Abstract
Aim
We report diverse phenotypic consequences of the delQKP-1507–1509 cardiac sodium channel mutation in three generations of a Chinese family.
Methods and results
Clinical and electrocardiographic (ECG), echocardiographic examination was followed by direct sequencing of SCN5A, KCNQ1, HERG, and LAMIN A/C to screen genomic DNA from blood samples. Of two mutation carriers, the proband was born with conduction disorders including second-degree atrioventricular (AV) block with prolonged QTc interval, additionally showing left anterior fascicular block (LAFB), incomplete right bundle-branch block (IRBBB), and intermittent third-degree AV block at 2 years, and clinical presentations of multiple syncope despite normal electroencephalograms at 8 years. Continuous ECG monitoring following presentation at 13 years revealed prolonged QTc and biphasic T-waves, multiple episodes of ventricular tachycardia, ventricular fibrillation, and torsades de pointes. Transthoracal echocardiography then revealed left ventricular dilatation and reduced systolic function. Another mutation carrier showed features of long QT syndrome type 3 (LQT3), LAFB, and dilated cardiomyopathy (DCM). Two additional subjects died suddenly at 13 and 33 years.
Conclusion
This data compliments and expands the spectrum of phenotypes resulting from this known gain-of-function mutation, including not only LQT3, cardiac conduction defects, and sudden death but also DCM, hitherto associated with loss-of-function mutations, for the first time.
Keywords: SCN5A, Mutation, LQT, Overlap phenotype, Dilated cardiomyopathy, Conduction disorder
Introduction
Mutations in SCN5A encoding the α-subunit of the human cardiac sodium channel have been associated with an increasingly wide range of cardiac rhythm disorders that include not only the long QT syndrome type 3 (LQT3) but also the Brugada syndrome (BrS), cardiac conduction diseases, idiopathic ventricular fibrillation, sinus node dysfunction including sick sinus syndrome (SSS), atrial standstill, dilated cardiomyopathy (DCM), and sudden infant death syndrome, each characterized by distinct electrocardiographic (ECG) and clinical features.1–6 Single, specific, SCN5A mutations can thus each result in either single or multiple phenotypes.
Mutations in SCN5A leading to LQT3 are in general associated with a gain-of-channel function with increased late sodium current leading to a delayed repolarization and prolonged action potentials. The first and the most extensively studied mutation in LQT3 has been the deletion of three amino acids KPQ at positions 1505–1507 in the cardiac sodium channel.7,8 Similarly, deletions of the three amino acid residues delQKP at position1507–1509 sharing the deletion of Q1507 with the delKPQ exclusively resulted in the same LQT3 phenotype.9 These mutations located in the DIII–DIV linker region of the cardiac sodium channel involve the inactivation process of the channel.10
The present study has identified a delQKP mutation at position 1507–1509 of the cardiac sodium channel through three generations of a Chinese family associated with an expanded spectrum of disorders: LQT3, cardiac conduction defects, DCM, and high incidence of youth sudden death. It also excluded possible mutations that would lead to amino acid changes in KCNQ1, HERG, and LAMIN A/C commonly associated with LQT1, LQT2, and DCM with conduction disorders on earlier occasions.11–13 The data thus compliments and expands the spectrum of phenotypes resulting from this known mutation, and associates the DCM phenotype with gain, and not only loss of sodium channel function for the first time.
Methods
Clinical investigation
All investigations conformed to principles defined in the Helsinki Declaration. Eleven members of a three-generation Chinese family were investigated (Figure 1), informed written consent having been obtained from each member. They included a complete medical history, physical examination, at least two 12-lead ECG recordings obtained at different times and echocardiographic scanning. Heart rates, widths of P wave, PR intervals, QRS durations, and QT intervals corrected for heart rate using Bazett's formula (QTc) were measured in limb lead II. QTc intervals were averaged from five consecutive beats of at least two 12-lead ECG recordings at different time points. Resting echocardiography was performed by experts. Global function was digitized using an IE33 system (Philips Medical Systems, Eindhoven, The Netherlands) with 1–5 MHz broadband phased-array transducer connected. Biplane end-diastolic and end-systolic volumes were calculated from the planimetered areas by computer software according to a modified Simpson's rule. Two hundred unrelated control individuals were randomly selected from a group of Chinese healthy volunteers with normal 12-lead ECGs and without reported cardiovascular history.
