Abstract
The zebrafish (Danio rerio) embryo has emerged as an important model of vertebrate development. As such, this model system is finding utility in the investigation of toxic agents that inhibit, or otherwise interfere with, developmental processes (i.e. developmental toxins), including compounds that have potential relevance to both human and environmental health, as well as biomedicine. Recently, this system has been applied increasingly to the study of microbial toxins, and more specifically, as an aquatic animal model, has been employed to investigate toxins from marine and freshwater microalgae, including those classified among the so-called “harmful algal blooms” (HABs). We have developed this system for identification and characterization of toxins from cyanobacteria (i.e. “blue-green algae”) isolated from the Florida Everglades and other freshwater sources in South and Central Florida. Here we review the use of this system as it has been applied generally to the investigation of toxins from marine and freshwater microalgae, and illustrate this utility as we have applied it to the detection, bioassay-guided fractionation and subsequent characterization of developmental toxins from freshwater cyanobacteria.
Keywords: Zebrafish, Danio rerio, Vertebrate development, Toxins, Algae, Model system, Cyanobacteria, Everglades
1. Introduction
Marine and freshwater microalgae, encompassing photosynthetic microbial representatives from both prokaryotic (e.g. cyanobacteria) and eukaryotic (e.g. dinoflagellates, diatoms) taxa, are known to produce a myriad of toxic or otherwise bioactive metabolites (Shimizu, 1996; Gerwick et al., 2001; Shimizu, 2003). These compounds are finding applications in biomedicine, including use as pharmacological and toxicological research tools, as well as development of potential pharmaceuticals (Mayer and Lehmann, 2001; Mayer and Gustafson, 2003). Moreover, as these microbes are ubiquitous in aquatic environments worldwide, the possible relevance of these toxic metabolites to human and environmental health is receiving growing attention (Duy et al., 2000; Hitzfield et al., 2000; Carmichael, 2001; Rao et al., 2002).
In particular, many of these toxigenic algae are categorized among the so-called “harmful algal blooms” (HABs) owing to the recognized impacts they have on human and environmental health when they occur in high abundance. Typically, HAB toxins are most closely associated with cases of acute poisoning, specifically including toxicoses resulting from direct exposure of humans to toxins via aerosolized seawater (e.g. Florida Red Tides) or contaminated drinking water, or from indirect exposure to toxins which bioaccumulate in fish and shellfish (e.g. paralytic shellfish poisoning, ciguatera). Based on such acute effects, toxicology of these metabolites most often focuses exclusively on those targets which interfere with immediate and pronounced cell-, tissue- or organ-specific function, including toxicoses related to neuronal action (i.e. neurotoxicity), gastrointestinal and hepatic functions (e.g. diarrhetic shellfish poisoning, hepatoenteritis), respiratory and pulmonary function (e.g. bronchoconstriction associated with Red Tide) and dermal responses (i.e. dermatotoxicity).
It is becoming increasingly clear, however, that HAB toxins, including many of the same species associated with acute poisonings, may additionally exert an array of more insidious, chronic effects. Indeed, toxic algal metabolites frequently have multiple targets or otherwise broad mechanisms of action such as inhibition of phosphatases and DNA damage that may potentially affect multiple cellular processes. Associated targets of the chronic effects of these toxins, and even some of the toxins themselves, may consequently remain largely unrecognized and uncharacterized when these effects are obscured by the more immediate acute effects.
Of particular concern are those toxins that either interfere with processes related to maintenance of cell-cycle control and cell differentiation, or cause DNA damage. Such effects may lead directly (via genotoxicity) or indirectly (via tumor promotion) to carcinogenesis. For example, the hepatotoxic microcystins, most widely associated with acute poisoning (Beasley et al., 2000; Fischer and Dietrich, 2000; discussed further below), are also recognized tumor promoters, and chronic exposure via contamination of drinking water by microcystin has been linked to high incidence of liver cancer in China (Yu, 1989).
There is also growing concern and evidence (e.g. Pilotto et al., 1999) that algal toxins, by interfering with the coordination of rapidly dividing and differentiating cells, may perturb pathways and processes as they relate to vertebrate development (i.e. “developmental toxins”). Such effects would have obvious direct relevance to human, animal and environmental health including possible concerns related to infertility, birth defects and other developmental abnormalities. Indeed, review of the human health impacts associated with HAB toxins (e.g. Hitzfield et al., 2000) indicates that children are often at particular apparent risk, suggesting perhaps that the relationship between toxicity and developmental stage may not be only limited to embryogenesis, but more generally to continuing vertebrate development.
Fish and other aquatic animals have been shown to be useful models for a variety of diseases such as cancers (Meierjohann et al., 2004; Rahn et al., 2004), anemia (Shafizadeh et al., 2004) and glaucoma (McMahon et al., 2004) as well as for toxicological studies including responses to environmental pollutants such as dioxin (Butler et al., 2004; Yamauchi et al., 2005) and heavy metals (Feng et al., 2003; Bielmyer et al., 2005). As a means to identify and characterize toxins from marine and freshwater microalgae, aquatic animals models have a number of practical advantages. In terms of investigating developmental toxins, embryos of various teleost fish have been specifically explored as potential models for vertebrate development (e.g. Berman et al., 2005; Drummond, 2005; Morris and Fadool, 2005; Puffett-Lugassay and Zon, 2005; Sherwood and Wu, 2005), and consequently for the investigation of toxins that disrupt the pathways and processes related to development. Of these, the embryo of the zebrafish (Danio rerio) is finding considerably utility owing to a number of practical advantages.
Advantages of the zebrafish embryo as a toxicological model, particularly in relation to developmental toxins, include a small size, a nearly transparent embryo, ease of husbandry, high fecundity, rapid embryogenesis and a growing understanding of its genome. The nearly transparent zebrafish embryos are approximately 1-mm in diameter (and surrounded by a nearly transparent chorion) making them easily observable with standard dissecting light-microscope, and conversely small enough to be utilized in a high-throughput (e.g. 96-well plate) format. The embryos can be reared in a wide array of media, and are reasonably tolerant to a variety of solvents and buffers used in toxin extraction and isolation. Laboratory breeding can produce more than sufficient numbers of embryos, typically on the order of hundreds and even thousands of eggs with a single daily breeding. The entire development of the embryo from a fertilized oocyte to a free-swimming hatched larval fish is approximately 5 days. Finally, the growing knowledge of the zebrafish genome, including a completed genome sequence, and an equally growing “library” of developmental mutants, enables toxicological findings to be increasingly related to the possible genes involved.
Here we review applications of the zebrafish embryo (as well as other freshwater fish models) as applied to the investigation of developmental toxins from marine and freshwater algae. In addition, we present primary data from our on-going study of cyanobacteria isolated from the Florida Everglades and other freshwater sources in South and Central Florida to illustrate the utility of this aquatic animal system for identification (i.e. screening), as well as subsequent purification (i.e. bioassay-guided fractionation) and characterization of, naturally occurring developmental toxins.
1.1. Freshwater fish as models for developmental toxins from marine dinoflagellates
Of the HAB toxins, perhaps the most widely recognized, and best characterized, are those from various marine protists, particularly including members of the Bacillariophyta (diatoms), Dinoflagellata (dinoflagellates) and related Raphidophyta phyla. Notable examples of these toxic HABs, and the toxins they produce, are given in Table 1. As discussed above, most of the focus on these HAB toxins has been on acute health effects, particularly including neurological symptoms (e.g. paralytic shellfish poisoning, amnesic shellfish poisoning), bronchial and pulmonary effects (e.g. aerosolized Red Tide), and gastrointestinal illness (e.g. diarrhetic shellfish poisoning).
Table 1.
