Abstract
Calcium waves among glial cells impact many central nervous system functions, including neural integration and brain metabolism. Here, we have characterized the modulatory effects of melatonin, a pineal neurohormone that mediates circadian and seasonal processes, on glial calcium waves derived from different brain regions and species. Diencephalic and telencephalic astrocytes, from both chick and mouse brains, expressed melatonin receptor proteins. Further, using the calcium-sensitive dye Fluo-4, we conducted real-time imaging analyses of calcium waves propagated among mammalian and avian astrocytes. Mouse diencephalic astrocytic calcium waves spread to an area 2-5 fold larger than waves among avian astrocytes and application of 10 nM melatonin caused a 32% increase in the spread of these mammalian calcium waves, similar to the 23% increase observed in chick diencephalic astrocytes. In contrast, melatonin had no effect on calcium waves in either avian or mammalian telencephalic astrocytes. Mouse telencephalic calcium waves radially spread from their initiation site among untreated astrocytes. However, waves meandered among mouse diencephalic astrocytes, taking heterogeneous paths at variable rates of propagation. Brain regional differences in wave propagation were abolished by melatonin, as diencephalic astrocytes acquired more telencephalon-like wave characteristics. Astrocytes cultured from different brain regions, therefore, possess fundamentally disparate mechanisms of calcium wave propagation and responses to melatonin. These results suggest multiple roles for melatonin receptors in the regulation of astroglial function, impacting specific brain regions differentially.
Keywords: circadian, glia, communication, hypothalamus, cortical
Introduction
The discovery of calcium wave propagation among glial cells has expanded the potential roles of glia in nervous system function. These new roles include the modulation of short-term plasticity at the neuromuscular junction (Colomar and Robitaille, 2004), neuronal signaling in hippocampal slice preparations (Volterra and Steinhauser, 2004), synaptic transmission and neuronal excitability in the mammalian retina (Newman, 2004), and neuronal proliferation during cortical development (Weissman et al., 2005). Since the observation that a rise in intracellular calcium in cultured astrocytes resulted in the release of glutamate (Parpura et al., 1994), a growing volume of studies have implicated glial calcium waves in the modulation of neuronal integration (Volterra et al., 2002) and a wide-range of other brain mechanisms, including the mediation of synchrony of neuronal activation (Fellin et al., 2004), the modulation of cerebrovascular constriction and microcirculation (Anderson and Nedergaard, 2003; Mulligan and MacVicar, 2004), and neocortical spreading depression (Peters et al., 2003). Besides mediating signaling among other glia, neurons, and vascular cells, glial calcium waves regulate the spatial coordination of glucose utilization and energy metabolism in response to localized synaptic activity (Bernardinelli et al., 2004) Given this emerging relationship between glial calcium waves and brain function and the fact that melatonin receptors are highly expressed in astrocytes (Reppert et al., 1995; Adachi et al., 2002), we have begun to investigate a role for melatonin modulation of glial calcium waves in the regulation of biological clock outputs and circadian rhythmicity (Peters et al., 2005).
Neurons of the suprachiasmatic nucleus (SCN) in mammals, and a homologous hypothalamic structure in birds, are considered the master pacemakers of vertebrate biological rhythms (Ebihara and Kawamura, 1981; Takahashi and Menaker, 1982; Moore, 1983; Welsh et al., 1995; Klein et al., 1997). Rhythmic SCN outputs activate hypothalamic-to-autonomic signaling pathways that culminate in sympathetic modulation (via the superior cervical ganglia) of the pineal gland (Klein et al., 1997; Moore & Silver 1998). This modulatory pathway regulates the rhythmic production and release of melatonin from the pineal, such that circulating blood levels of the neurohormone are low during the day and high during the night in both birds and mammals (Pang and Ralph, 1975; Cassone and Menaker, 1983; Cassone et al., 1986b). Day-night rhythms of melatonin release are entrained to environmental light-dark cycles by sensory inputs to the SCN from the neural retina via the retinohypothalamic tract (RHT; Rusak, 1979; Reppert et al., 1981; Cassone et al., 1990).
