Abstract
Aims
Pulmonary vein isolation (PVI) with cryoenergy delivered through a balloon is a new approach in the treatment of atrial fibrillation (AF), but long-term follow-up is lacking. The aim of this study was to provide insight in the success rate and the incidence of recurrences.
Methods and results
Patients with symptomatic AF despite anti-arrhythmic drugs (AADs) were treated with cryoballoon PVI. Daily transtelephonic ECG monitoring, 24 h Holter-ECG, and an arrhythmia-focused questionnaire were used to document AF. One hundred and forty-one patients completed a follow-up of 457 ± 252 days. Before ablation, Holter-ECG showed AF in 45%, including 16% continuous AF throughout the recording. Event recording revealed a median AF burden of 26%. The questionnaire showed a median of weekly AF complaints lasting for hours. All but one patient had successful PVI with a single procedure. After ablation, AF (defined as lasting for more than 30 s) was seen in 11% of Holter-ECGs, with 1% continuous AF. The event recording showed an AF burden of 9%. The median patient reported no more AF-related symptoms. Recurrence during the first 3 months was predictive for later recurrence. A second procedure was performed in 24 patients. The freedom of AF was 59% without AADs after 1,2 procedures. Four right phrenic nerve paralyses occurred, all resolving within 6 months. No PV stenoses were observed.
Conclusion
Pulmonary vein isolation with a cryothermal balloon is an effective treatment for paroxysmal AF, resulting in a clinical success rate comparable to studies involving radiofrequency ablation. Temporary right phrenic nerve paralysis is the most important complication.
Keywords: Ablation, Atrial fibrillation, Cryoablation, Cryoballoon, Follow-up
Introduction
Pulmonary vein isolation (PVI) has become an important treatment for patients with atrial fibrillation (AF). Reports that show more than 80% freedom of paroxysmal AF during long-term follow-up have been published.1 A large number of different approaches and techniques are currently routinely employed to achieve that goal, but the procedure remains technically challenging. The development of balloon cryoablation has recently proven to be safe and effective for PVI in animals2–5 and humans.6 While greatly simplifying the technical ablation aspects and showing 60% freedom of AF after a 3-month follow-up period, long-term results remained to be studied. The scope of our study is to report recurrences of AF during the first year and complications after a cryoballoon PVI, using a wide array of follow-up modalities.
Methods
Inclusion
Between August 2005 and August 2007, a cryothermal balloon approach was used for all consecutive patients who were selected for circumferential PVI because of paroxysmal AF. All patients signed an informed consent. Inclusion criteria for ablation were symptomatic paroxysmal AF without major structural heart disease (normal left ventricular ejection fraction, no or only minor mitral insufficiency, normal to slightly enlarged left atrial diameter, assessed in the long parasternal axis). None of the patients had previously been ablated in the left atrium, and all of them had episodes of AF despite concomitant anti-arrhythmic drug (AAD) treatment.
Screening before and assessment after ablation
Event recording
During 1 month before ablation, patients were instructed to use an event recorder for transmitting a daily transtelephonic 30 s ECG strip at a fixed hour. When symptoms were experienced, additional strips could be sent. This was continued until 3 months after ablation. The obtained ECG strips were coded as sinus rhythm, atrial flutter, atrial tachycardia, or AF. The AF burden was defined as the percentage of days on which an AF episode was transmitted. The compliance of patients with this follow-up method was monitored, and when no data were sent in, they were reminded to do so.
24 h Holter
All patients were scheduled for 24 h Holter recording at baseline and at 3 months follow-up. Thereafter, additional recordings were made at the physician's discretion as guided by patient complaints. Each Holter was analysed for the presence of AF, runs of atrial tachycardia, and atrial premature beats. Sustained AF was defined as lasting for more than 30 s. If AF was present during the entire recording, it was coded as continuous. The time in AF was measured.
Quality of life questionnaire
All patients were asked to fill out a questionnaire pertaining to their complaints before ablation and at 3 months after PVI. Both the frequency and the duration of AF-related complaints were graded according to a previously described and validated protocol.6,7 After ablation, they were asked to grade their overall improvement at 3 months.