Marine and freshwater HABs associated with human and environmental health concerns
| Organism(s) | Toxin(s) | “Target” | Toxicoses |
|---|---|---|---|
| Protista | |||
| Dinoflagellata (“dinoflagellates”) | |||
| Karenia brevis | Brevetoxins (PbTx) | Activates voltage-gated Na+ channels | “Florida Red Tide”, “Neurotoxic Shellfish Poisoning” |
| Gambierdiscus toxicus | Ciguatoxin (CTx) | Activates voltage-gated Na+ channels | “Ciguatera” |
| Alexandrium spp. | Saxitoxin (STx) | Inhibits voltage-gated Na+ channels | “Paralytic Shellfish Poisoning” |
| Dinophysis spp., Prorocentrum lima | Okadaic Acid (OA), Dinophysistoxins, Pectenotoxin, Yessotoxin | Phosphatase inhibition | “Diarrhetic Shellfish Poisoning” |
| Protoperidinium crassipesa | Azaspiracids (AZA) | Unknown | Gastrointestinal and neurological symptoms |
| Bacillariophyta (“diatoms”) | |||
| Pseudonitzchia spp. | Domoic Acid (DA) | Glutamate receptor agonist | “Amnesic Shellfish Poisoning” |
| Monera (Bacteria) | |||
| Cyanobacteria | |||
| Microcystis aeruginosa (and other genera/species) | Microcystins | Inhibit Ser/Thr phosphatases (PP1, PP2a) | Hepatotoxic, tumor promotion |
| Nodularia spumigens | Nodularin | Inhibit Ser/Thr phosphatases (PP1, PP2a) | Hepatotoxic, tumor promotion |
| Anabaena spp. | Anatoxin-a; STx | Mimics acetylcholine (see STx, above) | Neurotoxic |
| Aphanizomenon flos-aquae | Anatoxin-a(s); Anatoxin, STx | Acetylcholinesterase inhibition (see anatoxin-a and STx, above) | Neurotoxic |
| Cylindrospermopis raciborskii | Cylindrospermopsin | Inhibits protein synthesis | Hepatotoxic |
Species/genera and their toxins, as well as the associated targets and toxicoses, are given.
Proposed to be the algal source of azaspiracids, but not confirmed.
However, growing evidence suggests that these same toxins may either have mechanisms of action that affect multiple targets or alternative mechanisms that could potentially interfere with processes of cell maintenance, division and differentiation, as well as genotoxic (i.e. DNA damaging) effects. All of these mechanisms may have profound impacts on development. Okadaic acid (OA), for example, is well recognized as a potent inhibitor of the serine/threonine protein phosphatases, PP1 and PP2A (Bialojan and Takai, 1988). Typically, OA is most commonly associated with diarrhogenic effects (i.e. diarrhetic shellfish poisoning [DSP]), perhaps due to cell-specific cytotoxicity to intestinal cells, although the link between phosphatase inhibition and this effect is still unclear (Leira et al., 2003). Protein phosphatases are also recognized to be involved in wide array of signal transduction pathways and various other related cellular functions including those related to maintaining cellular viability and coordinating cell division. Accordingly, recent studies have demonstrated that OA impairs cell division, particularly via interference with F-actin (Fiorentini et al., 1996; Leira et al., 2003) and inhibition of chromosomal separation, leading to aneuploidy and polyploidy (Hégarat et al., 2005), both of which may be related to the general mechanism of phosphatase inhibition.
In other cases, such effects are presumably unrelated to the recognized modes-of-action commonly associated with the toxins. For example, brevetoxins (PbTx) from the Red Tide dinoflagellate, Karenia brevis, are typically associated with potent activation of voltage-gated sodium channels and consequent neurotoxicity, but have recently been shown to cause DNA damage in human leucocytes at picomolar concentrations (Sayer et al., 2005). Likewise, domoic acid (DA), most commonly associated with neurotoxic effects, specifically including so-called amnesic shellfish poisoning (ASP), is now recognized to exert genotoxic effects via DNA damage at equally low concentrations (Dizer et al., 2001).
Several of the marine HAB toxins have been investigated as developmental toxins, specifically including studies employing embryos of zebrafish or other freshwater fish species (see below). For example, recent studies of DA by Tiedeken et al. (2005) utilized microinjection of zebrafish eggs to demonstrate morphological and behavioral dysfunction in embryos treated with the toxin. Specifically, dose-dependent effects were observed on hatching rate, and neurobehavioral parameters, including convulsions, stereotypically incessant movement of pectoral fins and impairment of touch–swim response, as well various morphological deformities (e.g. pericardial swelling, curvature of the spine) that consequently decreased survival rate (Tiedeken et al., 2005). Given the recognized neuroexcitatory effects of DA via glutamate receptors, the observed behavioral dysfunction is perhaps not surprising, however, specific mechanisms for these and other effects, particularly including morphological abnormalities, have not been clarified. That said, observed behavioral effects of DA in zebrafish embryos parallel those reported for rodent models (Xi et al., 1997; Doucette et al., 2000) that specifically indicate structural changes in the hippocampus of DA-exposed neonatal rats (Dakshinamurti et al., 1993).
Similarly, Lefebvre et al. (2004) demonstrated, using the zebrafish embryo, apparent impairment of neurodevelopment by neurotoxic saxitoxin (STx), the causative agent of paralytical shellfish poisoning (PSP). Exposure of zebrafish embryos to STx in aqueous rearing media from 0 to 6 days post fertilization (dpf) resulted in consistent morphological abnormalities, including edemas of the eye, pericardium and yolk sac, as well as curvature of the spine, particularly at the highest concentrations (≥372 μg STx/L). Moreover, zebrafish exposed to all concentrations of STx showed stage-specific sensorimotor deficits, including reduced touch response and total paralysis. Interestingly, there was an apparent recovery from paralysis by 6 dpf at lower doses, and, furthermore, the observed developmental effects, including some degree of the physical abnormalities, were reversible following depuration in STx-free water (Lefebvre et al., 2004). These latter observations may provide insight to a possible mechanism. Specifically, the authors suggest that more pronounced inhibition of voltage-gated sodium channels in the Rohon–Beard (RB) subpopulation of neurons, versus less STx-sensitive dorsal root ganglions (DRGs) which replace the RB neurons after ∼5 dpf in developing embryos, may explain the eventual recovery from paralysis in the lower doses. Furthermore, the authors suggest, based on previous related observations, that some amount of the physical malformations may be related to impaired movement and paralysis. Specifically, it is proposed that this paralysis results in fluid imbalance, and subsequent edemas that, in turn, lead to craniofacial abnormalities; this mechanism would consequently explain the apparent reversibility of the observed edemas following depuration and recovery from paralysis (Lefebvre et al., 2003). Interestingly, based on these proposed explanations, the observed effects could consequently be attributed largely to neurotoxicity of STx (i.e. inhibition of neuronal function), rather than cytotoxicity, genotoxicity or other mechanism, as generally proposed above for developmental toxins.
In addition to the zebrafish embryo, developmental toxicity of several marine HAB toxins has been shown in other fish models of development, and specifically the Japanese medaka (Oryzia latipes) embryo. For example, both PbTx and the chemically related ciguatoxin (CTx) which both act as neurotoxins via activation of voltage-gated sodium channels, have been shown to impair development of medaka embryos (Edmunds et al., 1999; Kimm-Brinson and Ramsdell, 2001; Colman and Ramsdell, 2003; Colman et al., 2004). Developmental effects, following microinjection of medaka oocytes with both PbTx and CTx, were observed at sub-nanogram and -picogram (per egg) quantities, respectively, and included, in both cases, curvature of the spine, hyperkinesis and cardiovascular (i.e. heart rate) effects (Edmunds et al., 1999; Kimm-Brinson and Ramsdell, 2001; Colman and Ramsdell, 2003; Colman et al., 2004). Interestingly, there was an observed difference between two congeners of the brevetoxins tested, namely PbTx1 and PbTx3, in terms of their cardiovascular response; specifically, PbTx3-treated (as well as CTx-treated) embryos exhibited persistent tachycardia (Colman and Ramsdell, 2003), as opposed to delayed bradycardia in those exposed to PbTx1 (Kimm-Brinson and Ramsdell, 2001). In addition, both PbTx1 and PbTx3 lead to apparent dysfunction in hatching of embryos, specifically by abnormal “head-first hatching”, as opposed to normal, “tail-first” emergence, consequently resulting in increased mortality among the toxin-treated embryos (Kimm-Brinson and Ramsdell, 2001; Colman and Ramsdell, 2003).
Developmental toxicity of the recently discovered HAB toxin, azaspiracid-1 (AZA-1), which has been associated with severe human intoxication via consumption of contaminated shellfish, was also recently investigated utilizing the medaka embryo model (Colman et al., 2005). Dose-dependent effects were seen in terms of reduced cardiovascular function (i.e. bradycardia), delayed onset of blood circulation and pigmentation, and decreased hatching success, as well as general retardation of growth at higher concentrations (≥40 pg/egg). Interestingly, when extracts of AZA-contaminated mussels were evaluated in this system, additional hyperactivity of embryos was observed, perhaps due to other forms of the toxin (i.e. AZA-2 and -3) or other toxins (i.e. OA and dinophysistoxin-2) also detected in the sample.