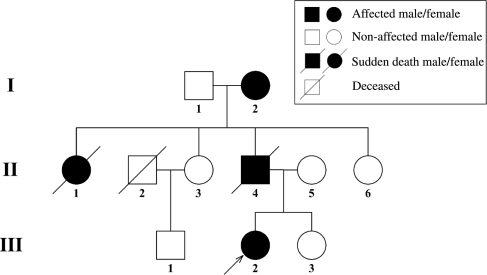
Figure 1.
Pedigree information of the family with the SCN5A-delQKP1507–1509 mutation. Filled squares denote affected male; filled circles denote affected female; open squares denote unaffected males; open circles denote unaffected females; pen square with a slash indicates deceased because of liver cancer; filled circles/square with a slash indicates sudden death and genotypes consequently are not available. Arrows indicate the proband.
SCN5A mutation analysis
Genomic DNA was extracted from 10 mL of whole blood using the Puregene Isolation kit (Gentra Systems, USA). The coding regions of SCN5A, KCNQ1, HERG, and LAMIN A/C and their exon–intron boundaries were amplified from genomic DNA by polymerase chain reaction (PCR) using a comprehensive set of primers and PCR conditions as described previously.7,13,14 Bidirectional sequencing was performed by an ABI automated cycle sequencer using an ABI BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, USA). Sequencing results were aligned with the respective wild-type sequences to detect genetic variations. The identified mutation was screened among the remaining family members by direct DNA sequencing. The mutations were further verified by single strand conformational polymorphism analysis (SSCP). The same mutation was also screened among 200 controls with the same ethnic background.
Results
Clinical features in the proband
The proband (Figure 1, subject III-2) is a 13-year-old girl, who was diagnosed with a cardiac conduction disorder identified as second-degree (Mobitz type II) atrioventricular (AV) block, prolonged QTc, and AV junctional ectopic beats, by a 12-lead ECG at 40 days after birth with heart rates ranging from 60 to 120 bpm. Further assessment at 2 months had revealed a bradycardic heart rate of 75 bpm, QTc prolongation, left axis deviation, and left anterior fascicular block (LAFB). At 4 months, she showed an atrial rate of 125 bpm and a third-degree AV block with a ventricular rate of 60 bpm. At the end of 2 years of life, the ECG conduction abnormalities consisted of a 2:1 second-degree AV block with intermittent episodes of third-degree AV block in successive ECG recordings, but an absence of significant atrial or ventricular arrhythmias. However, she did not present again with symptoms until 8 years of age, when she presented with a history of multiple episodes of syncope and seizure triggered by fits of anger that persisted over the subsequent 5 years, despite being normal to electroencephalographic examination. The episodes occurred reproducibly and were short-lived and followed by prompt and complete recovery. She also had recurrent nocturnal dyspnoeic episodes over this period.