Outpatient screening and follow-up
All patients were evaluated by one of two qualified physicians (L.J. and Y.V.B.) before ablation and at 3-month intervals after ablation. At these times, an extensive history, physical examination, and 12-lead ECG recordings were made. Transthoracic echocardiography and multislice CT were performed at baseline and at 3 months. Echocardiography was used to measure the left atrial dimensions and calculate the left atrial volume. Multislice CT was used to create a 3D anatomical reconstruction of the left atrium and to measure the ostial dimensions of the pulmonary veins. During these scheduled outpatient visits, additional rhythm registrations and cardiac imaging were performed at the physician's discretion to investigate complaints or register recurrence of AF. The same was done at unscheduled visits at the outpatient clinic and at the emergency department.
Procedure
A detailed description of the cryoballoon ablation procedure has been given in a previous report.6 A cavo-tricuspid isthmus ablation was performed in seven patients because of documented isthmus-dependent flutter. The cryoballoon size was selected upon availability until February 2007 and had to be larger than the PV diameter on the CT scan. From February 2007 on, the 28 mm size was preferred. Redo procedures were performed with the same protocol as the primary procedure.
Anti-arrhythmic drugs
Anti-arrhythmic drugs were stopped 1 week before ablation. After the PVI, patients were given their habitual drug regime until 3 months after ablation. If no recurrence during the first 3 months was observed, the AADs were stopped. If recurrences were limited to the first month or if a reduction of AF burden was obtained of more than 90%, AADs were stopped at 3 months. If not, either the drug regime was altered or a redo procedure was advised. If recurrence occurred after AAD cessation, they were restarted and altered if necessary.
Endpoints
Recurrence of AF was defined as the presence of at least one recording of AF after ablation, regardless of its origin (12-lead ECG, transtelephonic rhythm strip, 24 h Holter recording, unsolicited tracing). Additional endpoints were improvement of quality of life as perceived by the patient, reduction of AF burden with ≥90%, and disappearance of AF on the 24 h Holter recording. The advice for a second procedure was based on the presence of early and late symptomatic recurrence under AAD. Finally, the results were reported according to the HRS/EHRA/ECAS recommendations.1
Follow-up after the second procedure
The follow-up after a second PVI was performed in the same way as the first procedure, omitting the baseline Holter and event recording.
Statistical analysis
Continuous variables were expressed as mean ± SD if normally distributed, or otherwise by median. Continuous variables were evaluated using Student's t-test or one-way analysis of variance. Categorical variables were expressed as percentages. The χ2 test was used for the analysis of categorical variables. A paired non-parametric test was used when appropriate. The Wilcoxon rank test was used to compare the change in symptom frequency and duration scores over time for each patient. Actuarial event-free rates from atrial fibrillation were calculated according to the Kaplan–Meier method and were compared by use of the log-rank test. The level of statistical significance was set at 0.05.
Results
Patient description
The first 141 consecutive patients treated with this technique at our institution were included in this study. Two patients were excluded from the follow-up analysis: one was excluded due to equipment failure at the time of ablation, and one developed acute pulmonary oedema for which the procedure was aborted before ablation. The demographic data are presented in Table 1.
Table 1.
Patient demographics
| Patient number (n) | 141 |
| Male/female | 100/41 |
| Age (years) | 56 ± 9 |
| Follow-up duration (days) | 457 ± 242 |
| Left atrial diameter (mm) | 42 ± 7 |
| Valvular heart disease (n) | 5 |
| Arterial hypertension (n) | 19 |
| Thyroid disease (n) | 7 |
| Amiodarone treatment (n) | 35 |
Primary procedure results
Pulmonary vein isolation was achieved in 139 patients within one procedure. This was done with a 23 mm balloon in 33 patients, a 28 mm balloon in 99 cases, and both sizes were used in seven cases. A Freezor Max (Cryocath) was used to complete PVI in 56 patients (a total of 86 veins), on average 4 ± 2 applications were needed to complete the isolation. The mean procedure time was 207 ± 79 min and the mean fluoroscopy time 50 ± 28 min. A total number of 1243 applications was given with the balloon with a mean of 9 ± 3 per patient. With the Freezor Max, 242 applications were given. Eight patients experienced pericardial effusion, including one due to rupture of the left superior pulmonary vein caused by distal cryoballoon inflation and one had a haematopneumothorax. Right phrenic nerve paralysis was observed in spite of precautions in four patients.