Melatonin directly influences circadian rhythms. Rhythmic melatonin administration entrains locomotor activity, inhibits SCN metabolic activity in vivo, inhibits SCN electrical activity in vitro and phase shifts the SCN clock in vitro (Redman et al., 1983; Cassone et al., 1988; Shibata et al., 1989; Gillette & McArthur, 1996). Not surprisingly, two of the three G-protein-coupled melatonin receptors that have been identified, the Mel1a (MT1) and Mel1b (MT2) subtypes, are highly expressed in the mammalian SCN (Weaver et al., 1989; Reppert et al., 1996; von Gall et al., 2002; Dubocovich et al., 2003). In birds, melatonin receptor expression is high in many brain regions, including the hypothalamus and visual system structures (Rivkees et al., 1989; Weaver et al., 1989; Brooks & Cassone, 1992; Cassone et al., 1995). Thus, the same hypothalamic structures that drive or entrain rhythmic melatonin production and release, are direct targets of the neurohormone's modulatory action.
Identification of the avian melatonin receptor subtypes suggest that the Mel1a receptor is found mainly on neurons of the brain, while the Mel1c receptor is found on non-neuronal cells, primarily astrocytes (Reppert et al., 1995). In the chick, all astrocytes of the diencephalon express the mRNA of the Mel1c receptor, while only 25% express the Mel1a receptor (Adachi et al., 2002). None of these astrocytes appear to express the Mel1b receptor. Melatonin modulates glucose uptake and the release of glycolytic byproducts from astrocytic cultures (Adachi et al., 2002) and affects changes in brain metabolism in vivo (Lu and Cassone, 1993; Cantwell and Cassone, 2002). Melatonin also modulates resting levels of astrocytic calcium and facilitates the spread of intercellular calcium waves among astrocytes (Peters et al., 2005).
Because melatonin receptors are expressed in many brain regions (Rivkees et al., 1989; Weaver et al., 1989; Reppert et al., 1995), including telencephalic structures, we have tested whether melatonin affects differential responses in mouse and chicken astrocytes isolated from both the diencephalon and telencephalon. We hypothesized that the effects of melatonin on vertebrate brain physiology, in this case calcium signaling, would be greatest in diencephalic astrocytes.
Methods
Cell culture
Chick astrocyte culture
Glial cells were prepared from chick embryonic (E17) eggs (Hyline Hatcheries, Bryan, TX). Embryos were extracted, the brains were dissected, and meninges were removed. Telencephalic tissue and diencephalic tissue, where the SCN of the hypothalamus is located, were placed in separate petri dishes containing minimal essential medium with Earle's salts (MEM; Gibco/Invitrogen, Grand Island, NY) and 1% PSN antibiotic (penicillin, streptomycin, neomycin; Gibco/Invitrogen). Tissue was dissociated using gentle trituration with MEM containing 3U/ml dispase (Gibco/Invitrogen) while stirring at a low speed on a stir plate. Dissociated tissue in suspension was extracted after 10 minutes and placed into a 15 ml conical tube. Additional dissociation media was added to the non-dissociated tissue along with 80 μl of 8000 U/ml Dnase I solution (Sigma, St. Louis, MO) and allowed to stir for 10 more minutes. Another extraction of dissociated tissue was performed. Dissociated tissue from the 15 ml conical tubes was pooled into a 50 ml conical tube and centrifuged at 1000X g for 10 minutes at 4° C. Media was aspirated from the cell pellets, and cells were re-suspended in MEM with 10% fetal bovine serum (FBS) and 1% PSN antibiotic, and seeded into 75 cm2 tissue culture flasks. All astrocyte cultures were maintained in darkness at 37°C with 95% air and 5% CO2 in a humidified Napco CO2 incubator. Culture media was changed after 18-24 hours and then every three days until cell cultures reached confluence (approximately 10 – 14 days).
