wk after birth and progressed rapidly to end-stage kidney disease. Podocyte pathology included effacement, vacuolization, and hypertrophy with crescent formation. Despite normal expression of WT1, podocytes underwent dedifferentiation, exemplified by cytoskeletal disruption with early transcriptional downregulation of synaptopodin. These abnormalities differed from Cd2ap−/− mice, indicating they were not a general consequence of glomerular disease. Glomerular labeling of ezrin, moesin, and gelsolin was altered at 3 wk, but expression of nestin and α-actinin was unchanged. Abnormal cell proliferation or apoptosis was not responsible for the glomerular injury. Mutant podocytes were incapable of synthesizing mature miRNA, as revealed by their loss of miR-30a. In contrast, expression of glomerular endothelial and mesangial cell miRNAs (miR-126 and miR-145, respectively) was unchanged. These findings demonstrate a critical role for miRNA in glomerular function and suggest a pathway that may participate in the pathogenesis of kidney diseases of podocyte origin. The unique architecture of podocytes may make them especially susceptible to cytoskeletal alterations initiated by aberrant miRNA dynamics.
MicroRNAs (miRNAs) are regulatory RNAs that act as antisense posttranscriptional repressors by binding the 3′ untranslated region of target mRNAs. Eukaryotes express hundreds of miRNAs that can regulate thousands of mRNAs, yet they are not regarded as housekeeping genes. Rather, they serve critical roles in dictating cell- and tissue-specific gene expression and in this context reinforce differentiation by dampening fluctuations in target mRNAs essential for homeostasis.1,2 Primary miRNA transcripts are processed into approximately 70-nt stem-loop precursors in the nucleus by Drosha-DGCR8; these are then cleaved in the cytoplasm into approximately 22-nt duplexes by Dicer.3 One strand of the duplex is loaded into the RNA-induced silencing complex and directs the complex to its targets. When miRNA–mRNA pairing is near perfect, the target is cleaved by argonaute, a component of the RNA-induced silencing complex. More common, binding is imperfect, and complexes are recruited to cytoplasmic foci (P bodies), where the mRNAs are stored or degraded.4
The number and redundancy of miRNAs pose a challenge to studying their roles individually. One strategy has been to knock out all miRNAs by targeting Dicer. Dicer−/− mice die at embryonic day 7.5 (E7.5) with morphologic defects, whereas mutants homozygous for a hypomorphic allele survive to E14.5 with impaired angiogenesis.5,6 Conditional alleles permit targeted disruption of Dicer in mice using the Cre-loxP system. Knockout in embryonic limb causes developmental defects as a result of increased apoptosis and dysregulated gene expression.7 Knockout in developing lung disrupts epithelial-mesenchymal signaling and arrests branching morphogenesis.8 Others have used this strategy to demonstrate a requirement for miRNAs in T cell differentiation,9 epidermal organization,10 Purkinje cell survival,11 and heart function.12 Although miRNAs are critical for development and maintenance of many tissues, little is known about their roles in the kidney. Several miRNAs seem to be enriched in human kidney,13 and one has been implicated in the pathogenesis of diabetic nephropathy in mice.14
Ultrafiltration in the kidney occurs across the glomerular capillary wall. The outer aspect of the barrier is lined by podocytes, which envelop the capillaries by extending slender foot processes that interdigitate with those of neighboring podocytes, bridged by a slit diaphragm. Disruption of slit diaphragm–associated proteins (e.g., nephrin, podocin, CD2AP) uncouples this complex from the cytoskeleton, causing proteinuria, glomerular disease, and kidney failure.15 To define roles for miRNAs in podocytes, we disrupted their biogenesis in vivo using a conditional Dicer allele. Here we demonstrate that Dicer is essential for maintaining podocyte differentiation, foot process architecture, and the integrity of the glomerular filtration barrier.
RESULTS
Dicer Is Essential for Podocyte Structure and Function
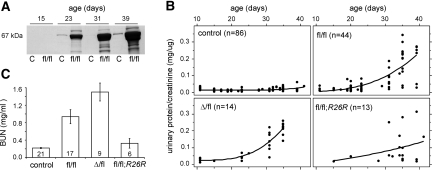
Podocyte-specific Dicer knockout mice were generated using a conditional allele (Dicerfl) containing loxP sites flanking an exon encoding most of the second RNAseIII catalytic domain.7 To target podocytes, we used a transgene (2.5P-Cre) that express Cre under control of the NPHS2 promoter. Cre is restricted to podocytes and is first active in immature podocytes of early capillary loop-stage glomeruli.16 Dicerfl/fl;2.5P-Cre mice developed albuminuria by 3 wk (Figure 1A) and progressed rapidly to end-stage kidney disease by approximately 6 wk. The disease followed a more aggressive course in some mutants, and urinary protein excretion suggested this was influenced by genotype (Figure 1B). Consistent with this, blood urea nitrogen levels at 5 to 6 wk indicated the phenotype was most severe in mutants carrying a null allele (DicerΔ/fl;2.5P-Cre) but delayed in those with a Cre reporter (Dicerfl/fl;2.5P-Cre; R26R; Figure 1C). This likely reflects the limited efficiency of Cre-mediated recombination, and it suggests podocytes are acutely sensitive to disruptions in Dicer activity. Unless otherwise indicated, the results herein are based on Dicerfl/fl;2.5P-Cre mice.