Similar evaluations of PbTx, CTx and AZA (and other marine dinoflagellate toxins) in the zebrafish system remain to be conducted, though it has been suggested in standardization studies that data for toxicity tests in zebrafish and medaka embryos are largely comparable (Braunbeck et al., 2005). Moreover, with continuing standardization of the zebrafish as a toxicological model more generally, it is highly likely that studies of these, and other marine HAB toxins, in the zebrafish system will soon emerge.
1.2. Freshwater fish as models for developmental toxins from cyanobacteria
In addition to these various eukaryotic marine species (discussed above), another group of HAB organisms, namely the prokaryotic cyanobacteria, or “blue-green algae”, is receiving growing attention in terms of human and environmental health concerns. The cyanobacteria are recognized to produce a diverse array of toxic or otherwise biologically active metabolites (see Gerwick et al., 2001). Although ubiquitous in their distribution, cyanobacteria as HABs are almost exclusively associated with freshwater environments where they have been linked to a range of acute and chronic illness, particularly via direct exposure to the toxin in contaminated drinking water or similar routes (Hitzfield et al., 2000). Examples of perhaps the best-recognized toxigenic cyanobacteria associated with various negative impacts as HABs are presented in Table 1.
Of these, the most widely recognized and best characterized are the microcystins (MCs). Approximately 70 variants of these heptapeptide toxins have been described, particularly from the freshwater species, Microcystis aeruginosa, although they have also been found in several other species/genera (Botes et al., 1982; Namikoshi et al., 1990, 1992). As potent inhibitors of protein phosphatases, and specifically the PP1- and PP2A-type catalytic subunits, the microcystins are associated with a range of acute effects, including arrest of cardiac and renal function and circulatory shock (Beasley et al., 2000; Fischer and Dietrich, 2000). However, as they accumulate primarily in the liver (specifically via ATP-dependent transporters in hepatocytes) where they cause hemorrhaging and various severe effects, the microcystins are largely considered hepatotoxins. Moreover, in addition to these acute effects, exposure to microcystins, as recognized tumor promoters, has also been associated specifically with increased incidence of primary liver cancer (Yu, 1989; discussed above).
Given this recognized biological activity, developmental toxicity of the microcystins has been investigated using zebrafish and other freshwater fish models in several studies spanning nearly ten years. The first reported study (Oberemm et al., 1997) investigated developmental toxicity of microcystin–LR (MC–LR), the most common of the microcystin variants, using the zebrafish embryo model. Specifically, these investigators utilized direct submersion of embryos in MC–LR treatments to assess developmental toxicity. Using this approach, it was shown that MC–LR had no overt effect on embryonic development (0 to 6 dpf), compared to controls, though subsequent rearing of the pre-exposed larvae in microcystin-free water for 21 days showed that MC–LR exposure decreased survival rate and body weight by as much as 40% and 25%, respectively, at the highest concentrations (5 and 50 μg/L), as well as minimally reducing body length (Oberemm et al., 1997).
More recently, however, the investigation of the developmental toxicity of microcystins has been furthered considerably by the use of microinjection of both medaka (Jaquet et al., 2004) and zebrafish (Wang et al., 2005) embryos. Unlike many of the marine HAB toxins (discussed above), microcystins are water-soluble metabolites. Recognizing that the chorion may present a significant barrier to the water-soluble toxin, Jaquet et al. (2004) employed microinjection techniques to expose medaka embryos to MC–LR by direct injection into one-cell stage embryos, and intravitelline injection during late neurulation (1 dpf) and early organogenesis (2 dpf) of embryos. At MC–LR concentrations as low as 100 pg/mL, injection of embryos at all three stages reduced survival (0−10 dpf) by as much as 96%, as well as reducing time-to-hatch by 1−3 days (Jaquet et al., 2004). Moreover, surviving embryos showed hepatobiliary abnormalities, including hypertrophy, hepatic hemorrhage and necrosis at later stages of development (Jaquet et al., 2004). Subsequent histological analysis of medaka embryos injected with sub-lethal concentrations of MC–LR (Huynh-Delerme et al., 2005) further revealed an array of development defects of the digestive system and related organs, including lack of mucus-secreting cells in the esophagus, decreased folding of the stomach and intestinal epithelium, reduced exocrine pancreas and liver, and lack of inflation of the swim-bladder, as well as inhibition of yolk resorption and depletion of hepatic glycogen stores.
Based on these studies in medaka, Wang et al. (2004) utilized similar microinjection techniques to investigate developmental toxicity of MC–LR in zebrafish embryos. Injection of zebrafish embryos at 2- to 4-cell stage significantly decreased survival (0−48 hpf) in a dose-dependent manner at concentrations as low as 300 nM (Wang et al., 2004). Assessment of embryos during cleavage indicated that MC–LR blocked the adherence and coherence of blastomeres in dividing cells, and subsequent histological analysis revealed that the toxin specifically interfered with localization of β-catenin and cadherin that are involved in cell–cell adhesion (Wang et al., 2004). At later stages of development, MC–LR injection led to a range of developmental deformities including bent tails/body axes, occasional cyclopia and severe edemas of the pericardium and hatching gland (Wang et al., 2004). Taken together with results from medaka studies (Jaquet et al., 2004; Huynh-Delerme et al., 2005), and previous zebrafish studies (Oberemm et al., 1997), these findings support a developmental toxicity of MC–LR, as well as clarifying the apparent role of the chorion as a potential barrier to water-soluble toxins, such as the microcystins.
With respect to this latter point, developmental toxicity of MC–LR has also been investigated in loach (Misguruns mizolepis), as an alternative freshwater fish model, by direct submersion of embryos (Liu et al., 2002). Interestingly, and in contrast to results with the zebrafish, exposure by immersion induced an array of embryonic abnormalities, including pericardial edema, tubular heart, bradycardia, homeostasis, poor yolk resumption, small head and curved body and tail, as well as a reduction in hatching rate. Moreover, the magnitude of these effects were apparently related to developmental stage of exposure such that embryos exposed during cleavage (approximately 32-cell stage) showed more pronounced effects than those exposed immediately post-fertilization, whereas those exposed even later, specifically during gastrulation, showed considerably less profound effects than those exposed during either post-fertilization or cleavage stages. Exposure of post-hatch larvae continued to reduce survival (i.e. increase mortality) of embryos, however, abnormalities were limited to ultrastructural effects, specifically interfering with subcellular organization of hepatocytes, including abnormalities of the nuclei and chromatin, as well as the endoplasmic reticula (Liu et al., 2000). No apparent morphological or cellular abnormalities were observed for exposed juveniles, although MC–LR did lead to significantly higher mortality compared to controls (Liu et al., 2000). Unlike studies using zebrafish, it was shown that larvae transferred to microcystin-free water following exposure to MC–LR did not show continued effects on survival rate or body size of juveniles (Liu et al., 2002). Clearly these results indicate that, although zebrafish is finding considerable utility, characterization of developmental toxicity may benefit from comparison with additional available fish models to address possible species-specificity.
Finally, in addition to the discussed application of the zebrafish and other freshwater fish embryos for characterizing developmental toxicity of known toxins from cyanobacteria and other HABs, it is becoming clear that this model may hold equal utility for identifying otherwise unknown, and potentially novel, toxins. Indeed, Oberemm et al. (1997) reported that crude extracts from cultures of M. aeruginosa and other species (e.g. Anabaena), as well as several field samples from cyanobacterial blooms (particularly dominated by Aphanizomenon flos-aquae), led to various severe developmental effects (Oberemm et al., 1997). However, these effects which included apparent inhibition of gastrulation, neural tube formation and organogenesis more generally did not seem to correlate with the presence of any of the recognized toxins (i.e. microcystins, anatoxin-1, aphantoxin) from these genera (Oberemm et al., 1997), indicating that observed developmental toxicity may potentially be related to otherwise unknown toxins.