A second chick cell culture approach, previously described for avian diencephalic astrocytes (Peters et al., 2005), was employed for comparative purposes. In this approach, diencephalic tissue was placed in Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO) and dissociated using titration with 1% trypsin. Cells were filtered through nylon mesh (180 mm pore diameter) and centrifuged for 5 minutes at 1000X g. The supernatant was removed and the pellet was re-suspended in DMEM with 10% fetal bovine serum (FBS) and 1% PSN antibiotic (penicillin, streptomycin, neomycin; Gibco/Invitrogen, Grand Island, NY). The suspension was filtered again through nylon mesh (77 mm pore diameter) and seeded into 75 cm2 tissue culture flasks. Initial isolation of the tissues and subsequent incubation conditions were identical to the first culture approach.
Mammalian astrocyte culture
One day old C57Bl/6J mice were killed by decapitation and primary astrocytes were isolated from cortex (telencephalon) or hypothalamus (diencephalon) essentially as previously described (Aschner et al., 1992; Erikson and Aschner, 2002). Tissue was rapidly dissected and meninges were removed under a stereomicroscope (Olympus) in isolation medium consisting of Modified Eagle's Medium containing Earle's salts (Invitrogen) and penicillin-streptomycin-neomycin (PSN) at twice the concentration present in normal growth medium (0.001 mg/ml for penicillin and streptomycine and 0.002 mg/ml for neomycin, respectively). Tissue extractions were performed in isolation medium containing 1.5 U/ml dispase (Sigma). The number of extractions was reduced by half to accommodate the lower yield of tissue from mice as compared to rats. Astroglial cultures were maintained at 37 C, 5% CO2 in Minimal Essential Medium (MEM) supplemented with Earle's salts, 10% FBS, and PSN, and grown 18 days to confluence prior to experiments. All studies utilizing primary cultured astrocytes from mice were carried out under approved animal use protocol number 2001-209 at Texas A&M University.
Calcium imaging
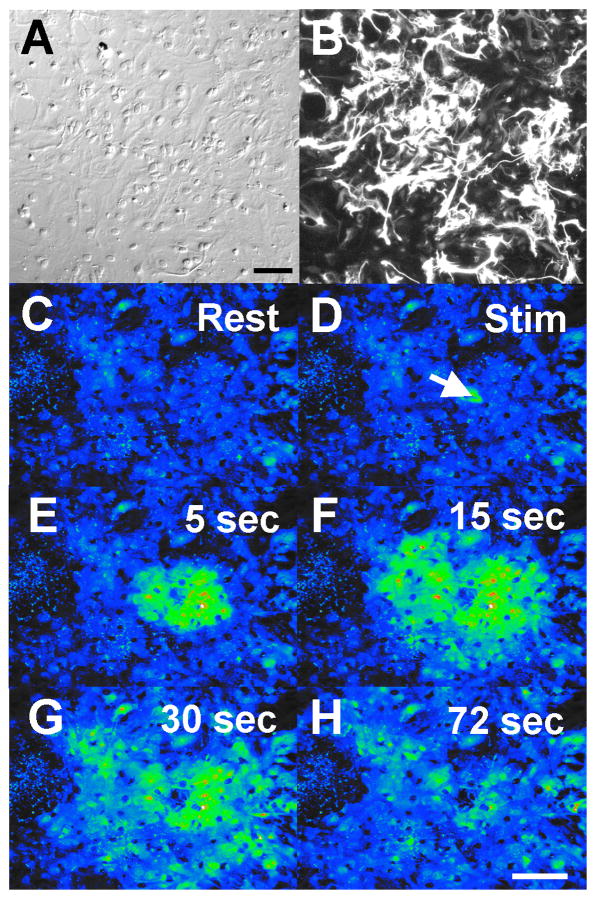
Confluent astrocyte cultures were sub-cultured onto coverglass chamber slides (Lab-Tek) and allowed to grow to confluence (6-7 days). Cells were loaded with 4 μM Fluo-4 (diluted from a 2 mM Fluo-4 stock in DMSO; Molecular Probes, Eugene, OR) in imaging medium (MEM with HEPES and L-glutamine and without phenol red) for 45 minutes at 37° C in a 5% CO2 cell culture incubator. Cells were then washed twice and treated with melatonin (Sigma) or DMSO vehicle (0.01%) for 30 minutes prior to imaging. Calcium imaging was conducted at room temperature with an Olympus IX70 inverted microscope and 10X PlanAprochromat air objective. Images were acquired using an Orca-ER CCD camera (Hamamatsu) and an entire field of cells (approximately 1 mm2) was designated as a region of interest (ROI) for analysis. Resting calcium levels were first obtained from a ROI and then a single glial cell was stimulated using mechanical stimulation with a micromanipulated, glass micropipette