Figure 1.
Podocyte-specific Dicer knockout mice develop proteinuria by 3 wk and progress rapidly to end-stage kidney disease. (A) Analysis of urine from controls (C) and Dicerfl/fl;2.5P-Cre mice (fl/fl) by SDS-PAGE reveals progressive albuminuria in mutants by approximately 3 wk. (B) Dicerfl/fl;2.5P-Cre mutants show a corresponding increase in urinary protein/creatinine ratios and progress to end-stage kidney disease by approximately 6 wk. The phenotype was more severe in DicerΔ/fl;2.5P-Cre mutants (Δ/fl) carrying a null allele, and by 5 wk, most showed signs of uremia, including ascites, weight loss, and lethargy uncommon in Dicerfl/fl;2.5P-Cre mice at this age. Conversely, the phenotype was less severe in Dicerfl/fl;2.5P-Cre; R26R mutants (fl/fl; R26R) carrying a Cre-dependent reporter. The data include serial measurements on the number of mice indicated. (C) Blood urea nitrogen values were comparable in Dicerfl/fl;2.5P-Cre;R26R mutants and controls at 5 to 6 wk, whereas the differences between all other genotypes were statistically significant (P < 0.05). Disease progression is correlated with the number of “floxed” genomic loci, likely reflecting the limited efficiency of conditional targeting. Germline and podocyte-specific Dicer heterozygotes (DicerΔ/+ and Dicerfl/+;2.5P-Cre, respectively) had normal urinary protein/creatinine ratios and no overt phenotype.
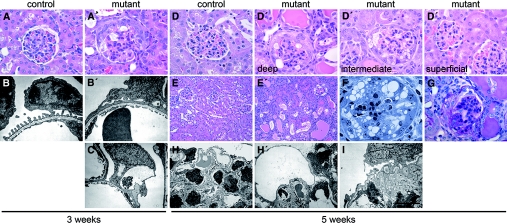
Mutants showed no renal pathology up to 2 wk of age. At 3 wk, superficial glomeruli were normal, but corticomedullary glomeruli displayed focal hypertrophy and vacuolization of podocytes and parietal epithelium, as well as pseudocrescents (Figure 2A). The zonal pattern of these lesions correlates with the state of glomerular maturity and likely reflects the timing of Dicer knockout in glomeruli, which develop in a temporally staggered manner. Podocytes in deep glomeruli also showed segmental foot process effacement, lipid droplets, and cytoplasmic vacuoles (Figure 2, B and C). Glomerular basement membrane (GBM) abnormalities included focal splitting, inclusions, and subepithelial projections. By 5 wk, all but the most superficial glomeruli showed pathologic changes (Figure 2D). Tubules were dilated and held luminal protein casts and nuclei (Figure 2E), suggesting detachment of epithelial cells of tubular and/or glomerular origin. There was prominent podocyte vacuolization (Figure 2F), as well as FSGS, periglomerular fibrosis, and interstitial inflammation consistent with late-stage disease (Figure 2G). Mutant podocytes showed extensive effacement and microvillous transformation, and many had large, clear cytoplasmic vacuoles or were degenerating (Figure 2H). In rare cases, we noted collapsing lesions with prominent GBM wrinkling and capillary occlusion (Figure 2I), but in other regions, the glomerular endothelium was normally flattened and fenestrated.
Figure 2.
Dicer is essential for maintenance of podocyte structure. (A and A′) Mutants at 3 wk show focal hypertrophy and vacuolization of epithelial cells in corticomedullary glomeruli, as well as pseudocrescents. (B, B′, and C′) Electron microscopy (EM) reveals segmental podocyte foot process effacement and vacuolization in affected glomeruli. (D and D′) The pathology is zonal in nature, and even at 5 wk, deep and intermediate glomeruli show changes, whereas superficial glomeruli are normal. (E′, F, and G) At advanced stages, mutants have tubular dilation with luminal protein casts and nuclei (E′), prominent vacuolization of glomerular epithelial cells (F), and FSGS and periglomerular fibrosis (G). (H, H′, and I) EM at 5 wk reveals global podocyte foot process effacement and prominent vacuolization (H′), whereas some glomeruli show collapsing lesions (I). Bar = 50 μm in A, A′, D, and D′; 150 μm in E and E′; 30 μm in F; 1.45 μm in B and B′; 3.13 μm in C and I; and 7.41 μm in H and H′; n ≥ 13 and n ≥ 4 mice for each light microscopy and EM panel, respectively.
X-gal staining was used to track the fate of podocytes in mutants 4 to 6 wk of age carrying the R26R allele (Supplemental Figure 1). Podocytes in deep glomeruli enveloped the periphery of the glomerular tuft or were localized to crescents, whereas labeling in superficial glomeruli was normal. Staining was weak and limited to superficial glomeruli in mutants with severe proteinuria, and podocytes that could be identified were mislocalized.