Likewise, the zebrafish system was used to identify the novel lactone, mueggelone, along with the known compound, lupenyl acetate, from blooms of A. flos-aquae, as developmental toxins (Papendorf et al., 1997). At concentrations of 10 μg/L purified mueggelone, exposed zebrafish embryos showed signs of impaired development of the circulatory system, as well as pericardial edema and thrombosis, and reduced (45%) survival. Lupenyl acetate, on the other hand, although it showed similar effects (e.g. pericardial edema), as well as unrelated effects (e.g. bent tails), was active only at higher concentrations (i.e. 100 μg/mL).
These studies clearly demonstrate that the zebrafish has considerable utility for investigating both otherwise unrecognized developmental toxicity of known HAB toxins, as well identification and characterization of potentially novel toxic metabolites from marine and freshwater algae. Accordingly, we have employed the zebrafish embryo model as a means to screen cyanobacteria isolates, specifically from the Florida Everglades and other South/Central Florida freshwater sources, that are rich in microbial (and particularly cyanobacterial) diversity. This approach is used to generate “leads” to toxins based on observed inhibition of development, and has subsequently been employed to direct purification (i.e. bioassay-guided fractionation) and eventual chemical and biological characterization of compounds identified in this way. Here we present data on developmental toxins from freshwater cyano-bacteria identified using the zebrafish embryo model, and discuss the utility of this assay for isolation and characterization of such cyanobacterial metabolites.
2. Materials and methods
2.1. Isolation and culture of freshwater cyanobacteria
All strains of cyanobacteria were isolated from samples of water or algal assemblages (e.g. periphyton mats) collected from the Florida Everglades and other freshwater sources in South and Central Florida. Strains were isolated by standard microbiological procedures (Rippka et al., 1979), specifically employing filtration of samples through a 0.45 μm membrane filter, subsequently transferred to BG11/agar (1.5%) plates which are selective for cyanobacteria and certain “green algae” (Chlorophyta). Plates with filters were incubated at room temperature (24 °C) under continuous light (25 μE/m2/s), and individual colonies picked, and subsequently grown as non-axenic, unialgal cultures in BG11 medium buffered with 2-morpholinoethanesulfonic acid (MES), pH 7.2, to obtain sufficient biomass (see below) for extraction. All isolates were taxonomically identified (to genus) by microscopic observation using classical morphological criteria given in Komarek and Anagnostidis (1986, 1989).
2.2. Extraction of toxins from cyanobacterial isolates
For screening, biomass from cyanobacterial cultures was sequentially extracted in CHCl3 and 30% EtOH in order to ensure sufficient representation of non-polar (i.e. lipophilic) and “semi-polar” compounds, respectively. Specifically, biomass was collected by centrifugation, filtration or direct “harvesting” (e.g. collection with forceps), and freeze-dried. Freeze-dried biomass (approximately 100 mg) was extracted by sonication in CHCl3 (10 mL), followed by filtration and evaporation of the extraction solvent. Subsequently, the extracted biomass was extracted similarly in 30% EtOH, followed by filtration and evaporation of the extraction solvent. Dried CHCl3 and 30% EtOH extracts were re-dissolved to 1 mg crude extract per mL in absolute EtOH and 30% EtOH, respectively, for screening.
2.3. Rearing and breeding of zebrafish for embryos
Zebrafish of the D and DGL lines (Gibbs and Schmale, 2000) were bred and maintained in the UM NIEHS Marine and Freshwater Biomedical Sciences (MFBS) Center's In Vitro and In Vivo Fish Culture Facility Core. Zebrafish were maintained in 30-L tanks at 28 °C with 14 h:10 h light/dark cycle. To breed, male and female adult zebrafish (typically ca. 10−30 individuals) were collected (within 15 min of the end of the dark cycle), held above 10-L tanks in mesh enclosures and allowed to breed freely. Following successful breeding (typically 3−5 h), eggs fell through the mesh, and were subsequently collected from the bottom of tanks. Following collection and washing, eggs were transferred to plates containing “embryo rearing solution” (ERS) prepared fresh by diluting 25 mg of neomycin sulfate, 50 μL stock solution (35% w/v) of sodium thiosulfate, 125 μL Amquel® and 200 μL of stock solution (0.1% w/v) of methylene blue in 1 L of tap water. Eggs containing dead or obviously poor quality embryos were removed. The remaining embryos were used, usually within 2 h post-fertilization (hpf), for developmental toxicity assays (see below).
2.4. Zebrafish embryo developmental toxicity assay
Screening of zebrafish embryo developmental toxicity was typically conducted in 24-well plates. Prior to adding embryos, cyanobacterial extracts were either diluted (typically 100, 50, 20 and 10 μg/mL final concentration of crude extracts) in wells containing 1 mL of ERS, or alternatively added to wells, and placed in a fume-hood to allow solvents to evaporate, prior to adding ERS. In addition, appropriate controls of solvent vehicle, along with untreated controls, were included in each assay. Subsequently, embryos at the 4- to 32-cell stage (except where otherwise noted; see below) were collected and transferred to test wells (typically 5 embryos per well), by pipetting to minimize damage to eggs.
Embryo development was observed daily (for 5 dpf) using a dissecting microscope, and any abnormal developmental phenotypes (particularly morphological abnormalities), as well as mortalities, were recorded and photographically documented. Morphological abnormalities were assessed based primarily on developmental features described by Kimmel et al. (1995), and generally categorized when possible as early-, mid- and late-stage phenotypes. “Early-stage” phenotypes included apparent inhibition of cleavage, blastulation and gastrulation (0−24 hpf); “mid-stage” phenotypes included interference with segmentation, organogenesis and pharyngulation (24−48 hpf); “late-stage” phenotypes include those developmental dysfunctions that manifested primarily post-hatching (2−5 dpf). Assays were repeated as necessary to confirm any observed developmental effects. Furthermore, to ensure that developmental toxicity was consistent and reproducible, these effects were specifically recorded as “positive” when observed in 80% (i.e. 4 of 5, or 8 of 10) of embryos tested. For untreated embryos, mortality and spontaneous developmental dysfunction typically occurred at relatively low frequency (approximately 5% of embryos).
3. Results
To-date, over 122 strains of cyanobacteria isolated from the Florida Everglades and other freshwater sources in South and Central Florida have been screened with the zebrafish developmental toxicity assay. Approximately 17% of the strains evaluated were found to produce compounds that inhibit embryo development (Table 2). Developmental toxicity was observed for both lipophilic (CHCl3) and polar (30% EtOH) extracts, although considerably more lipophilic fractions were active (i.e. 71% vs. 41%). Taxonomically, active strains represented six families from four orders of cyanobacteria. Some taxa were more represented than others; in particular, 7 of the active strains belonged to the genus, Nostoc, and 8 belong to the family Nostocaceae (Table 2).
Table 2.
Isolates of freshwater cyanobacteria identified to produce developmental toxins in the zebrafish embryo assay
| Isolate | Order: Family | Extract |
|---|---|---|
| Anabaena 66−2 | Nostocales: Nostocaceae | CHCl3 |
| Calothrix 3−27 | Nostocales: Rivulariaceae | CHCl3 |
| Calothrix 22−7 | Nostocales: Rivulariaceae | CHCl3 |
| “Coccoid” 21−11 | Chroococcales: N.A. | 30% EtOH |
| Fischerella 52−1 | Stigonemetales: Stigonemetaceeae | CHCl3 |
| Leptolyngbya 69−5 | Oscillatoriales: Pseudanabaenaceae | CHCl3 |
| Limnothrix 60−1 | Oscillatoriales: Pseudanabaenaceae | CHCl3 |
| Nostoc Ev-1 | Nostocales: Nostocaceae | 30% EtOH |
| Nostoc 23−2 | Nostocales: Nostocaceae | 30% EtOH |
| Nostoc 53−2 | Nostocales: Nostocaceae | 30% EtOH |
| Nostoc 58−2 | Nostocales: Nostocaceae | 30% EtOH |
| Nostoc 31−4 | Nostocales: Nostocaceae | CHCl3 |
| Nostoc 33−10 | Nostocales: Nostocaceae | CHCl3 |
| Nostoc 15−7b | Nostocales: Nostocaceae | CHCl3 |
| Oscillatoria 48−3 | Oscillatoriales: Oscillatoriaceae | CHCl3 |
| Plectonema 33−7 | Oscillatoriales: Oscillatoriaceae | CHCl3 |
| Pseudoanabaena 69−7 | Oscillatoriales: Pseudanabaenaceae | CHCl3 |
| Scytonema 26−1 | Nostocales: Scytonemataceae | 30% EtOH, CHCl3 |
| Spirulina 21−0 | Oscillatoriales: Oscillatoriaceae | CHCl3 |
| Synechococcus 21−10a | Chroococcales: N.A. | 30% EtOH |
| Tolypothrix 30−1−4 | Nostocales: Nostocaceae | CHCl3 |
All strains were isolated, by standard microbiological methods (Rippka et al., 1979), from freshwater sources in South and Central Florida. Isolates were cultured and extracted in CHCl3 and 30% EtOH, and evaluated for developmental toxicity, using 4- to 32-cell stage embryos, from 1 hpf to 6 dpf (see Materials and methods).