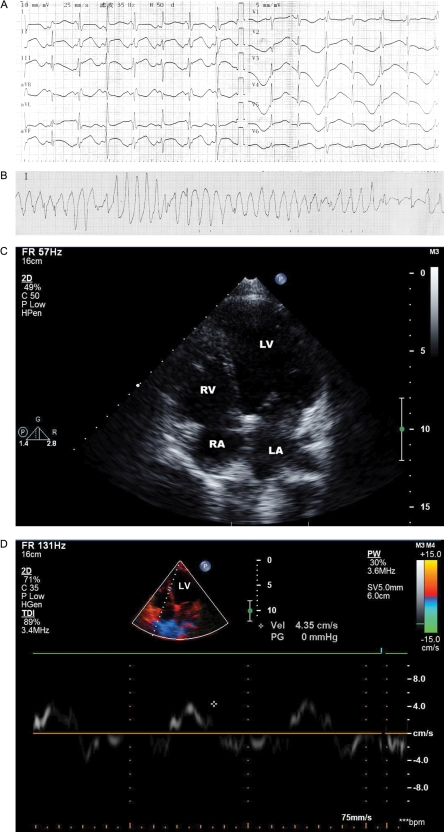
At the end of this period, she was a paediatric cardiological patient, presenting with multiple episodes of syncope and seizure. Within hours following admission, continuous ECG monitoring during such multiple episodes led to a diagnosis of ventricular fibrillation and torsade de pointe (TdP) that was reversed by electrode defibrillation. Subsequent 12-lead ECG recording revealed a sinus rate with a relative risk interval of 680 ms, which on some follow-up measurements increased to as much as 1132 ms. In addition, there was a borderline PR interval (200 ms) and normal QRS durations (100 ms), features of intraventricular conduction delay consisting of LAFB and incomplete right bundle-branch block (IRBBB). QTc interval was prolonged (750 ms) and T-waves were biphasic with a delayed onset (Figure 2A). Continuous ECG monitoring revealed self-terminating episodes of TdP (Fig. 2B). Transthoracic echocardiography revealed a LV dilatation with end-diastolic and end-systolic volumes (LVEDV and LVESV) of 156 mL and 98 mL, respectively, low ejection fraction (EF) of 37% (Table 1) and a thin ventricular wall (Figure 2C). There was a clear reduction of LV myocardial motion on Doppler tissue imaging to 4 mm/s (Figure 2D). Immediate treatment involved insertion of a temporary cardiac pacemaker; this was succeeded by β-adrenergic blocker maintenance therapy.
Figure 2.
Twelve-lead electrocardiography (ECG) and transthoracal echocardiography results of proband. (A) 12-lead ECG (25 mm/s). Leads I, II, III, aVR, aVL, aVF (10 mm/mV), and V1–V6 (5 mV/mm) showed prolonged QTc, with the former showing biphasic T waves, and the latter alternating upright and inverting T-waves (T-wave alternans). ECG records also show intraventricular conduction delay of left anterior fascicular block (LAFB) and incomplete right bundle-branch block (IRBBB). (B) An example of an episode of torsades de pointes. (C) Transthoracal echocardiographic image demonstrating dilated left ventricle by apex position. (D) Colour Doppler image showed dysfunction of the left ventricular wall (wall velocity approximately 4.35 cm/s).
Table 1.
Summary of electrocardiographic, echocardiography parameters, and clinical information of live members of the family pedigree
| Subject | Sex | Age (years) | HR (bmp) | PR interval (ms) | QRS duration (ms) | QTc (ms) | LEVDV (mL) | LEVSV (mL) | EF (%) |
|---|---|---|---|---|---|---|---|---|---|
| I-1 | M | 68 | 58 | 200 | 100 | 400 | 122 | 50 | 59 |
| I-2 | F | 65 | 88 | 200 | 120, LAFB | 550 | 188 | 124 | 34 |
| II-2 | F | 44 | 71 | 160 | 80 | 430 | 101 | 38 | 62 |
| II-4 | F | 40 | 65 | 120 | 90 | 430 | 106 | 41 | 61 |
| II-6 | F | 35 | 70 | 140 | 90 | 430 | 88 | 32 | 64 |
| III-1 | M | 18 | 75 | 160 | 100 | 440 | 92 | 34 | 64 |
| III-2 | F | 13 | 92 | 120 | 100; LAFB; IRBBB | 750 | 156 | 98 | 37 |
| III-3 | F | 11 | 73 | 140 | 90 | 430 | 136 | 54 | 60 |
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LAFB, left anterior fascicular block; IRBBB, incomplete right bundle-branch block; EF, ejection fraction. Simpson's method was used to record the data.
Family pedigree
Subject I-2 had a history of multiple episodes of syncopy at an age <55 years, but not at ages beyond this. The 12-lead ECGs revealed borderline PR intervals (200 ms) and QRS durations (120 ms), LAFB, prolonged QTc intervals (550 ms) with a significant leftward shift of the electrical axis. Echocardiographic examination demonstrated a LVEDV of 188 mL, a LVESV of 124 mL and an EF of 34% (Table 1). Subject II-4, the proband's father had already died suddenly at 33 years of age during a Valsalva manoeuvre despite having no history of syncope. Subject II-1, one of the proband's aunts had died suddenly at age 13 years after an angry argument. Subjects II-1, II-3, II-5, II-6, III-1, and III-3 appeared normal with no history of syncope, sudden death, or cardiovascular events. The 12-lead ECG's revealed normal QTc intervals and transthoracal echocardiography revealed normal cardiac structure and function. None of the subjects fulfilled criteria for arrhythmogenic right ventricular dysplasia. Table 1 shows a summary of ECG, echocardiography parameters, and clinical information about the live members of the family pedigree.