Pre-miRNA Processing Is Abolished in Dicer-Deficient Podocytes
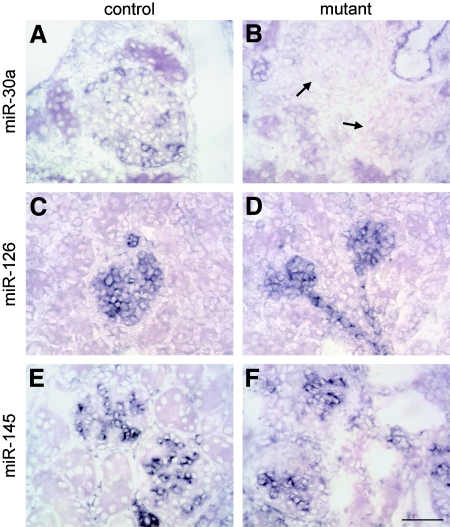
In situ hybridization for mature miRNA was used to assay Dicer activity. To identify miRNAs enriched or specific to podocytes, we analyzed data from a recent survey17 and tested several candidates in 3-wk-old mice. miR-30a was detected only in collecting duct epithelial cells and podocytes in normal kidney (Figure 3A). Tubular staining was comparable in mutants, but labeling of all podocytes was much weaker at 2 wk and absent by 3 wk (Figure 3B). This indicates that miRNA biogenesis was disrupted by 3 wk, a time point that coincides with the onset of the phenotype. miR-30a was also detected in normal lung, but signals were weak or undetectable in other adult organs. miR-126 was detected in glomerular and peritubular capillary endothelial cells in normal kidney, and labeling was comparable in mutants (Figure 3, C and D). miR-126 was restricted to endothelium in all tissues, consistent with the fact that it is encoded within an intron of the endothelial-specific gene Egfl7. miR-145 was expressed only by mesangial and vascular smooth muscle cells in normal kidney (Figure 3E), and labeling was indistinguishable in mutants (Figure 3F). Interestingly, miR-145 was limited to vascular and visceral smooth muscle in all tissues surveyed. miR-200b and miR-204 were detected in tubular epithelial cells in normal kidney, and the latter was additionally expressed by glomerular parietal epithelium. Labeling for both miRNAs in mutants was comparable to controls. miR-10b and miR-194 were not detected in mutant or control kidneys.
Figure 3.
miRNA processing is abolished in mutant podocytes. miRNA expression was evaluated in the kidneys of 3-wk-old by in situ hybridization. (A) miR-30a was expressed by collecting duct epithelium and podocytes in controls. (B) Podocytes in mutant glomeruli (arrows) were negative, a finding consistent with loss of Dicer activity, whereas tubular labeling was comparable to controls. This involved all podocytes, including those populating superficial glomeruli that show no pathology. (C and D) Signals for miR-126 were detected in glomerular and peritubular capillary endothelial cells in controls (C), and labeling was comparable in mutants (D). (E and F) miR-145 was expressed by mesangial and vascular smooth muscle cells in normal kidney (E), and labeling was indistinguishable in mutants (F). Bar = 50 μm; n ≥ 4 mice for all panels.
Mutant Podocytes Undergo Dedifferentiation Marked by Early Loss of Synaptopodin and Altered Glomerular Expression of ERM Proteins
miRNAs are posttranscriptional regulators, so the consequences of Dicer knockout should be readily apparent at the protein level. To seek changes underlying the phenotype, we analyzed glomeruli isolated from four pairs of 19- to 20-d-old mutant and control mice by two-dimensional-difference gel electrophoresis. Of approximately 2600 distinct proteins constituting the glomerular proteome, 195 differed significantly in abundance between the samples, and approximately 10% of these were isolated for identification by matrix-assisted laser desorption ionization time of flight mass spectrometry. Many proved to be either structural or regulatory cytoskeletal proteins, including actin, myosin light chain, tropomyosin, moesin, gelsolin, and caldesmon. Most glomeruli are normal by light microscopy at this age, and only podocytes in deep glomeruli have foot process effacement. This suggests that cytoskeletal disruption might cause the mutant phenotype, rather than be secondary to it.