Cyanobacterial toxins inhibited zebrafish embryogenesis at various stages of development including early, middle and late phases (see Materials and methods). Chloroform extracts of Oscillatoria 48−3, for instance, inhibited early stages of development. At 1 dpf, greater than 80% of embryos, treated at cleavage (4- to 32-cell stage) with 20 μg/mL of crude extract, were halted at mid-blastula transition (MBT; Fig. 1A) or gastrulation (Fig. 1B). Approximately 20% of embryos treated at 20 μg/mL were able to proceed beyond gastrulation at 1 dpf, however, the development of these embryos was notably retarded compared to control embryos (Fig. 1E), including truncated tails and poorly formed eyes and other organs (Fig. 1C). To assess the affect of developmental stage during exposure, embryos were additionally exposed at 1-cell, 64- to 128-cell and MBT stages. Again, approximately 80% of embryos exposed to 20 μg/mL of extract prior to MBT (i.e. 1-cell and 64- to 128-cell stages) were halted at MBT or gastrulation (data not shown). On the other hand, all embryos exposed at MBT (also at 20 μg/mL) developed normally (Fig. 1D), and appeared similar to control embryos (Fig. 1E), indicating that the toxin specifically affects developmental pathways preceding MBT. At concentrations of 50 μg/mL or greater, all embryos, regardless of developmental stage during exposure, were dead within 1 dpf. Embryos treated at lower concentrations (i.e. 10 μg/mL), generally developed normally, though embryos were slightly smaller compared to control embryos (data not shown).
Fig. 1.
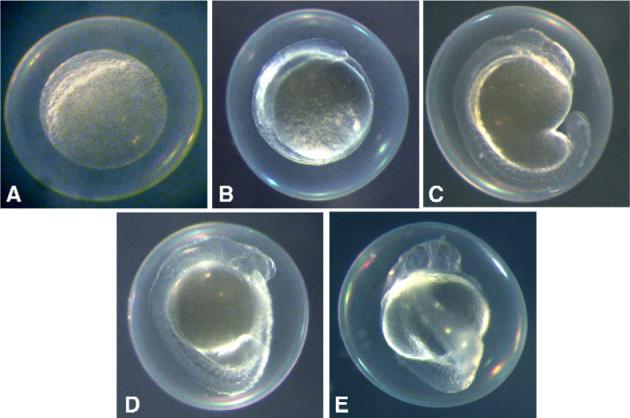
Developmental toxicity of Oscillatoria 48−3 extracts. All embryos shown (A–E) are 1 dpf. Within 1 dpf, zebrafish embryos exposed at 4- to 32-cell stage to chloroform extracts (20 μg/mL) of Oscillatoria 48−3 (A–C) arrest development at mid-blastula transition (MBT; A), or are slow to progress to gastrulation (B), compared to control embryos treated with solvent only (E). In some cases, embryos develop beyond gastrulation (C), but are notably slowed compared embryos treated at the same time with solvent only (E). Embryos treated at MBT (D) developed normally as solvent controls (E).
Extracts of Plectonema 33−7 and Tolypothrix 30−1−4, on the other hand, apparently affected embryos during (or perhaps prior to) pharyngulation (1−2 dpf), and specifically appear to inhibit pathways of organogenesis and differentiation more generally (Fig. 2A, B, D and F, respectively) when compared to controls (Fig. 2C, E and G). As shown in Fig. 2, eyes failed to develop beyond a rudimentary stage in embryos treated with chloroform extracts from either of the two strains (at 50 and 100 μg/ml, respectively). Likewise, in both cases, embryos developed only rudimentary heads and truncated tails, along with a generally malformed body axis (Fig. 2A–B, D). Embryos, however, survived up to 3−4 dpf, and were responsive to light stimulation by stereotypical twitching behavior.
Fig. 2.
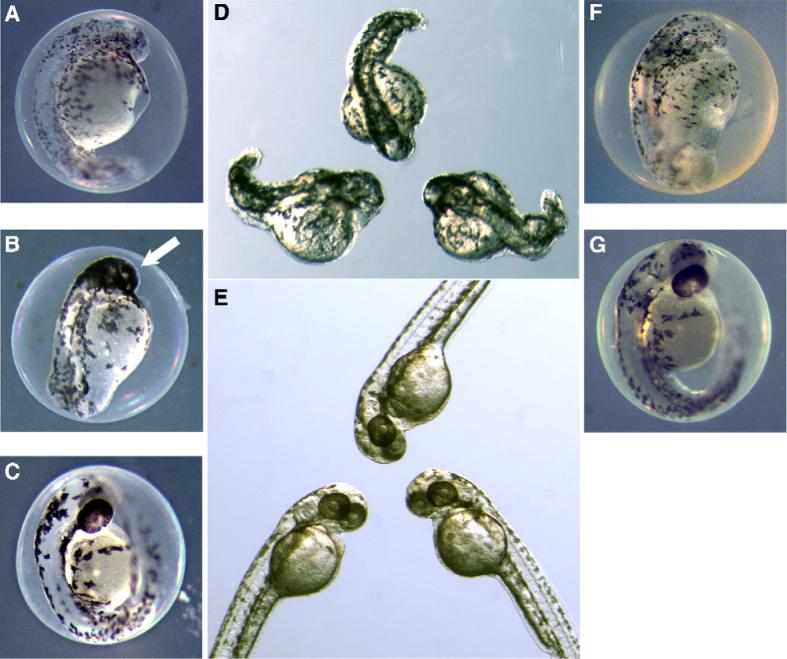
Developmental toxicity of Plectonema 33−7 and Tolypothrix 30−1−4 extracts. Within 2 dpf, zebrafish embryos exposed at 4- to 32-cell stage to 50 μg/mL of chloroform extracts from Plectonema 33−7 (A, B and D) and 100 μg/mL of chloroform extracts from Tolypothrix 30−1−4 (F), respectively, show inhibition of pharyngulation, including organogenesis (e.g. formation of the eye) and general cell-differentiation, compared to embryos treated with either 50 μL CHCl3/mL of ERS (C and E), as solvent-only control for A, B and D, or 100 μL of CHCl3/mL of ERS (G), as solvent-only control for F. In the case of Plectonema 33−7, there is additionally an apparent lack of migration of melanophores from neural crest (B) relative to the solvent-only control (C and E). Note: D and E are the same magnification.
In the case of embryos treated with extracts of Plectonema 33−7, the phenotype was specifically characterized by an apparent failure of melanophores to migrate from their origin in the neural crest, consequently aggregating in and around the head (Fig. 2A, B and D). This phenotype was highly consistent and reproducible (Fig. 2D), indicating inhibition of a specific developmental pathway(s), rather than general dysfunction. Exposures to Plectonema 33−7 extract was repeated using eggs at 1-cell, 64- to 128-cell and MBT stages, however, no difference was observed (data not shown), indicating that the toxin targeted pathways later in the development (i.e. post-MBT).
Chloroform extracts (100 μg/mL) of Calothrix 33−2 (Fig. 3) induced various morphological deformities in embryos at later stages that largely manifested only post-hatching (ca. 4−5 dpf). Although embryos developed otherwise normal body plans, and organs generally elaborated as normal, embryos were consistently and reproducibly characterized by severe edemas of pericardial and peritoneal cavities, and considerably bent tails and/or body axis (Fig. 3). Embryos, however, successfully hatched and were otherwise seemingly normal behaviorally – swimming and righting themselves, for example, as well as otherwise responding to stimuli (e.g. touch, light) similarly to control embryos. However, eventual survival of the extract-exposed larvae to adults was significantly lower than control embryos (data not shown).
Fig. 3.