Genetic analysis
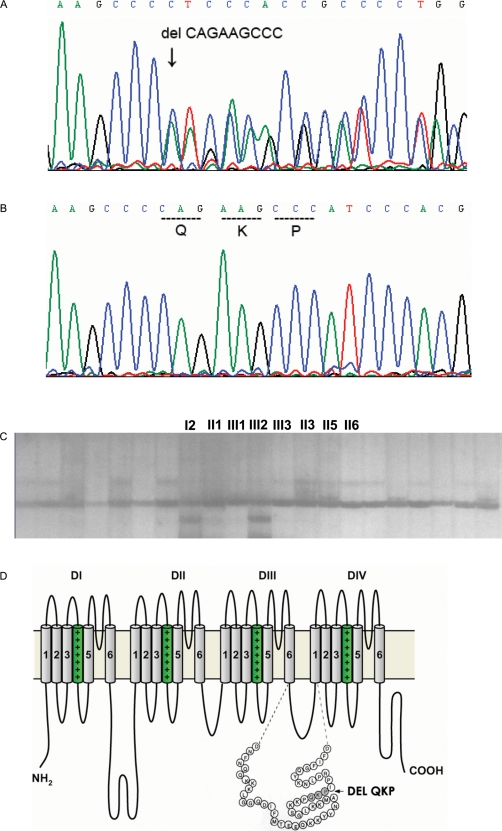
A heterozygous deletion of nine base pairs (CAGAAGCCC) in exon 26 of SCN5A (Figure 3A), corresponding to the three amino acid residues Gln1507–Lys1508–Pro1509 (QKP) was found (Figure 3B) in the proband (subject III-2) and his grandmother (subject I-2) and excluded in the remaining living subjects in this family. This mutation was confirmed using SSCP (Figure 3C). The inframe-deletion disrupts the coding sequence of QKP at the terminal part of exon 26 and is localized in the linker region between DIII and DIV of the Nav1.5 sodium channel (Figure 3D). Similar analysis excluded possible mutations that would lead to amino acid changes in KCNQ1, HERG, and LAMIN A/C commonly associated with LQT1, LQT2, and DCM with conduction disorders on earlier occasions:11–13 we did not find new mutations that would lead to amino acid changes. Three base substitutions were observed in HERG: 1480C→T, 1552C→T, and 1705A→G, but these would not have altered the encoded amino acid.
Figure 3.
delQKP1507–1509 mutation of SCN5A sodium channel. (A) A heterozygous deletion of nine base pairs (CAGAAGCCC) in exon 26 of SCN5A resulting in the deletion of three amino acids QKP Gln1507–Lys1508–Pro1509 compared with the corresponding control segments (B) of exon 26 of SCN5A. (C) SSCP confirmed two mutation carriers (proband and subject I-2). No labelled bands are control samples. (D) Position of the delQKP1507–1509 mutation on Nav1.5 channel.
Discussion
This paper associates multiple clinical cardiac phenotypes with the gain-of-function, delQKP1507–1509 sodium channel mutation, in the linker region between DIII and DIV, associated with channel inactivation, for the first time. This adds to previous reports and identifications of this mutation through three generations of a family pedigree that had then observed only a single, LQT3, phenotype.9 Previous biophysical studies of this delQKP1507–1509 mutation had revealed persistent residual sodium currents that were 1–1.5% of the maximum current magnitude measured at −30 mV nearly completely blocked by the sodium-channel blockers, tetrodotoxin and lidocaine. These findings were additionally associated with significant positive shifts of steady-state activation9 and negative shifts in inactivation.9 Such features correlated with a LQT phenotype, consistent with previous studies of other SCN5A mutations causing LQTS.7,15–17 However, persistent inward currents associated with LQT-related mutations have also been reported to result in bradycardia and sinus pauses resulting from sinoatrial node cells failing to repolarize under conditions of extra net inward current suggesting the possibility of additional phenotypic characteristics.18 The present clinical report fulfills such biophysical predictions.