to elicit a calcium wave. The area of the spread of the wave was calculated using SimplePCI 4.0 imaging software (Compix, Inc., Cranberry Township, PA). Images were collected every 0.5 seconds for 1.5 minutes in order to permit recording of the initiation, rate of spread, and maximal extent of the spread of the calcium wave through each ROI. Even using the 10× objective, the wave occasionally spread beyond the field of view, indicating that the calculated areas of cells involved in the wave are likely somewhat underestimate the full extent of wave propagation (Fig. 1A).
Figure 1.
Ca+2 wave propagation through a confluent mammalian astrocyte culture. A-B. DIC image of a region of interest of telencephalic mammalian astrocytes (A). The same field of view as in A is illustrated for anti-GFAP fluorescence (B). Astrocyte cultures were 85% glial as determined by GFAP immunocytochemistry. Bar equals 100 μm (for panels A-B). C-H. Pseudo-colored image of an intercellular calcium wave. A region of telencephalic mouse astrocytes, loaded with Fluo-4 AM, shows selected frames from a calcium wave sequence. After resting levels of calcium were obtained, a single cell was activated by mechanical stimulation (arrow in D; Stim) and this touch stimulation initiated a Ca+2 wave that spread to surrounding glial cells. Bar equals 200 μm (for panels C-H).
For each of the four astrocytic culture groups used in calcium wave experiments, 8-10 embryonic brains (either mouse or chick) were used to establish initial cultures. Each calcium data set included three independent initial cultures. Thus, brain tissue from approximately 30 chicks and 30 mice contributed to the calcium wave studies. Astrocytes were passaged 1-2 times in culture flasks prior to expansion on multi-well, experimental dishes. The number of regions of interest (i.e., calcium waves) collected from each culture well was restricted to ensure that that all cells were naïve prior to activation as part of an intercellular wave. That is, a criterion was used where no cells involved in a calcium wave were used for a subsequent data point.
Immunocytochemistry and cell counts
Cell cultures were plated on glass-bottom dishes for 5-7 days. Wells were fixed with 4% paraformaldehyde fixative for 10 minutes, and were then washed three times in PBS (pH 7.6) for 10 minutes each. Next they were washed with PBS with 1% Triton-X (PBT) for 10 minutes and blocked with 5% goat serum in PBT (PBS-GT) for 10 minutes. They were reacted with glial fibrillary acidic protein antibody (GFAP; Incstar, Stillwater, MN; 1:1000) in PBT-GT overnight at 4°C. Controls were incubated overnight with block (PBS-GT) instead of primary antibody. The cells were then put through a series of 10 minutes washes with block, PBT, and PBS. The cells were incubated in FITC-tagged, goat anti-rabbit antibody (1:1000) in PBS-GT overnight at 4°C. Slides were then rinsed with PBS and viewed on an inverted fluorescence microscope using FITC optical configurations. Images were obtained of the cells using the Orca ER CCD camera.