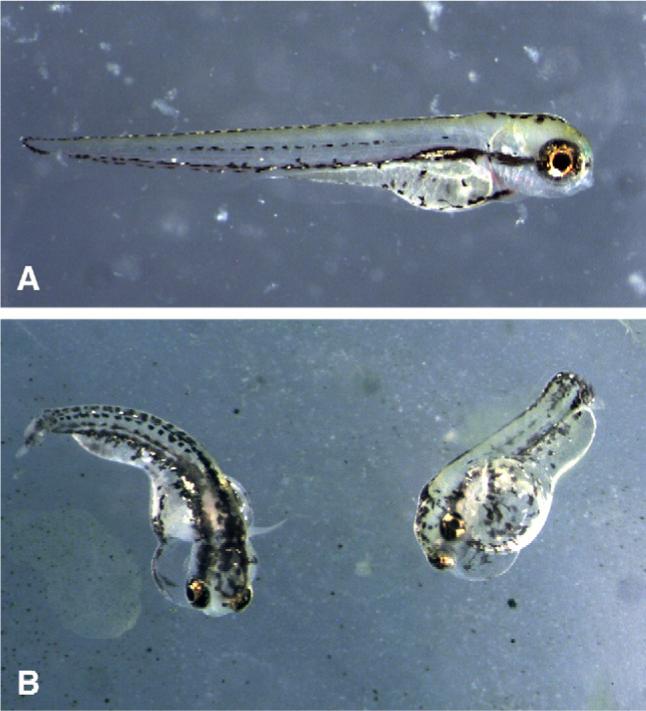
Developmental toxicity of Calothrix 33−2 extracts. Within 4 dpf, zebrafish embryos exposed at 4- to 32-cell stage to 100 μg/mL chloroform extracts of Calothrix 33−2 (B) show morphological deformities, including pericardial and peritoneal edemas, as well as bent tails/body axes, compared to control embryos treated with solvent (i.e. CHCl3) only (A).
One isolate identified by screening, Fischerella 52−1, was specifically selected for subsequent isolation and characterization of the developmental toxin. Embryos treated with chloroform extracts of Fischerella 52−1 culture biomass, or alternatively culture medium, consistently and reproducibly developed a characteristic phenotype termed “puffy”, characterized by a generally swollen appearance and lack of pigmentation (Fig. 4)which developed within approximately 1−2 dpf (followed by 100% mortality within 2−3 dpf). This developmental phenotype was used to guide purification (i.e. bioassay-guided fractionation) of a group of developmental toxins from Fischerella 52−1 for subsequent chemical and biological characterization. Interestingly, certain aspects of the phenotypes for purified compounds (Fig. 5) were not identical to that of the crude extract, although other features, such as the lack of pigmentation, and particularly melanophores, were observed in both cases. However, as with the crude extracts, the observed phenotype for embryos exposed to the purified compounds was equally consistent for a given set of treatment conditions (Fig. 5). Details of the purification and chemical characterization are presented elsewhere (Berry et al., submitted for publication).
Fig. 4.
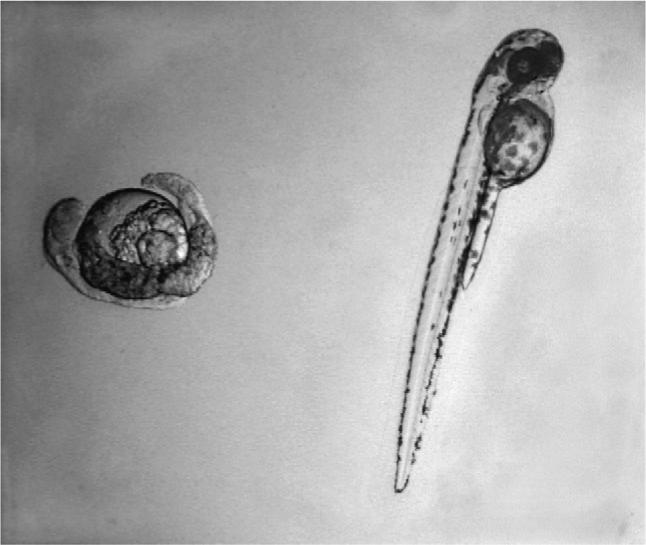
Developmental toxicity of crude extracts from Fischerella 52−1. Within 1 dpf, zebrafish embryos exposed at 4- to 32-cell stage to chloroform extracts of Fischerella 52−1 culture medium (left) show a developmental phenotype, termed “puffy” ,characterized by swollen appearance and general lack of pigmentation, compared to control embryos treated with solvent only (right).
Fig. 5.
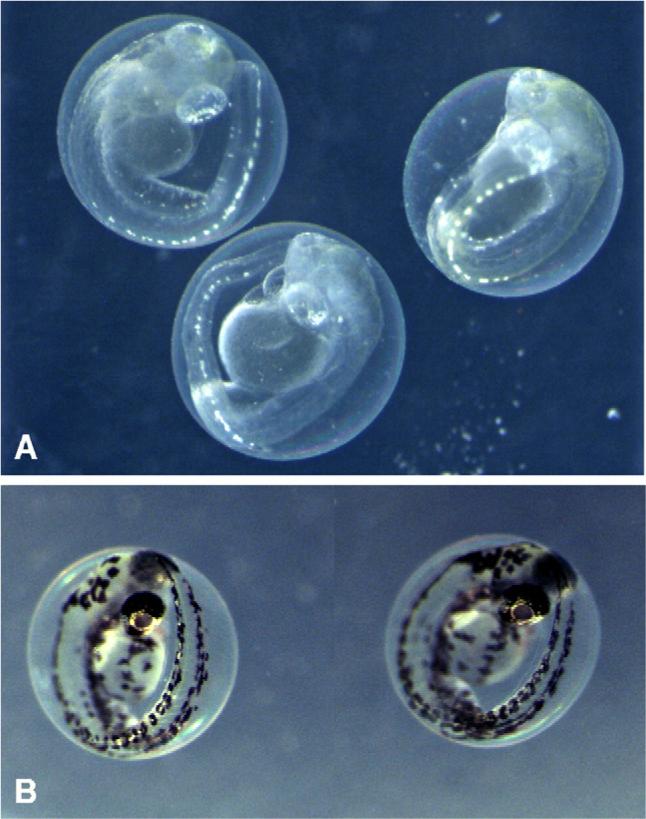
Developmental toxicity of a compound purified from Fischerella 52−1. Within 3 dpf, zebrafish embryos exposed at 4- to 32-cell stage to 100 μg/mL of a pure compound isolated from Fischerella 52−1 (A) show a developmental phenotype characterized, in particular, by lack of melanophores, compared to control embryos (B) treated with solvent (i.e. CHCl3) only. Moreover, this phenotype is consistent for all embryos exposed to the toxin under a given set of conditions (e.g. toxin concentration, stage and line of fish).
4. Discussion
The zebrafish embryo (along with other teleost fish models, e.g. medaka) is finding growing utility in the investigation of HAB toxins, particularly with respect to the effects of these toxins on processes of vertebrate development (i.e. developmental toxicity). This model system has been effectively employed to identify developmental toxicity associated with a number of well-recognized toxins from marine and freshwater HABs (Oberemm et al., 1997; Edmunds et al., 1999; Kimm-Brinson and Ramsdell, 2001; Liu et al., 2002; Colman and Ramsdell, 2003; Colman et al., 2004, 2005; Lefebvre et al., 2004; Tiedeken et al., 2005). Equally, this system is being increasingly utilized to identify and characterize novel, or otherwise unrecognized, developmental toxins from algal sources (e.g. Papendorf et al., 1997).
We have specifically applied this model system to the identification and characterization of developmental toxins from freshwater cyanobacteria, including over 100 isolates from the Florida Everglades and other freshwater sources in South and Central Florida. Evaluation of these isolates has demonstrated that potential developmental toxins are widespread. Indeed, extracts from approximately 17% of isolates tested, representing a considerable diversity of the cyanobacteria (Table 2), inhibited development of the zebrafish embryo.