These findings also complement other reports that single sodium channel mutations can result in multiple phenotypes giving rise to an ‘overlap syndrome’ as well as unique isolated phenotypes in which a mutation expected to result in a gain-of-function results in phenotypes that include changes suggestive instead of a loss-of-function.19–22 Thus, the C-terminal SCN5A mutation (1795insD) is associated with several clinical ECG features of bradycardia, conduction disease, LQT3, and BrS.23,24 There is a similar bradycardia, right ventricular conduction slowing, and QT prolongation in the corresponding Scn5a1798insD/+ murine model,25 which shows 39% reductions in peak sodium current density resulting in a similar reduction in action potential upstroke velocity accounting for conduction disorder; delays in fast inactivation, and persistent sodium currents resulting in action potential prolongation. The persistent inward current may also account for the observed bradycardia and sinus pauses resulting from sinoatrial node cells failing to repolarize under conditions of extra net inward current.18 Similarly, patients carrying a deletion of a lysine in the intracellular DIII–DIV linker (delK1500), located close to the LQT3-associated mutation delKPQ1505–1507, have also been shown to display a heterogeneous clinical phenotype with symptoms of long-QT syndrome, BrS, and conduction disease.21
However, there have been relatively few clinical reports associating Na+ channel abnormalities with DCM, most strikingly observed in 13-year-old proband, and all of these have consistently resulted in loss- rather than gain-of- function.26 Olson et al. localized a form of congenital DCM (CMD1E) characterized by progression toward atrial dilatation, frequently followed by right and, sometimes left ventricular dilatation and dysfunction27 to a heterozygous mutation (3823 G→A) in exon 21 leading to a D1275N substitution in the S3 transmembrane region of domain III of SCN5A.6 This had previously been mapped to chromosome 3p22–p25.28 It resulted in a +3.8 mV shift in sodium conductance activation curves in a Xenopus oocyte expression system.4 A more extensive analysis then identified 156 unrelated DCMpatients negative for mutations in other associated genes including those encoding cardiac actin, α-tropomyosin, and metavinculin. However, they showed alterations in four highly conserved residues in the transmembrane domains of SCN5A. Such patients also showed a range of phenotypes including SSS, atrial and ventricular arrhythmias, and congenital conduction disease. In contrast, the present findings associated DCM with a gain-of-function, delQKP1507–1509, mutation for the first time.
In summary, the present study identified a gain-of- function, delQKP, mutation at position 1507–1509 of the cardiac sodium channel in a three generation Chinese family associated with an expanded spectrum of disorder: LQT3, cardiac conduction defects, DCM and high incidence of youth sudden death. These data compliment previous reports of an expanding spectrum of phenotypes of this known mutation, as well as associating the DCM phenotype with gain-of-function sodium channel mutations for the first time.
Conflict of interest: none declared.
Funding
Grants from National Natural Science Foundation of China (NSFC, numbers 30371571 and 30672209) and grant 2003DF000037 from Ministry of Science and Technology of the People's Republic of China, and from the Wellcome Trust, Medical Research Council, and British Heart Foundation (UK). Funding to pay the Open Access publication charges for this article was provided by Wellcome Trust 077156/Z/05/Z.