For estimation of cell numbers, differential interference contrast (DIC) optical images were captured using Simple PCI software (C-Imaging, Inc). Image files were imported into Adobe Photoshop and were contrast-enhanced for maximal resolution of cell nuclei (Fig. 1A). Dual captures of DIC images and anti-GFAP staining were used to estimate the percentage of glial cells in a culture. In all experiments, regions of interest (ROIs) equaled 1000 × 103 μm2. Using empirically-determined cell counts for each culture type (i.e., chick diencephalic, etc.), the number of cells involved in a calcium wave was estimated by multiplying the average number cells per ROI (cells/μm2) times the area of the wave (μm2).
Western blot analysis
Protein was extracted from confluent cell cultures with Trizol reagent using Invitrogen Trizol protocol, quantified with Total Protein Assay (Sigma), and 30ug was loaded and run on Tris-HCl (4-20% gradient; Bio-Rad) gels. Protein was transferred to nitrocellulose membrane. Membrane was blocked with blocking buffer (10% dry milk in PBS; pH 7.6) for 30 minutes and incubated with melatonin receptor antibody (chick Mel1a or Mel1c; 1:1000 and mammalian Mel1a (MT1); 1:1000 or Mel1b (MT2); 1:250; Lifespan Biosciences) in blocking buffer overnight at 4°C. Membrane was washed three times with PBS for 10 minutes each wash and then incubated with biotinylated goat anti-rabbit antibody (1:500) in blocking buffer for 1 hour at room temperature. It was then washed three times with PBS for 10 minutes each wash and then incubated with Avidin-Biotin Complex (ABC kit; Vector labs) for 1 hour at room temperature. The membrane was washed three times with PBS for 10 minutes each wash and then incubated with DAB until there was a color contrast (about 10-15 minutes).
Statistics
As mentioned above, approximately 30 embryonic brains contributed to the initial cultures representing each species and brain region. Subsequently, multiple culture expansions were conducted to generate the experimental culture wells. Multiple ROIs were collected from a single well only if the first calcium wave had not invaded this region of cells. n throughout the paper equals the total number of ROIs studied across culture wells. Calcium imaging data was analyzed using Microsoft Excel and treatment groups statistically compared using t-tests or one-way ANOVA. Student-Newman-Keuls posthoc test was used when significant differences were found. In all cases p<0.05 level was used to determine minimum significance.
Results
Following dissociation of mouse and avian brain tissues, telencephalic and diencephalic cell cultures of each were grown in conditions that promote the enrichment of astrocytes and were brought to confluence (Fig. 1A). Cultures derived from the two brain regions of mouse embryos contained similar cell numbers (based on counts of nuclei) when confluent. Mouse telencephalic and diencephalic cultures contained 842 ± 22 and 816 ± 67 cells in an area of 1000 × 103 μm2, respectively (mean ± s.e.m.; p=0.65; n=3). Chick telencephalic and diencephalic cultures were more disparate in there cell counts, but were still not significantly different (p=0.08). The telencephalic and diencephalic cultures possessed 783 ± 36 and 667 ± 14 cells in 1000 × 103 μm2, respectively. Each of the four cell culture groups exhibited wide-spread expression glial fibrillary acidic protein (GFAP), demonstrating that the cultures were composed predominantly of astrocytes (Fig. 1B). Using dual DIC and anti-GFAP imaging, the estimated percentage of glial cells in each of the four cultures ranged from 84.0 ± 4.9 to 87.1 ± 4.8%. Thus, whether derived from chick or mouse brain, or diencephalic or telencephalic tissues, the cultures used in these studies had similar total cell numbers, the vast majority of which were glial.