Moreover, developmental toxins identified from cyanobacteria in the present study appear to affect a range of developmental stages (Figs. 1-3), and it is thereby becoming increasingly clear that stage of exposure to toxins may play an important role in the manifestation of these toxicoses. Lipophilic extracts of Oscillatoria 48−3, for example, appear to specifically inhibit pathways up to mid-blastula transition (MBT), as treated embryos are largely arrested at MBT, or slowed in their progress to gastrulation and subsequent stages, and treatment of embryos at equivalent concentrations after MBT has no apparent effect on development (Fig. 1). Others, such as Plectonema 33−7 and Tolypothrix 30−1−4, appear to inhibit processes of pharyngulation (1−2 dpf), including organogenesis and cell-differentiation more generally (Fig. 2). In other cases, such as Calothrix 33−2 (Fig. 3), developmental defects are not apparent until embryos hatch (∼4−5 dpf) and morphological malformations (e.g. bent body axis, edema) are observable.
In addition, the zebrafish embryo has been successfully used for identification, purification and characterization of developmental toxins. As an example, utilizing the zebrafish embryo model for bioassay-guided fractionation, we have recently purified developmental toxins from the isolate, Fischerella 52−1. Using either crude extracts or pure compounds, consistent developmental phenotypes were observed, though some degree of variability was observed between the phenotype associated with the crude extracts compared to the purified compounds (see Figs. 4 and 5). Details of the purification and characterization of these compounds is reported elsewhere (Berry et al., submitted for publication), however, chemical characterization has identified these compounds as belonging to the hapalindoles, a group of indole alkaloids previously identified from Fischerella and related genera (Moore et al., 1984, 1987; Smitka et al., 1992; Stratmann et al., 1994). Although previous work (Moore et al., 1997; Smitka et al., 1992; Doan et al., 2000; Doan et al., 2001) has characterized biological activity of these compounds (e.g. antimicrobial activity, inhibition of RNA polymerase), this is the first identification of apparent developmental toxicity associated with these compounds. This may be of particular importance as these compounds are currently being developed as anticancer agents (Smith et al., 1995; Zhang and Smith, 1996). Moreover, through our studies it was found that these toxins are abundant in extracellular components (i.e. culture medium) of cultures (Gantar et al., submitted), and may be released by naturally occurring algal populations, representing a potential environmental and even human health concern.
Perhaps the most striking outcome of this research was the consistent phenotype, with very little observable morphological differences between embryos, seen after exposure to either crude extracts, such as those from Plectonema 33−7 (Fig. 2B–C) or Fischerella 52−1 (Fig. 4), or a purified compound (Fig. 5). This consistency clearly suggests that these toxins are inhibiting a specific developmental pathway, or set of pathways and processes, rather than resulting from general dysfunction of embryogenesis. In each case, the consistent phenotype was found with a specific concentration given to same stage embryos of one particular line of fish. Although these phenotypic outcomes are reproducible within these conditions, further work is needed to determine the range of conditions that yield consistent phenotypic outcomes. Likely key variables include toxin concentration, stage of application and line of fish.
Marine and freshwater HABs are recognized to produce a vast array of toxic or otherwise biologically active metabolites. Although most research has focused on the acute toxicoses associated with these toxins, it is becoming clear that the same compounds may exert, via identical, related or alternative targets, more insidious and often unrecognized outcomes. Such effects as developmental toxicity may have profound implications for human and environmental health. The zebrafish embryo model is finding a considerable role in our understanding of these impacts.
Acknowledgements
The authors would like to thank the National Institute of Environmental Health Sciences (NIEHS) for financial support of this research through an NIEHS ARCH grant (S11 ES11181). This research was additionally supported, in part, by an NIEHS Marine and Freshwater Biomedical Sciences Center Grant (ES 05705) to the University of Miami. We would also like to thank Drs. Kathleen Rein, Minglei Wang and Robert E. Gawley who have contributed to the on-going chemical characterization of cyanobacterial toxins.
Footnotes
This paper is based on a presentation given at the conference: Aquatic Animal Models of Human Disease hosted by the University of Georgia in Athens, GA, USA, October 30–November 2, 2005.
References
- Beasley VR, Lovell RA, Holmes KR, Walcott HE, Schaeffer DJ, Hoffmann WE, Carmichael WW. Microcystin–LR decreases hepatic and renal perfusion, and causes circulatory shock, severe hypoglycemia and terminal hyperkalemia in intravascularly doses swine. J. Toxicol. Environ. Health, Part A. 2000;61:281–303. doi: 10.1080/00984100050136599. [DOI] [PubMed] [Google Scholar]
- Berman JN, Kanki JP, Look AT. Zebrafish as a model for myelopoiesis during embryogenesis. Exp. Hematol. 2005;33:997–1006. doi: 10.1016/j.exphem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielmyer GK, Gatlin D, Isely JJ, Tomasso J, Klaine SJ. Responses of hybrid striped bass to waterborne and dietary copper in freshwater and saltwater. Comp. Biochem. Physiol. C. 2005;140:131–137. doi: 10.1016/j.cca.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Botes DP, Kruger H, Viljoen CC. Isolation and characterization of four toxins from the blue-green algae Microcystis aeruginosa. Toxicon. 1982;20:945–954. doi: 10.1016/0041-0101(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Braunbeck T, Boettcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N. Towards an alternative for the acute fish LC50 test in chemical assessment: the fish embryo toxicity test goes multi-species – an update. ALTEX. 2005;22:87–102. [PubMed] [Google Scholar]
- Butler RA, Kelley ML, Olberding KE, Gardner GR, Van Beneden RJ. Aryl hydrocarbon receptor (AHR)-independent effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on softshell clam (Mya arenaria) reproductive tissue. Comp. Biochem. Physiol. C. 2004;138:375–382. doi: 10.1016/j.cca.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Carmichael WW. Health effects of toxin-producing cyanobacteria: the cyanohabs. Hum. Ecol. Risk Assess. 2001;7:1393–1407. [Google Scholar]
- Colman JR, Ramsdell JS. The type B brevetoxin (PbTx-3) adversely affects development, cardiovascular function and survival in Medaka (Oryzias latipes) embryos. Environ. Health Perspect. 2003;111:1920–1925. doi: 10.1289/ehp.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman JR, Dechraoui MY, Dickey RW, Ramsdell JS. Characterization of the developmental toxicity of Caribbean ciguatoxins in finfish embryos. Toxicon. 2004;44:59–66. doi: 10.1016/j.toxicon.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Colman JR, Twiner MJ, Hess P, McMahon T, Satake M, Yasumoto T, Doucette GJ, Ramsdell JS. Teratogenic effects of azaspiracid-1 identified by microinjection of Japanese medaka (Oryzias latipes) embryos. Toxicon. 2005;45:881–890. doi: 10.1016/j.toxicon.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J. Neurosci. 1993;13:4486–4495. doi: 10.1523/JNEUROSCI.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizer H, Fischer B, Harabawy AS, Hennion MC, Hansen PD. Toxicity of domoic acid in the marine mussel Mytilus edulis. Aquat. Toxicol. 2001;55:149–156. doi: 10.1016/s0166-445x(01)00178-3. [DOI] [PubMed] [Google Scholar]
- Doan NT, Rickards RW, Rothschild JM, Smith GD. Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fisherella and Calothrix. J. Appl. Phycol. 2000;12:409–416. [Google Scholar]
- Doan NT, Stewart PR, Smith GD. Inhibition of bacterial RNA polymerase by the cyanobacterial metabolites 12-epi-hapalindole E isonitrile and calothrixin A. FEMS Microbiol. Lett. 2001;196:135–139. doi: 10.1111/j.1574-6968.2001.tb10554.x. [DOI] [PubMed] [Google Scholar]
- Doucette TA, Strain SM, Allen GV, Ryan CL, Tasker RA. Comparative behavioral toxicity of domoic acid and kanaic acid in neonatal rats. Neurotoxicol. Teratol. 2000;22:863–869. doi: 10.1016/s0892-0362(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Drummond IA. Kidney development and disease in the zebrafish. J. Am. Soc. Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- Duy TN, Lam PK, Shaw GR, Connell DW. Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev. Environ. Contam. Toxicol. 2000;163:113–186. doi: 10.1007/978-1-4757-6429-1_3. [DOI] [PubMed] [Google Scholar]