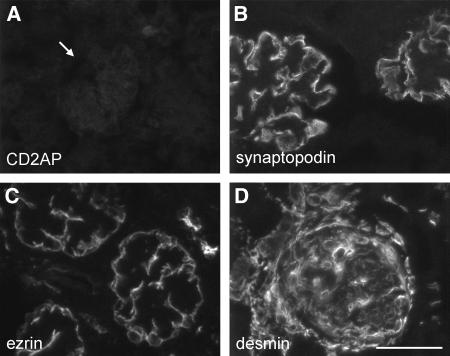
One of the earliest and most striking changes in mutants was downregulation of the actin-associated protein synaptopodin. In normal kidney, an antibody to synaptopodin labeled podocytes and reacted weakly with interstitial vessels. Podocyte staining in mutants was markedly decreased or absent in deep glomeruli at 3 wk (Figure 4A), and most glomeruli were negative by 5 wk. In situ hybridization revealed a corresponding loss of synaptopodin RNA in mutant glomeruli, a finding that could reflect either transcriptional downregulation or increased mRNA degradation (Figure 4B). Ezrin, which links podocalyxin to the podocyte cytoskeleton and can be a marker of podocyte injury,18 was detected in podocytes and proximal tubules in controls but was markedly reduced in podocytes of deep glomeruli of 3-wk-old mutants (Figure 4C). Podocytes and endothelial cells stained for podocalyxin in normal mice, but labeling was reduced in mutant glomeruli lacking synaptopodin, and the remaining signal was consistent with expression by endothelial cells. Disruption of ezrin and podocalyxin was expected, given their interaction. Moesin, a functionally related ERM (ezrin-radixin-moesin) family member, was downregulated in the proteomic screen of mutant glomeruli. It is expressed by all glomerular cell types except podocytes in the rat.19 Moesin localized primarily to endothelial cells in normal glomeruli, as determined by dual labeling for synaptopodin or the endothelial marker CD31, and its expression was reduced in deep glomeruli of 3-wk-old mutants (Figure 4D). Moesin may be disrupted indirectly through alterations in podocyte-endothelial signaling. Mutant podocytes stained normally for α-actinin-4 and nestin at 3 wk (Figure 4, E and F). Those in deep glomeruli also labeled prominently for desmin, a marker of injured podocytes (Figure 4G).
Figure 4.
Mutant podocytes undergo dedifferentiation marked by early loss of synaptopodin and altered glomerular expression of ERM proteins. (A) The actin-associated protein synaptopodin is expressed by podocytes in normal mice, and staining was markedly reduced in deep glomeruli of 3-wk-old mutants. (B) In situ hybridization revealed synaptopodin was downregulated at the transcriptional level. (C and D) Podocyte labeling for ezrin was reduced in mutants (C), whereas the related protein, moesin (D), that localizes primarily to endothelial cells in normal mice was also downregulated. (E through G) Mutant podocytes stain normally for α-actinin-4 and nestin at 3 wk (E and F), and those in deep glomeruli also labeled prominently for desmin (G), a marker of cell injury. (H) The actin-severing protein gelsolin was upregulated in mutant podocytes. Bar = 50 μm; n ≥ 5 mice for all panels.
The cytoskeletal changes could stem from abnormal expression of the structural molecules themselves or from altered expression of regulatory molecules governing cytoskeletal dynamics. An example of the latter is the actin-severing protein gelsolin, which was downregulated in the proteomic screen. Gelsolin is expressed by most tubular epithelial cells in normal kidney and has also been localized to glomerular endothelium.20 Using the same gelsolin antibody, we noted diffuse labeling in normal mice, consistent with expression by all glomerular cell types, including podocytes. Gelsolin was upregulated in mutant podocytes, whereas labeling of other glomerular cells was decreased (Figure 4H).
These changes could reflect a specific cellular response to the loss of miRNAs or, alternatively, could be a nonspecific consequence of glomerular disease. To investigate this, we profiled glomeruli from CD2AP knockout mice that develop nephrotic syndrome as a result of the loss of this slit diaphragm–associated protein (Figure 5A). Cd2ap−/− mice have foot process effacement and proteinuria by 3 wk, making them a suitable model for comparison with Dicer mutants, because both follow a similar clinical course and stem from a primary podocyte defect. Glomeruli of 1-mo-old Cd2ap−/− mutants stained for synaptopodin (Figure 5B). The intensity of labeling was comparable to controls, although its distribution was altered, reflecting glomerular capillary distension and sclerosis common at this age. Ezrin was retained in mutant glomeruli (Figure 5C), and podocytes also stained prominently for desmin (Figure 5D), suggesting a similar degree of cell injury as in Dicer mutants. Thus, the cytoskeletal abnormalities in Dicer-deficient podocytes are not part of a general response to foot process effacement or proteinuria.
Figure 5.
The cytoskeletal changes in Dicer mutants are not an indirect consequence of proteinuria. (A and B) Mice lacking the slit diaphragm-associated protein CD2AP (A) develop nephrotic syndrome as a result of a primary podocyte defect and follow a clinical course comparable to Dicer mutants, yet they retain glomerular expression of synaptopodin (B). (C and D) Podocyte staining for ezrin is also preserved in Cd2ap−/− mice (C), despite a similar degree of cell injury as revealed by prominent labeling for desmin (D). Bar = 50 μm; n = 3 mice for all panels.
Dicer-Deficient Podocytes Lose Many Defining Antigens Despite Normal Expression of WT1 but Do not Undergo Mesenchymal Transdifferentiation
Staining for nephrin was reduced in mutant glomeruli that lacked synaptopodin at 3 wk (Figure 6A) but was normal in superficial glomeruli up to 5 wk. Labeling for podocin and CD2AP followed a similar pattern (Figure 6B and data not shown), an expected finding given their association with nephrin. In situ hybridization revealed podocin was downregulated at the RNA level (Figure 6C). Podocytes normally express vascular endothelial growth factor (VEGF), which maintains the health of the underlying glomerular endothelium.21 In controls, there was intense expression of VEGF mRNA by podocytes, and weak signals were also detected in some tubular epithelial cells. Podocyte labeling was markedly decreased in deep glomeruli of 3-wk-old mutants (Figure 6D), whereas superficial glomeruli were normal. Curiously, no endothelial abnormalities expected in the setting of reduced VEGF (e.g., loss of fenestrations, endotheliosis) were noted, and glomerular staining for CD31 in mutants was comparable to controls even at 5 wk. Mutant podocytes might synthesize VEGF at levels below the limit of detection, or the GBM may serve as a reservoir for sufficient VEGF to maintain endothelial homeostasis.