Follow-up duration
The mean follow-up in this prospective study was 457 ± 252 days, until 31st March 2008.
Event recording
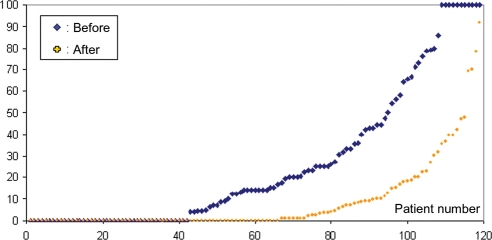
A total number of 2019 rhythm strips before and 7986 after ablation were available, of which 534 of 2019 (26%) before and 686 of 7986 (9%) after ablation showed AF. Of the entire group, 119 patients had reliably transmitted transtelephonic rhythm strips before and after ablation. On average, they transmitted 17 ± 7 strips/month before ablation and 20 ± 9 strips/month after ablation. Of this group, 42 (35%) patients showed no AF episodes on the baseline event recording. Their AF burden before and after ablation is represented in Figure 1. The reduction in AF burden was highly significant (P < 0.0001) (Table 2). In total, 66 patients (55%) did not have any recurrence of AF after ablation on this event recording (P < 0.005). When looking at patients with recurrence, the AF burden still showed a significant reduction after ablation (P < 0.0001). The baseline burden between those with and without recurrence did not differ significantly.
Figure 1.
Atrial fibrillation (AF) burden as calculated from transtelephonic ECG recordings sorted ascendingly by the burden before pulmonary vein isolation (before) and burden sorted ascendingly after isolation (after). The area between both curves represents the reduction in AF burden for the entire group.
Table 2.
Results of event recording 1 month before and 3 months after ablation
| Total group |
Recurrence |
No recurrence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | ||
| Proportion with AF | n | 77/119 | 53/119 | <0.005 | 41/53 | 53/53 | NA | 36/66 | 0/66 | NA |
| AF burden (%) | Mean ± SD | 27 ± 32 | 9 ± 17 | <0.0001 | 33 ± 34 | 19 ± 21 | <0.0005 | 21 ± 33 | 0 | NA |
| Median (range) | 14 (0–100) | 0 (0–92) | 20 (0–100) | 10 (1–92) | 5 (0–100) | 0 (0–0) | ||||
Data from daily transtelephonic event recording for the patients with recordings before and after the intervention. Burden means the percentage of days with AF present in a recording. AF, atrial fibrillation; NA, not applicable; n, number; SD, standard deviation.
24 h Holter recording
In total, 128 patients had performed a 24 h Holter recording before and 129 patients 3 months after ablation. Before ablation, 58 (45%) patients had AF documented on their Holter recording, of whom 21 (16%) patients had continuous AF during the entire 24 h. After ablation, this decreased to 14 (11%) patients with AF on the Holter, of whom 1 (1%) patient had continuous AF during the entire 24 h. The median time in AF for those with paroxysmal AF decreased from 19 to 8%. Heart rate during sinus rhythm did not change significantly before and after ablation (67 ± 11 vs. 68 ± 10 b.p.m.).
Quality of life
Patients assessed the frequency, duration, and improvement of their complaints. The median scores (as explained in Table 3) for frequency and duration before were, respectively, 3 (range 1–5) and 3 (range 1–5), being weekly episodes lasting for one or more hours. After ablation this was significantly reduced to median values of 1 and 1 (ranges for both 1–5), being no more complaints (P < 0.01). Paired data were available in 125 patients. After a 3-month follow-up period, 90 (72%) of the patients considered themselves improved, 22 (18%) considered their symptoms as equal, and 13 (10%) considered their symptoms as worse.
Table 3.