- Edmunds JS, McCarthy RA, Ramsdell JS. Ciguatoxin reduces larval survivability in finfish. Toxicon. 1999;37:1827–1832. doi: 10.1016/s0041-0101(99)00119-1. [DOI] [PubMed] [Google Scholar]
- Feng Q, Boone AN, Vijayan MM. Copper impact on heat shock protein 70 expression and apoptosis in rainbow trout hepatocytes. Comp. Biochem. Physiol. C. 2003;135:345–355. doi: 10.1016/s1532-0456(03)00137-6. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Matarrese P, Fattorossi A, Donelli G. Okadaic acid induces changes in the organization of F-actin in intestinal cells. Toxicon. 1996;34:937–945. doi: 10.1016/0041-0101(96)00025-6. [DOI] [PubMed] [Google Scholar]
- Fischer WJ, Dietrich DR. Pathological and biochemical characterization of microcystin-induced hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000;164:73–81. doi: 10.1006/taap.1999.8861. [DOI] [PubMed] [Google Scholar]
- Gerwick WH, Tan LT, Sitachitta N. Nitrogen-containing metabolites from marine cyanobacteria. Alkaloids: Chem. Biol. Perspect. 2001;57:75–184. doi: 10.1016/s0099-9598(01)57003-0. [DOI] [PubMed] [Google Scholar]
- Gibbs PDL, Schmale MC. GFP as a genetic marker scorable throughout the life cycle of transgenic zebra fish. Mar. Biotechnol. 2000;2:107–125. doi: 10.1007/s101269900014. [DOI] [PubMed] [Google Scholar]
- Hégarat LL, Orsiere T, Botta A, Fessard V. Okadaic acid: chromosomal non-disjunction analysis in human lymphocytes and study of aneugenic pathway in CHO-K1 cells. Mutat. Res. 2005;578:53–63. doi: 10.1016/j.mrfmmm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hitzfield BC, Höger SJ, Dietrich DR. Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000;108:113–122. doi: 10.1289/ehp.00108s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm-Brinson KL, Ramsdell JS. The red tide toxin, brevetoxin, induces embryo toxicity and developmental abnormalities. Environ. Health Perspect. 2001;109:377–381. doi: 10.1289/ehp.01109377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes 2-Chroococcales. Arch. Hydrobiol., Suppl.bd. Algol. Stud. 1986;43:157–226. [Google Scholar]
- Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes 2-Nostocales. Arch. Hydrobiol., Suppl.bd. Algol. Stud. 1989;56:247–345. [Google Scholar]
- Lefebvre KA, Trainer VL, Scholz NL. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat. Toxicol. 2004;66:159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Leira F, Alvarez C, Cabado AG, Vieites JM, Vieytes MR, Botana LM. Development of a F-actin-based live-cell fluorometric microplate assay for diarrhetic shellfish toxins. Anal. Biochem. 2003;317:129–135. doi: 10.1016/s0003-2697(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Song L, Li X, Liu T. The toxic effects of microcystin–LR on embryo-larval and juvenile development of loach, Misguruns mizolepis Gunthe. Toxicon. 2002;40:395–399. doi: 10.1016/s0041-0101(01)00173-8. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int. J. Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Lehmann VK. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res. 2001;21:2489–2500. [PubMed] [Google Scholar]
- McMahon C, Semina EV, Link BA. Using zebrafish to study the complex genetics of glaucoma. Comp. Biochem. Physiol. C. 2004;138:343–350. doi: 10.1016/j.cca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M, Volff J-N. Genetic, biochemical and evolutionary facets of Xmrk-induced melanoma formation in the fish Xiphophorus. Comp. Biochem. Physiol. C. 2004;138:281–290. doi: 10.1016/j.cca.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Moore RE, Cheuk C, Patterson GML. Hapalindoles: new alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 1984;106:6456–6457. [Google Scholar]
- Moore RE, Cheuk C, Xu-Qiang GY, Patterson GML, Bonjouklian R, Smitka TA, Mynderse JS, Foster RS, Jones ND, Swartzendruber JK, Deeter JB. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987;52:1036–1043. [Google Scholar]
- Morris AC, Fadool JM. Studying rod photoreceptor development in zebrafish. Physiol. Behav. 2005;86:306–313. doi: 10.1016/j.physbeh.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikoshi M, Rinehart KL, Sakai R, Sivonen K, Carmichael WW. Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue-green alga) Nostoc sp. Strain 152. J. Org. Chem. 1990;55:6135–6139. [Google Scholar]
- Namikoshi M, Sivonen K, Evans WR, Carmichael WW, Sun F, Rouhrianen L, Luukkainen R, Rinehart KL. Two new L-serine variants of microcystins–LR and –RR from Anabaena sp. Strains 202 A1 and 202 A2. Toxicon. 1992;30:1457–1464. doi: 10.1016/0041-0101(92)90521-6. [DOI] [PubMed] [Google Scholar]
- Oberemm A, Fastner J, Steinberg CEW. Effects of microcystin–LR and cyanobacterial crude extracts on embryo-larval development of zebra-fish (Danio rerio). Water Res. 1997;31:2918–2921. [Google Scholar]
- Papendorf O, Konig GM, Wright AD, Chorus I, Oberemm A. Mueggelone, a novel inhibitor of fish development from the freshwater cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 1997;60:1298–1300. doi: 10.1021/np970231s. [DOI] [PubMed] [Google Scholar]
- Pilotto LS, Kliewer EV, Davies RD, Burch MD, Attewell RG. Cyanobacterial (blue-green algae) contamination in drinking water and perinatal outcomes. Austral. New Zealand. J. Public Health. 1999;23:154–158. doi: 10.1111/j.1467-842x.1999.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Puffett-Lugassay NN, Zon LI. Analysis of hematopoietic development in zebrafish. Methods Mol. Med. 2005;105:171–189. doi: 10.1385/1-59259-826-9:171. [DOI] [PubMed] [Google Scholar]
- Rahn JJ, Gibbs PDL, Schmale MC. Patterns of transcription of a virus-like agent in tumor and non-tumor tissues in bicolor damselfish. Comp. Biochem. Physiol. C. 2004;138:401–409. doi: 10.1016/j.cca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rao PV, Gupta N, Bhaskar AS, Jayaraj R. Toxins and bioactive compounds from cyanobacteria and their implications on human health. J. Environ. Biol. 2002;23:215–224. [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- Sayer A, Hu Q, Bourdelais AJ, Baden DG, Gibson JE. The effect of brevenal on brevetoxin-induced DNA damage in human lymphocytes. Arch. Toxicol. 2005;79:683–688. doi: 10.1007/s00204-005-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafizadeh E, Peterson RT, Lin S. Induction of reversible hemolytic anemia in living zebrafish using a novel small molecule. Comp. Biochem. Physiol. C. 2004;138:245–250. doi: 10.1016/j.cca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Wu S. Developmental role of GnRH and PACAP in a zebrafish model. Gen. Comp. Endocrinol. 2005;142:74–80. doi: 10.1016/j.ygcen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. Microalgal metabolites: a new perspective. Annu. Rev. Microbiol. 1996;50:431–465. doi: 10.1146/annurev.micro.50.1.431. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003;6:236–243. doi: 10.1016/s1369-5274(03)00064-x. [DOI] [PubMed] [Google Scholar]
- Smith CD, Zilfou JT, Stratmann K, Patterson GM, Moore RE. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]
- Smitka TA, Bonjouklian R, Doolin L, Jones ND, Deeter JB, Yoshida WY, Prinsep MR, Moore RE, Patterson GML. Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 1992;57:857–861. [Google Scholar]
- Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricate. Relationship to fisherindoles and hapalindoles. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]
- Tiedeken JA, Ramsdell JS, Ramsdell AF. Developmental toxicity of domoic acid in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2005;27:711–717. doi: 10.1016/j.ntt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Xi D, Peng YG, Ramsdell JS. Domoic acid is a potent neurotoxin to neonatal rats. Nat. Toxins. 1997;5:74–79. doi: 10.1002/(SICI)(1997)5:2<74::AID-NT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kim EY, Iwata H, Tanabe S. Molecular characterization of the aryl hydrocarbon receptors (AHR1 and AHR2) from red seabream (Pagrus major). Comp. Biochem. Physiol. C. 2005;141:177–187. doi: 10.1016/j.cca.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Yu S-Z. Drinking water and primary liver cancer. In: Tang ZY, Wu MC, Xia SS, editors. Primary Liver Cancer. Academic Publishers/Springer; New York: 1989. pp. 30–37. [Google Scholar]
- Zhang X, Smith CD. Microtubule effects of welwistatin, a cyanobacterial indolinone that circumvents multiple drug resistance. Mol. Pharmacol. 1996;49:288–294. [PubMed] [Google Scholar]