Figure 6.
Mutant podocytes lose many defining antigens despite normal WT1 expression but do not undergo mesenchymal transdifferentiation. (A and B) Staining for the slit diaphragm components nephrin (A) and podocin (B) was attenuated in glomeruli of 3-wk-old mutants that lacked synaptopodin. (C and D) In situ hybridization revealed podocin (C) and VEGF (D) were downregulated at the transcriptional level. (E and F) Despite this, mutant podocytes still express WT1 protein (E) and mRNA (F) at levels comparable to controls. (G and H) By 5 wk, mutants showed periglomerular labeling for FSP-1 (G) and α-smooth muscle actin (H), consistent with late stage disease, but signals were not detected in podocytes. Bar = 50 μm; n ≥ 4 mice for all panels.
WT1 is a transcription factor restricted to podocytes in adult kidney. At 3 wk, podocytes in mutant glomeruli with little synaptopodin showed nuclear WT1 staining comparable to controls (Figure 6E). In situ hybridization revealed WT1 was also expressed at the RNA level (Figure 6F). This suggests that although certain podocyte mRNAs are downregulated in mutants, there is not a general repression of transcriptional activity. Some mutant podocytes expressed WT1 at 5 wk, although most in deep glomeruli were negative. Loss of miRNAs might cause podocytes to revert to a primitive state and reexpress markers of their mesenchymal precursors; however, one such marker, PAX2, was not detected in mutant podocytes by immunostaining at 3 wk, and by in situ hybridization, they were also negative for its transcriptional targets, GDNF and WNT4. Glomeruli of all mice were negative for the fibroblast marker FSP-1/S100A4 and for α-smooth muscle actin at 3 wk, but by 5 wk, there was prominent periglomerular labeling for both antigens in mutants (Figure 6, G and H). Myofibroblast accumulation is typical in advanced disease and consistent with fibrosis noted by histology. Occasional cells in mutant glomeruli at this age were positive for α-smooth muscle actin. Although their identity could not be firmly established given the lack of podocyte markers, their localization was consistent with expression by parietal epithelium.
Aberrant Podocyte Proliferation or Apoptosis Does not Contribute to the Initiation of Glomerular Injury
Knockout of Dicer has been associated with increased apoptosis, whereas altered expression of certain miRNAs promotes proliferation. Either process would be injurious to normally quiescent podocytes. Bromodeoxyuridine (BrdU) labeling revealed proliferating tubular and interstitial cells in 2- to 3-wk-old controls and mutants, reflecting ongoing postnatal nephrogenesis. Positive cells were noted in glomeruli, but their number did not differ significantly between mutants and controls (Figure 7). In contrast, at 5 to 6 wk, tubular and interstitial proliferation was prominent only in mutants. There was also focal BrdU labeling in glomeruli that was most evident in those with severe structural changes, and quantification revealed an overall increase in glomerular proliferation compared with controls (Figure 7). On the basis of location, at least some of this represented parietal epithelium. The contribution by podocytes is unclear, because there was concomitant loss of WT1 as assessed by dual staining. Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL)-positive cells were only rarely noted in 2- to 3-wk-old mutants and controls, yet there was a small but significant increase in the number of apoptotic cells in mutants (Figure 7). By 5 to 6 wk, many apoptotic cells were present in the interstitium and within tubular lumens in mutants, and there was a marked increase in glomerular apoptosis compared with controls (Figure 7). Abnormal cell proliferation and apoptosis occurs late in the course of the disease and cannot account for the early glomerular lesions.
Figure 7.
Abnormal cell proliferation or apoptosis does not contribute to the initiation of glomerular injury. The number of BrdU-positive proliferative cells per glomerular cross-section did not differ significantly between mutants and controls at 2 to 3 wk but was increased in mutants at 5 to 6 wk. Some of this reflected proliferation of parietal epithelium, but the contribution of podocytes could not be established because of the loss of cell-specific markers. TUNEL-positive apoptotic cells were rarely noted in normal kidneys. There was a small but significant increase in glomerular apoptosis in mutants at 2 to 3 wk and a striking increase by 5 to 6 wk. Data are means ± SEM. *P < 0.05; ***P < 0.001.
DISCUSSION
In this study, we demonstrated a requirement for miRNAs in the maintenance of podocyte structure and function. Knockout of Dicer in podocytes leads to cytoskeletal disorganization and dedifferentiation, causing progressive glomerulonephritis and death by approximately 6 wk. The severity of the phenotype was influenced by the number of “floxed” alleles, whether Dicer or R26R. This likely reflects the limited efficiency of Cre-mediated recombination, and it highlights an important factor to consider when deleting multiple conditional alleles, whether in podocytes or other cell types.
