Abstract
A homeostatic control mechanism that monitors and reacts to the need for sleep has been thought to function independently of the brain's circadian clock in previous studies. Now simultaneous recordings of sleep stages and electrical activity in the suprachiasmatic nucleus in behaving animals reveal feedback from sleep centers to the circadian pacemaker.
Models of sleep regulation1 have emphasized two distinct processes: a sleep-control mechanism, or sleep homeostat, and a circadian oscillator. The circadian oscillator, based in the suprachiasmatic nucleus (SCN), is responsible for the tendency to sleep during certain phases of the 24-hour cycle and the consolidation of sleep and wake into distinct episodes. The sleep homeostat is responsible for monitoring and reacting to the need for sleep, causing the urge to sleep to depend on prior amounts of sleep or wakefulness. Experimental evidence from humans and other organisms has broadly supported this dualistic view of the control of sleep2,3. Yet as anyone who has stayed up all night knows, the increase in alertness you feel in the morning is followed by a sleepy afternoon during which the desire to nap can become overwhelming. Thus, ultimately, the sleep homeostat and the circadian system must interact to regulate the propensity, duration, and intensity of sleep. Previous studies have examined this issue from the perspective of identifying the mechanisms by which the SCN or clock genes can modulate sleep or arousal4–6. Now, Meijer and colleagues report the first physiological evidence that sleep centers can, in turn, regulate neural activity in the SCN7.
Previous work clarified some of the mechanisms by which the circadian system can influence the sleep/wake cycle. For day-active animals, the SCN is thought to regulate arousal through a signal that increases throughout the day and then declines during the night. For night-active animals, like the rodents in Meijer et al.’s study7, the sign of the signal is reversed—as arousal peaks during the night. The neural circuitry mediating this signal is beginning to be understood. For example, a recent study8 identified an indirect projection from the SCN to a major brain arousal system in the locus coeruleus (LC) and demonstrated that this circuit rhythmically drives electrical activity in the LC (Fig. 1). Other arousal systems in the serotonergic raphe nuclei, the histominergic tuberomammillary nucleus, the hypocretin/orexin hypothalamic neurons, cholinergic pedunculopontine nuclei and laterodorsal tegmental area likely also receive information from the SCN9–12. But as the case is being built for circadian regulation of sleep/wake centers, the anatomical, physiological or functional evidence for communication from the sleep/wake centers back to the circadian system has been hard to find. One hint that such a connection occurs comes from a study finding that sleep deprivation can alter the phase of the circadian clock13. Nevertheless, until now the basic question of whether the sleep/wake centers communicate directly with the SCN has gone unanswered.
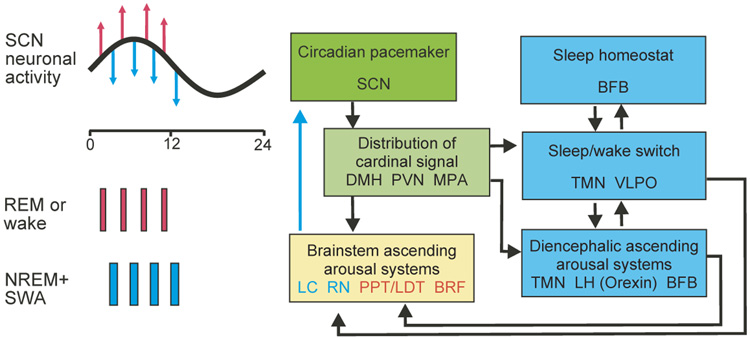
Figure 1.
Possible connections between the circadian pacemaker and the sleep/wake control systems. The circadian timing signal generated in the suprachiasmatic nucleus (SCN, green) is transmitted through nuclei in the anterior hypothalamus to sleep/wake control systems in the diencephalon (blue) and then to structures in the brainstem controlling REM–NREM cycling (yellow). A direct pathway from the dorsal medial hypothalamus (DMH) to the locus coeruleus (LC) has also been documented; both the circadian pacemaker and the sleep homeostat can influence sleep structure through these pathways. REM (red)–NREM (blue) cycling is controlled by reciprocal interactions of LC and raphe nuclei (RN) with pedunculopontine tegmental nucleus (PPT), laterodorsal tegmental nucleus (LTD) and the brainstem reticular formation (BRF). REM and NREM stages depicted on the left side correlate with acute changes in SCN neural activity7. Stage-selected sleep deprivation prohibited this modulation in firing rate7, suggesting a functional feedback pathway from the brainstem to the SCN; other pathways may be involved as well. PVN, paraventricular nucleus; BFB, basal forebrain; MPA, medial preoptic area; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic area; LH, lateral hypothalamus.