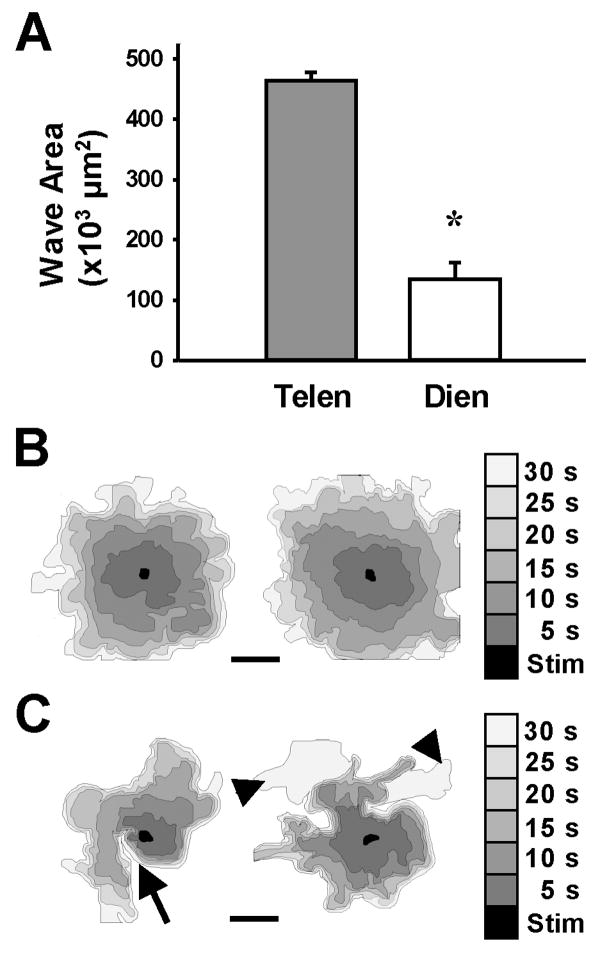
Astrocyte cultures, whether telencephalic or diencephalic, elicited intercellular calcium waves when a single cell was stimulated by the touch of a micropipette. Calcium waves spread from the stimulated cell to neighboring cells and propagated among large numbers of glial cells in the confluent culture (Fig. 1C-H). The spread of the calcium waves varied dramatically between avian and mammalian astroglial cultures. In mouse telencephalic cultures, waves spread to an area 3.4-fold larger than the mouse diencephalic waves (466.1 ± 13.7 × 103 μm2 versus 135.8 ± 26.7 × 103 μm2; mean ± s.e.m.; Fig. 2A). Wave spread in the telencephalic cultures was radial in nature, with the leading edge of the wave advancing concentrically (Fig. 2B). In diencephalic cultures, wave spread was characterized by an uneven rate of wave advance (Fig. 2C, black triangles) and the direction of the wave spread was irregular throughout the ROI, often making turns that left large areas of the culture uninvolved in the wave (Fig. 2C, arrow).
Figure 2.
Differential spread of calcium waves in mammalian astrocyte cultures. A. Following stimulation of mammalian astrocytes, Ca+2 waves had spread farther in telencephalic (Telen; *, p<0.0001, n=10) than in diencephalic cultures (Dien; n=10) after 90 seconds of propagation. B-C. The spread of Ca+2 waves in the telencephalic cultures (B) was radial and more synchronous than in the diencephalic cultures (C), where waves spread irregularly. Two representative waves from each group indicate the area of wave spread in 5-second intervals (shades of gray) from touch stimulation site (black area). Calcium waves among diencephalic astrocytes made hairpin turns (arrow) and unequal advances of the wave front (arrowheads). These features were not seen in telencephalic cultures. Bar equals 200 μm.