Immunostaining or Western blotting is often used in knockout studies to confirm mutant tissue lacks the targeted protein; however, the Dicerfl mutation generates an in-frame deletion and a mutant protein that cannot reasonably be differentiated from control. More important, because of protein and nucleic acid half-lives, low levels of Dicer activity and/or mature miRNAs should persist in genetically null cells; therefore, the timing of Dicer knockout was evaluated by in situ hybridization using probes that target mature miRNAs. Screening several candidates, we identified a miRNA “signature” for three glomerular cell types: miR-126 was expressed by the endothelium, miR-145 by mesangial cells, and miR-30a by podocytes. miR-30a was not restricted to podocytes but was enriched in the kidney, consistent with an earlier report in zebrafish.22 (We note that the probe to miR-30a cannot discriminate the closely related family members miR-30e and miR-30d that differ only by 1 nt, so any of these could be expressed.) Signals for miR-30a were undetectable in all mutant podocytes at 3 wk of age, as expected after inactivation of Dicer. Although this finding could reflect transcriptional downregulation of miR-30a rather than loss of processing by Dicer, this is unlikely because podocytes in superficial glomeruli expressed all other podocyte transcripts assayed at normal levels. Only one of seven miRNAs surveyed herein was detected in normal podocytes, but we hypothesize that they express a unique repertoire of miRNAs that collectively act to reinforce their differentiated state. It is likely that all of these are either absent or disrupted to some degree, depending on their stability and baseline expression, in Dicer mutants.
A number of podocyte proteins were downregulated in mutants, a finding seemingly at odds with inhibition of miRNA-mediated gene silencing. Loss of translational repression is presumably the initiating insult, but the consequences are likely complex; it is easy to envisage how disruption of transcriptional networks or cell signaling pathways that are themselves regulated by miRNAs indirectly affect the expression of downstream genes important for podocyte function. In this respect, it is noteworthy that the molecular changes in mutants manifest at a time when expression of WT1 by podocytes is normal. Downregulation of synaptopodin was one of the earliest changes noted. The relevance of this finding is unclear, because podocyte ultrastructure and function are normal in Synpo−/− mice, although a novel truncated form of synaptopodin is expressed in these mutants.23 Loss of synaptopodin is a hallmark of the dedifferentiated podocyte phenotype in idiopathic and HIV-associated collapsing glomerulonephritis24; however, in these disorders, there is also dramatic podocyte proliferation, a feature not observed in Dicer mutants. Human nephrotic syndrome is not invariably associated with loss of synaptopodin; neither are experimental models of the disease, as shown herein using Cd2ap−/− mice. Common molecular pathways, yet to be identified, may lead to disruption of synaptopodin in Dicer-deficient podocytes and in collapsing glomerulonephritis.
The cytoskeleton is critical for elaboration and maintenance of podocyte foot processes, and it serves as a platform for transmitting signals from the extracellular environment and neighboring podocytes. Disruption of the cytoskeleton may be an early consequence of altered miRNA dynamics because its constituents are more labile than other classes of proteins, such as matrix molecules. Cytoskeletal abnormalities have not been the focus of most other Dicer knockout studies, but this was a defining feature of Dicer-deficient cardiomyocytes that abnormally express various contractile and intermediate filament proteins.12
We expect that miRNAs will prove critical for many aspects of kidney development and function. Those with a developmentally restricted expression pattern in mouse kidney or that are limited to specific cell types therein might be considered candidates for involvement in human kidney disease. Polymorphisms in the 3′ untranslated region of genes implicated in kidney disease might disrupt miRNA-mediated silencing and influence gene expression. Collectively, this study establishes an essential role for miRNA in the maintenance of podocyte differentiation and for functional integrity of the glomerular filtration barrier. Our findings provide a starting point to investigate novel pathways that may underlie the pathogenesis of kidney diseases characterized by podocyte dysfunction and in doing so point to miRNAs as potential therapeutic targets.
CONCISE METHODS
Mice
Podocyte-specific Dicer knockout mice were generated using a conditional allele (Dicerfl) that deletes most of the second RNAseIII domain.7 Podocytes were targeted using 2.5P-Cre transgenic mice.16 Where indicated, mice were heterozygous for R26R25 or carried a Dicer null allele (DicerΔ) generated using β-actin–Cre.26 Cd2ap−/− mice have been described previously.27 Genotyping was performed by PCR of tail DNA. Mice were studied on a 129 × C57BL/6J background, and experiments were approved by the Washington University Animal Studies Committee.
Clinical Chemistry and Urinalysis
Urine from mutants or control littermates was pooled according to genotype (n > 3 at each age) for analysis by SDS-PAGE. Blood urea nitrogen, urinary protein, and creatinine concentrations were measured with a Cobas MiraPlus analyzer (Roche, Somerville, NJ).