Meijer and colleagues7 tackled this question by simultaneously recording the electrical activity in the SCN and monitoring the sleep stages from rats in vivo. Although conceptually straightforward, this dual long-term recording in freely moving animals is technically quite difficult, in large part because the SCN is near the bottom surface of the brain. Specifically, the authors asked whether the central circadian pacemaker that regulates the sleep/wake switch also gets feedback about the specific sleep state. Recall that sleep can be recognized as REM (rapid eye movement) or non-REM-sleep (NREM). Within the NREM-stages, deep sleep episodes are marked by low-frequency brain waves and defined as slow-wave sleep (SWS). The amount of SWS increases after sleep deprivation and decreases as a result of sleep. For these reasons and others2,3, SWS is considered a marker for the restorative and homeostatically regulated sleep processes.
Strikingly, Meijer et al. found a clear correlation between sleep states and the neuronal activity in the SCN. The frequency of electrical activity in the SCN undergoes a daily rhythm, with higher activity found during the day in both day- and night-active animals (Fig. 1). On top of this circadian modulation of firing rate, the SCN neurons fired at lower rates during NREM sleep, and higher rates during REM sleep. A closer look at the NREM stages revealed a significant negative correlation between the SWS and the SCN activity, but no correlation with NREM sleep containing higher-frequency waves. The transitions between vigilance states were tightly paralleled by changes in SCN firing rate.
Meijer and her colleagues then put this correlation to the test by examining the effect of sleep deprivation on SCN activity. SWS or REM sleep was prevented over a two-hour period by briefly disturbing the animals as they entered these sleep stages. Neuronal activity in the SCN was significantly higher during the SWS deprivation compared to undisturbed SWS episodes. In contrast, REM deprivation led to a decrease in the mean SCN firing rate compared to controls during REM sleep. These findings are consistent with the suggestion that SWS inhibits the firing rate of neurons in the SCN, whereas REM sleep increases the firing rate. The results provide strong evidence that information about these sleep states is transmitted to the SCN.
Although the current work of Meijer and colleagues provides no direct evidence for the underlying anatomical pathways and functional significance of this feedback loop, it certainly raises some interesting possibilities. A recent study demonstrated that the firing rate of individual SCN neurons is highly correlated with the degree of expression of one of the circadian clock genes, Period14. By altering the firing rates of SCN neurons, information about sleep states can influence the molecular feedback loops that lie at the heart of the circadian timing system. This communication between the sleep homeostat and the circadian oscillator might allow the circadian system to track the amount of SWS and REM sleep during the previous daily sleep/wake cycle. Perhaps the circadian system responds to a night of insufficient sleep by making it easier to go to bed early the following day?
Unraveling the neurobiological mechanisms underlying sleep has broad implications for industrial and post-industrial societies. By some estimates, 50% of the adult population suffers from difficulties sleeping at night and staying awake during the day (2003 Sleep in America poll, National Sleep Foundation: http://www.sleepfoundation.org/NSAW/2003presskit/pk_pollhighlights.html). In older people and in patients with psychiatric and neurological disorders, this percentage is far higher. Although it would be premature to claim that the present study will have an immediate clinical impact, sleep disorders can arise from dysfunction in the circadian system, the sleep homeostat, or in communications between the two. With the work of Meijer and colleagues, we are a step closer to understanding the neurobiological basis of the coupling between the sleep homeostat and the circadian system. Understanding the basic neurobiology of sleep provides the opportunity to develop treatments that target the pathophysiology of sleep disorders rather than just the symptoms. There is a huge need for such improvements in treatments; sleep dysfunction has been estimated to cost the US economy alone around $18 billion annually due to lost productivity. Given the scale of this problem, the question is not if we can afford to sleep in this 24/7 society, but rather if we can afford not to sleep. The least we can do is to promote the research that will enable us to get a good night’s sleep.
References
- 1.Borbély AA, Achermann P. J. Biol. Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Lockley SW. J. Appl. Physiol. 2002;9:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Duffy JF, Czeisler CA. Chronobiol. Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 4.Wurts SW, Edgar DM. J. Neurosci. 2000;20:4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naylor E, et al. J. Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley CA, et al. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 7.Deboer T, Vansteensel MJ, Détári L, Meijer JH. Nat. Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 8.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. Nat. Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 9.Sutcliffe JG, de Lecea L. Nat. Rev. Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 10.Haas H, Panula P. Nat. Rev. Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 11.Pace-Schott EF, Hobson JA. Nat. Rev. Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 12.Saper CB, Chou TC, Scammell TE. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 13.Antle MC, Mistlberger RE. J. Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. J. Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]