Score for AF episode frequency and duration
| Score | Frequency of AF episodes | Duration of AF episodes |
|---|---|---|
| 1 | None | None |
| 2 | Monthly | Minutes/seconds |
| 3 | Weekly | Hours |
| 4 | Daily | Days |
| 5 | Incessant | Incessant |
AF, atrial fibrillation.
Clinical long-term follow-up results
From the initial procedure, until redo or 31st March 2008, an additional 597 rhythm strips and 191 Holter recordings were obtained from the entire group either at the routine follow-up visits or when presenting with complaints. On average, a patient had 10 ± 9 rhythm strips taken during the rest of his follow-up and had 1.3 ± 0.7 Holters performed after the initial 3 months.
Freedom from recurrent atrial fibrillation
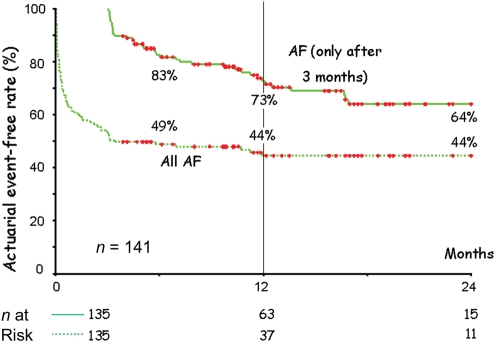
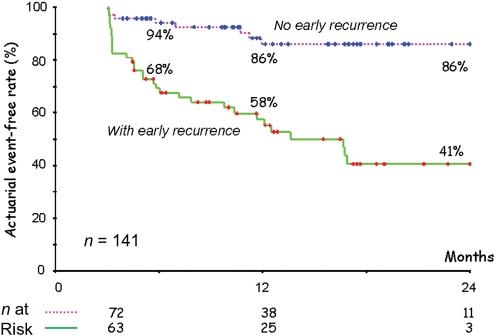
Combining all these resources, actuarial event-free rates from any AF were calculated (Figure 2). The event-free survival rate at 365 days was 44%. When all events in the first 3 months were blanked, and the curve was constructed from 90 days on, the event-free survival rate at 365 days was 73% (P < 0.0001). When patients with and without a recurrence in the first 3 months were compared (Figure 3), it became clear that a recurrence in the first 3 months was highly predictive for recurrence after 3 months, whereas the absence of events in the first 3 months was highly predictive for a low recurrence rate (log-rank 23, P < 0.0001).
Figure 2.
Event-free survival curve for atrial fibrillation (AF) after a single ablation procedure. The two curves represent the same patient population. The upper curve (AF only after 3 months) represents the event-free survival after a 3-month blanking period; the second curve (All AF) represents the event-free survival without the 3-month blanking period. The patient numbers of both groups are represented at the bottom.
Figure 3.
Event-free survival curve for atrial fibrillation (AF) after a single ablation procedure, employing 3-month blanking period. The upper curve (no early recurrence) is the patient population that did not have recurrence of AF during the blanking period. The lower curve (with early recurrence) represents the group that experienced recurrence of AF during the 3-month blanking period. Patient numbers of both groups are represented at the bottom.
Recurrence of atrial fibrillation and anti-arrhythmic drug treatment
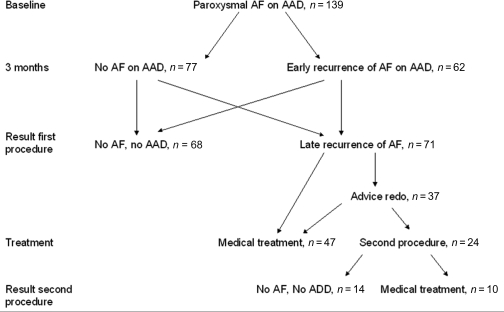
After one procedure, 49% (68/139) were free from AF without AADs. Of the remaining 51% (71/139) with AF recurrence, 27% (37/139) were advised to undergo a second procedure and 24% (34/139) continued on medical treatment due to a decrease in AF burden. In total, 34% (47/139) continued on medical treatment and 17% (24/139) agreed to undergo a second procedure. These data are represented in Figure 4.
Figure 4.
Graphical representation of the overall 1-year clinical outcome after a mean of 1,2 procedures, presented as patient numbers. AF, recurrence of atrial fibrillation; AAD, anti-arrhythmic drugs.