Histology, Immunostaining, and In Situ Hybridization
Paraffin sections were stained with hematoxylin or periodic acid-Schiff reagent. X-gal histochemistry and electron microscopy were performed as described previously.28,29 Cryosections were immunostained using a panel of antibodies (Table 1); dilutions and modifications to a standard staining protocol28 are indicated therein. DIG-labeled riboprobes were generated by in vitro transcription of cDNA encoding mouse VEGF-A (nt 327-1169 of NM_009505), synaptopodin,30 podocin,29 and WT1.31 In situ hybridization was performed as described previously,32 except hybridization and stringency washes were at 57°C. Sense probes gave no signal above background. miRNAs were detected using DIG-labeled locked nucleic acid probes to mouse miR-10b, miR-30a-5p, miR-126–3p, miR-145, miR-194, miR-200b, and miR-204 (Exiqon, Woburn, MA). Locked nucleic acid probes (50 nM) were hybridized using the same protocol and stringency washed three times for 15 min in 2× SSC, then two times for 30 min in 0.2× SSC. After color development, slides were stained with Nuclear Fast Red or immunostained as already described.
Table 1.
Antibodies used for immunohistochemistrya
| Antibody | Dilution | Species | Reference or Supplier |
|---|---|---|---|
| α-actinin-4 | 750 | Rabbit anti-human | 33 |
| cd2ap | 2500b | Rabbit anti-mouse | 34 |
| Desmin (clone D33) | 400b | Mouse anti-human | Dako (Carpenteria, CA) |
| Ezrin (clone TB4) | 1000b | Mouse anti-chick | 35 |
| S100A4/FSP-1/MTS1 | 200 | Rabbit anti-human | Dako (Carpenteria, CA) |
| Gelsolin | 500b | Rabbit anti-mouse | 36 |
| Moesin (Q480) | 75b | Rabbit anti-human | Cell Signaling (Danvers, MA) |
| Nephrin | 1000 | Rabbit anti-mouse | 37 |
| Nestin | 10 | Mouse anti-rat | DSHB, University of Iowa |
| PAX2 | 300b | Rabbit anti-mouse | Covance (Berkeley, CA) |
| CD31/PECAM (clone MEC13.3) | 100 | Rat anti-mouse | BD Pharmingen (San Jose, CA) |
| Podocalyxin | 1000 | Rabbit anti-human | 38 |
| Podocin | 2000 | Rabbit anti-human | 39 |
| Smooth muscle actin (clone 1A4) | 200 | Mouse anti-human | Sigma (St. Louis, MO) |
| Synaptopodin | 300 | Mouse anti-rat | 40 |
| WT1 | 150 | Rabbit anti-human | Santa Cruz Biotechnology (Santa Cruz, CA) |
The following goat antibodies were used for detection: Cy3-conjugated anti-rabbit or anti-rat IgG (1:500), and Alexa488-conjugated anti-mouse IgG1 (1:200, Invitrogen). DSHB, Developmental Studies Hybridoma Bank; PECAM, platelet-endothelial cell adhesion molecule.
Paraformaldehyde fixation.
Cell Proliferation and Apoptosis
Cell proliferation was assessed by injecting BrdU (0.15 mg/g body wt) intraperitoneally into mice 1.5 h before being killed, and the label was detected by peroxidase staining of paraffin sections with a BrdU antibody (Accurate Chemical, Westbury, NY). Apoptosis was evaluated in the same specimens by TUNEL labeling (ApopTag; Chemicon, Temecula, CA). One hundred glomeruli from each animal were scored in a blinded manner, and results are reported as the number of positive cells per glomerular cross-section.
Statistical Analysis
Data were analyzed by one-way ANOVA with Tukey post hoc testing (Figure 1) or two-sample t test (Figure 7) using GraphPad InStat software (GraphPad, San Diego, CA). Differences were considered significant at P < 0.05
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by a KRESCENT Fellowship to S.J.H. from the Kidney Foundation of Canada, by a grant from the National Kidney Foundation of Eastern Missouri & Metro East to S.J.H., and by grants from the National Institutes of Health (R01DK064687 and R01GM060432) to J.H.M. J.H.M. is an Established Investigator of the American Heart Association.
We thank Lawrence Holzman, Marcus Moeller, and Andrey Shaw for supplying mice; Paul Thorner and Helen Liapis for consultation; Dan Martin, Jennifer Richardson, Gloriosa Go, and the WUSM Mouse Genetics Core (C06RR015502), DDRCC Morphology Core (P30DK052574), and WUCKDR O'Brien Center (P30DK079333) for technical assistance. We are grateful to Carola Haas, Roy Morello and Brent McCright for supplying plasmids and to the many generous investigators who provided antibodies.
Published online ahead of print. Publication date available at www.jasn.org.
S.J.H.'s current affiliation is Inserm “Avenir” U574, Hôpital Necker-Enfants Malades, Paris, France.
Supplemental information for this article is available online at http://www.jasn.org/.
See related Occasional Observation, “Dicer Cuts the Kidney,” on pages 2043–2046.