References
- 1.Tan HL, Bezzina CR, Smits JP, Verkerk AO, Wilde AA. Genetic control of sodium channel function. Cardiovasc Res. 2003;57:961–73. doi: 10.1016/s0008-6363(02)00714-9. [DOI] [PubMed] [Google Scholar]
- 2.Akai J, Makita N, Sakurada H, Shirai N, Ueda K, Kitabatake A, et al. A novel SCN5A mutation associated with idiopathic ventricular fibrillation without typical ECG findings of Brugada syndrome. FEBS Lett. 2000;479:29–34. doi: 10.1016/s0014-5793(00)01875-5. [DOI] [PubMed] [Google Scholar]
- 3.Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112:1019–28. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, Sandkuijl L, et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. doi: 10.1161/01.res.0000050585.07097.d7. [DOI] [PubMed] [Google Scholar]
- 5.Wang DW, Desai RR, Crotti L, Arnestad M, Insolia R, Pedrazzini M, et al. Cardiac sodium channel dysfunction in sudden infant death syndrome. Circulation. 2007;115:368–76. doi: 10.1161/CIRCULATIONAHA.106.646513. [DOI] [PubMed] [Google Scholar]
- 6.McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–7. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, et al. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4:1603–7. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- 9.Keller DI, Acharfi S, Delacretaz E, Benammar N, Rotter M, Pfammatter JP, et al. A novel mutation in SCN5A, delQKP 1507–1509, causing long QT syndrome: role of Q1507 residue in sodium channel inactivation. J Mol Cell Cardiol. 2003;35:1513–21. doi: 10.1016/j.yjmcc.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Patton DE, West JW, Catterall WA, Goldin AL. Amino acid residues required for fast Na(+)-channel inactivation: charge neutralizations and deletions in the III–IV linker. Proc Natl Acad Sci USA. 1992;89:10905–9. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 12.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 13.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–24. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 14.Syrris P, Murray A, Carter ND, McKenna WM, Jeffery S. Mutation detection in long QT syndrome: a comprehensive set of primers and PCR conditions. J Med Genet. 2001;38:705–10. doi: 10.1136/jmg.38.10.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehrens XH, Abriel H, Cabo C, Benhorin J, Kass RS. Arrhythmogenic mechanism of an LQT-3 mutation of the human heart Na(+) channel alpha-subunit: a computational analysis. Circulation. 2000;102:584–90. doi: 10.1161/01.cir.102.5.584. [DOI] [PubMed] [Google Scholar]
- 16.Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, et al. Multiple mechanisms of Na+ channel–linked long-QT syndrome. Circ Res. 1996;78:916–24. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 17.Wang DW, Yazawa K, George AL, Jr, Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci USA. 1996;93:13200–5. doi: 10.1073/pnas.93.23.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldkamp MW, Wilders R, Baartscheer A, Zegers JG, Bezzina CR, Wilde AA. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res. 2003;92:976–83. doi: 10.1161/01.RES.0000069689.09869.A8. [DOI] [PubMed] [Google Scholar]
- 19.Smits JP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, Bhuiyan ZA, et al. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J Mol Cell Cardiol. 2005;38:969–81. doi: 10.1016/j.yjmcc.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Rossenbacker T, Carroll SJ, Liu H, Kuiperi C, de Ravel TJ, Devriendt K, et al. Novel pore mutation in SCN5A manifests as a spectrum of phenotypes ranging from atrial flutter, conduction disease, and Brugada syndrome to sudden cardiac death. Heart Rhythm. 2004;1:610–5. doi: 10.1016/j.hrthm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, et al. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest. 2002;110:1201–9. doi: 10.1172/JCI15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyndt F, Probst V, Potet F, Demolombe S, Chevallier JC, Baro I, et al. Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation. 2001;104:3081–6. doi: 10.1161/hc5001.100834. [DOI] [PubMed] [Google Scholar]
- 23.van den Berg MP, Wilde AA, Viersma TJW, Brouwer J, Haaksma J, van der Hout AH, et al. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:630–6. doi: 10.1046/j.1540-8167.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- 24.Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, et al. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85:1206–13. doi: 10.1161/01.res.85.12.1206. [DOI] [PubMed] [Google Scholar]
- 25.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–94. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 26.Tan HL. Sodium channel variants in heart disease: expanding horizons. J Cardiovasc Electrophysiol. 2006;17:S151–S157. doi: 10.1111/j.1540-8167.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenlee PR, Anderson JL, Lutz JR, Lindsay AE, Hagan AD. Familial automaticity-conduction disorder with associated cardiomyopathy. West J Med. 1986;144:33–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Olson TM, Keating MT. Mapping a cardiomyopathy locus to chromosome 3p22–p25. J Clin Invest. 1996;97:528–32. doi: 10.1172/JCI118445. [DOI] [PMC free article] [PubMed] [Google Scholar]