Recurrence of atrial fibrillation after a second procedure
Of the 37 patients to whom a redo procedure was advised (always after 3 months), 24 underwent a second procedure. On average, there was reconduction to 3 ± 1 PV. Reconduction was found in 20/24 (83%) left superior PVs, in 22/24 (92%) left inferior PVs, in 17/24 (71%) right superior PVs, and in 14/24 (58%) right inferior PVs. During this second procedure, all reconducting veins were successfully isolated with the same technique. The average follow-up after the second procedure was 225 ± 137 days. During this follow-up, eight patients had an early recurrence after a mean interval of 15 days; three had a late recurrence after 3 months, making the total number of patients with recurrence after a redo procedure 46% (11/24).
Overall recurrence of atrial fibrillation
After a mean of 1,2 procedures, 82/139 (59%) of the patients were free from AF without AADs and 57/139 (41%) of the patients were under medical treatment, with a reduced AF burden.
Long-term complications
Multislice CT scan showed that there was no significant difference in PV diameter before and after the procedure. The mean PV diameter was 18.0 ± 3.8 mm before the procedure vs. 18.1 ± 3.7 mm after. Two patients complained of haemoptysis during the first month after PVI, but without PV stenosis on the multislice CT scan. In both of these patients, the problem did not recur after temporary cessation of the anticoagulation therapy. The haematopneumothorax resolved completely. Two patients needed transfusion, because of a haematoma in the groin and a retroperitoneal bleeding, respectively. Two arteriovenous fistula were reported. Four asymptomatic right phrenic nerve paralyses were observed, persisting at discharge. Three patients had recovery of their diaphragm movement at 3 months; all four had recovered at 6 months. One perimitral flutter was documented and successfully ablated.
Discussion
We describe in this paper the clinical 1-year follow-up of a large consecutive group of patients treated with a cryothermal balloon approach. The major finding is that we had a clinical freedom from AF comparable to studies using radiofrequency (RF) ablation. Further, early recurrence was indicative for later clinical failure and associated with reconduction to the veins.
Complications
The potential advantages of cryoenergy were already described in animals and have been suggested for humans as well.3,8 No thromboembolism was seen in our group, which seems equivalent to similar RF populations. In contrast to ostial RF ablation,1 comparison of ostial PV diameters obtained from serial CT shows again that cryothermal energy, although being delivered at the antral and ostial regions, causes no PV stenosis.8 Although a rare complication in RF ablation, we found a 4% incidence of right phrenic nerve paresis, with complete recovery in all of them at the end of follow-up. A large multicentre study found this in around 8% of the cases, also reporting complete recovery in all. This complication was also reported in different balloon delivery systems, independent of the energy used (ultrasound, high intensity ultrasound).9,10 In RF ablation, the complete recovery of this nerve can only be expected in 66% and partial recovery in 17%.11
Freedom of atrial fibrillation
The event-free survival in our analysis is comparable with data in the worldwide survey on RF ablation of paroxysmal and persistent AF.12 After a single procedure, 73% reported symptom improvement, coinciding roughly with the advice for a second procedure in 23%, which was given after objective recurrences in spite of AAD therapy after 3 months. Previously published cryoablation studies in the literature show success rates varying between 56% freedom of AF after 1 year (including 21% on AAD)8 and 71% freedom of AF after 4 years (including 22% on AAD) with a segmental isolation.13 A cryoballoon study with a very limited number of patients shows freedom of AF in 90% of the cases after 6 months,14 whereas a multicentre study reveals sinus rhythm in 74% of the patients after 1 year without AAD, in paroxysmal AF, and 42% without AAD in persistent AF.15 Our study yields a lower success rate, with 59% being free from AF at 1 year without the use of AAD. This is probably due to a difference in follow-up method, since no large difference in patient characteristics is obvious.
Reconduction
Previous studies have shown AF recurrence to be associated with reconduction in the PVs.16–18 This was also true for our study. In the patients considered for a repeat procedure, we found high rates of reconduction from the left atrium to the PVs.