REFERENCES
- 1.Kloosterman W, Plasterk R: The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bartel D, Chen C: Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5: 396–400, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kim V: MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Valencia-Sanchez M, Hannon G, Parker R: MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7: 719–723, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein E, Kim S, Carmell M, Murchison E, Alcom H, Li M, Mills A, Elledge S, Anderson K, Hannon G: Dicer is essential for mouse development. Nat Genet 35: 215–217, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Yang D, Na S, Sandusky G, Zhang Q, Zhao G: Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 280: 9330–9335, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Harfe B, McManus M, Mansfield J, Hornstein E, Tabin C: The RNAseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A 102: 10898–10903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris K, Zhang Z, McManus M, Harfe B, Sun X: Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A 103: 2208–2213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muljo S, Ansel K, Kanellopoulou C, Livingston D, Rao A, Rajewsky K: Aberrant T cell differentiation in the absence of dicer. J Exp Med 202: 261–269, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi R, O'Carroll D, Pasolli H, Zhang Z, Dietrich F, Tarakhovsky A, Fuchs E: Morphogenesis in the skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38: 356–362, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer A, O'Carroll D, Tan C, Hillman D, Sugimori M, Llinas R, Greengard P: Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med 204: 1553–1558, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Murchison E, Tang R, Callis T, Tatsuguchi M, Deng Z, Rojas M, Hammond S, Schneider M, Selzman C, Meissner G, Patterson C, Hannon G, Wang D: Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A 105: 2111–2116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Koo S, White N, Peralta E, Esau C, Dean N, Perera R: Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 32: e188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M, Zhang L, Wang M, Lanting L, Yuan H, Rossi J, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnstone D, Holzman L: Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol 2: 271–282, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Moeller M, Sanden S, Soofi A, Wiggins R, Holzman L: Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Landgraf P, Rusu M, Sherida R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst A, Landthaler M, Lin C, Socci N, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller R, Schermer B, Bissels U, Inman J, Phan O, Chien M, Weir D, Choksi R, De Vita G, Frezzetti D, Trompeter H, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler C, Nagle J, Ju J, Papavasiliou F, Benzing T, Lichter P, Tam W, Brownstein M, Bosio A, Borkhardt A, Russo J, Sander C, Zavolan M, Tuschl T: A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129: 1401–1414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda T, McQuistan T, Orlando R, Farquhar M: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo C, Hugo C, Pichler R, Gordon K, Schmidt R, Amieva M, Couser W, Furthmayr H, Johnson R: The cytoskeletal linking proteins, moesin and radixin, are upregulated by platelet-derived growth factor, but not basic fibroblast growth factor in experimental mesangial proliferative glomerulonephritis. J Clin Invest 97: 2499–2508, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lueck A, Brown D, Kwiatkowski D: The actin-binding proteins adseverin and gelsolin are both highly expressed but differentially localized in kidney and intestine. J Cell Sci 111: 3633–3643, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber H, Kikkawa Y, Miner J, Quaggin S: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wienholds E, Kloosterman W, Miska E, Alvarez-Saavedra E, Berezikov E, De Bruijn E, Horvitz H, Kauppinen S, Plasterk R: MicroRNA expression in zebrafish embryonic development. Science 309: 310–311, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Asanuma K, Kim K, Oh J, Giardino L, Chabanis SF, C, Reiser J, Mundel P: Synaptopodin regulates the actin-bundling activity of α-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barisoni L, Kopp J: Modulation of podocyte phenotype in collapsing glomerulopathies. Microsc Res Tech 57: 254–262, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Lewandoski M, Meyers E, Martin G: Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol 62: 159–168, 1997 [PubMed] [Google Scholar]
- 27.Shih N, Li J, Karpitskii V, Nguyen A, Dustin M, Kanagawa O, Miner J, Shaw A: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Miner J, Li C, Mudd J, Go G, Sutherland A: Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131: 2247–2256, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Miner J, Morello R, Andrews K, Li C, Antignac C, Shaw A, Lee B: Transcriptional induction of slit diaphragm proteins by Lmx1b is required in podocyte differentiation. J Clin Invest 109: 1065–1072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bas Orth C, Vlachos A, Del Turco D, Burbach G, Haas C, Mundel P, Feng G, Frotscher M, Deller T: Lamina-specific distribution of synaptopodin, an actin-associated molecule essential for the spine apparatus, in identified principal cell dendrites of the mouse hippocampus. J Comp Neurol 487: 227–239, 2005 [DOI] [PubMed] [Google Scholar]
- 31.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T: Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128: 491–502, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen N, Miner J, Pierce P, Senior R: Laminin α5 is required for lobar septation and visceral pleural basement membrane formation in the developing lung. Dev Biol 246: 231–244, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan J, Kim S, North K, Rennke H, Correia L, Tong H, Mathis B, Rodriguez-Perez J, Allen P, Beggs A, Pollak M: Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Dustin M, Olszowy M, Holdorf A, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe P, Allen P, Shaw A: A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94: 667–677, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi M, Yamagata M, Noda M: Specific expression of ezrin, a cytoskeletal-membrane linker protein, in a subset of chick retinotectal and sensory projections. Eur J Neurosci 11: 545–558, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Azuma T, Witke W, Stossel T, Hartwig J, Kwiatkowski D: Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J 17: 1362–1370, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holzman L, St John P, Kovari I, Verma R, Holthofer H, Abrahamson D: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Takeda T, Go W, Orlando R, Farquhar M: Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell 11: 3219–3232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler M, Antignac C: Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160: 131–139, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mundel P, Gilbert P, Kriz W: Podocytes in glomerulus of rat kidney express a characteristic 44 kD protein. J Histochem Cytochem 39: 1047–1056, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.