Limitations
The major limitation of this prospective study is that it is observational, and therefore, substantive conclusions cannot be drawn regarding its relative advantages or disadvantages compared with RF ablation. For this a randomized head-to-head comparison would be required.
A second limitation is the less intense follow-up after the initial 3-month period. Although a large effort was made to document long-term clinical efficacy, daily transtelephonic event recording over a very long period proved to decrease patient compliance dramatically, so that this was not a feasible method.
Conclusions
The data we present indicate that cryoablation with a balloon delivery system yields similar results to those reported on RF ablation, and comparable to other cryoballoon trials. An acceptable complication rate was observed.
Funding
Funding to pay the Open Access publication charges for this article was provided by the Department of Clinical Electrophysiology, Thoraxcentre, Erasmus Medical Centre, Rotterdam.
Conflict of interest: The first author received a minor consultancy fee from Cryocath.
References
- 1.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Avitall B, Lafontaine D, Rozmus G, Adoni N, Dehnee A, Urbonas A, et al. Ablation of atrial-ventricular junction tissues via the coronary sinus using cryo balloon technology. J Interv Card Electrophysiol. 2005;12:203–11. doi: 10.1007/s10840-005-0339-5. [DOI] [PubMed] [Google Scholar]
- 3.Avitall B, Lafontaine D, Rozmus G, Adoni N, Le KM, Dehnee A, et al. The safety and efficacy of multiple consecutive cryo lesions in canine pulmonary veins-left atrial junction. Heart Rhythm. 2004;1:203–9. doi: 10.1016/j.hrthm.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 4.Garan A, Al-Ahmad A, Mihalik T, Cartier C, Capuano L, Holtan D, et al. Cryoablation of the pulmonary veins using a novel balloon catheter. J Interv Card Electrophysiol. 2006;15:79–81. doi: 10.1007/s10840-006-8519-5. [DOI] [PubMed] [Google Scholar]
- 5.Sarabanda AV, Bunch TJ, Johnson SB, Mahapatra S, Milton MA, Leite LR, et al. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol. 2005;46:1902–12. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Van Belle Y, Janse P, Rivero-Ayerza MJ, Thornton AS, Jessurun ER, Theuns D, et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28:2231–7. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 7.Scholten MF, Thornton AS, Mekel JM, Jordaens LJ. Targets and endpoints in ablation therapy for atrial fibrillation in the light of pathophysiological mechanisms. J Interv Card Electrophysiol. 2006;15:27–33. doi: 10.1007/s10840-006-6334-7. [DOI] [PubMed] [Google Scholar]
- 8.Tse HF, Reek S, Timmermans C, Lee KL, Geller JC, Rodriguez LM, et al. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol. 2003;42:752–8. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]
- 9.Natale A, Pisano E, Shewchik J, Bash D, Fanelli R, Potenza D, et al. First human experience with pulmonary vein isolation using a through-the-balloon circumferential ultrasound ablation system for recurrent atrial fibrillation. Circulation. 2000;102:1879–82. doi: 10.1161/01.cir.102.16.1879. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Antz M, Ernst S, Ouyang F, Falk P, Chun JK, et al. Pulmonary vein isolation by high-intensity focused ultrasound: first-in-man study with a steerable balloon catheter. Heart Rhythm. 2007;4:575–84. doi: 10.1016/j.hrthm.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Sacher F, Monahan KH, Thomas SP, Davidson N, Adragao P, Sanders P, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47:2498–503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 12.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 13.Moreira W, Manusama R, Timmermans C, Ghaye B, Philippens S, Wellens HJ, et al. Long-term follow-up after cryothermic ostial pulmonary vein isolation in paroxysmal atrial fibrillation. J Am Coll Cardiol. 2008;51:850–5. doi: 10.1016/j.jacc.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 14.Klein G, Oswald H, Gardiwal A, Lusebrink U, Lissel C, Yu H, et al. Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008;5:802–6. doi: 10.1016/j.hrthm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273–8. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Callans DJ, Gerstenfeld EP, Dixit S, Zado E, Vanderhoff M, Ren JF, et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1050–5. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 17.Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226–9. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 18.Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–35. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]