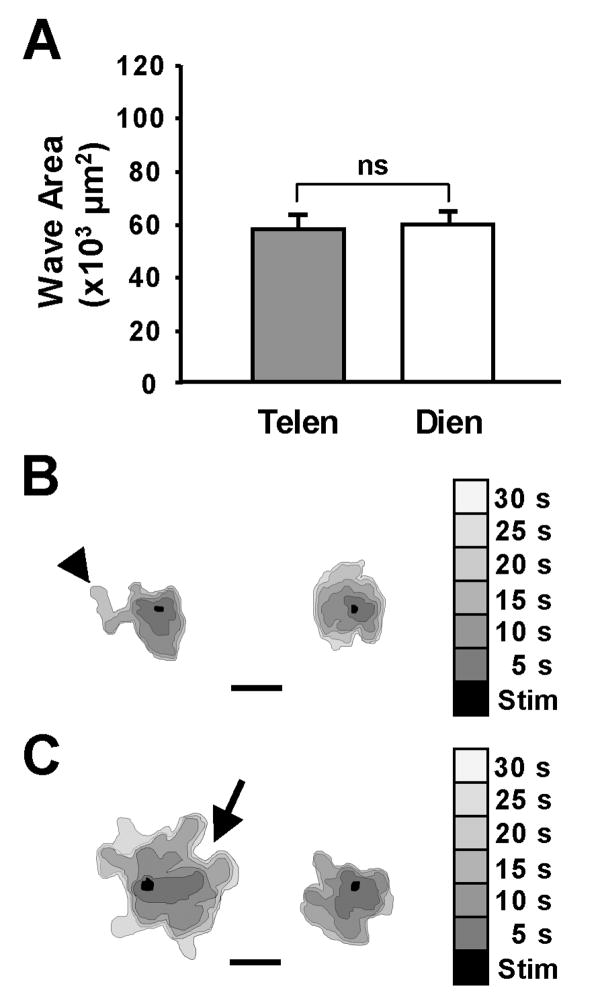
In chick astrocyte cultures, the area of the spread of the calcium wave was similar in both the telencephalic and diencephalic groups (58.0 ± 4.5 × 103 μm2 and 59.6 ± 4.5 × 103 μm2, respectively; Fig. 3A). The telencephalic (Fig. 3B) and the diencephalic (Fig. 3C) waves spread from the point of stimulation in a highly variable fashion. That is, the rate of spread was uneven, with the wave advancing into some areas of the ROI rapidly, but more slowly into others.
Figure 3.
The spread of calcium waves among avian astrocytes was homogeneous. A. In chick astrocyte cultures, the spread of the Ca+2 waves after 90 seconds was the same in telencephalic (Telen; n=12) and diencephalic (Dien; n=27) cultures. However the spread of the waves was dramatically reduced from those seen in the mammalian astrocytes. Note the scale of the y-axis. B-C. The nature of the spread of the wave was similar in both the chick telencephalic (B) and diencephalic (C) cultures. Two representative waves from each group indicate the area of wave spread in 5-second intervals (shades of gray) from the touch stimulation site (black area). Calcium waves among the astrocyte cultures made hairpin turns (arrow) and had unequal advances of the wave front (arrowhead). Bar equals 200 μm.
Differences in calcium waves among astrocytes of mammals and birds
Calcium waves in mouse astrocytes involved many more cells (Fig. 4A) and spread more rapidly (Fig. 4B) than waves in chick astrocytes. The total estimated number of cells involved in the wave, following an increase in intracellular calcium in a single stimulated cell, was greatest in the telencephalic mouse cultures with approximately 400 cells involved compared to 100 cells in the diencephalic mouse cultures (Fig. 4A). Thus, mouse telencephalic waves involved almost that 4 times the number of astrocytes, compared to diencephalic waves. In addition, mouse astrocytic calcium waves involved 4 to 10 times the number of cells involved in chick astrocytic waves. Chick telencephalic and diencephalic cultures had essentially the same number of cells involved, 45 and 40, respectively (Fig. 4A). Calcium waves in mouse astrocyte cultures spread faster than those of chick astrocytes (Fig. 4B). The rate of the spread was approximately 9 cells/second (telencephalic) and 5 cells/second (diencephalic) for mouse astrocytes as compared to 2 cells/second in both telencephalic and diencephalic chick astrocytes.
Figure 4.
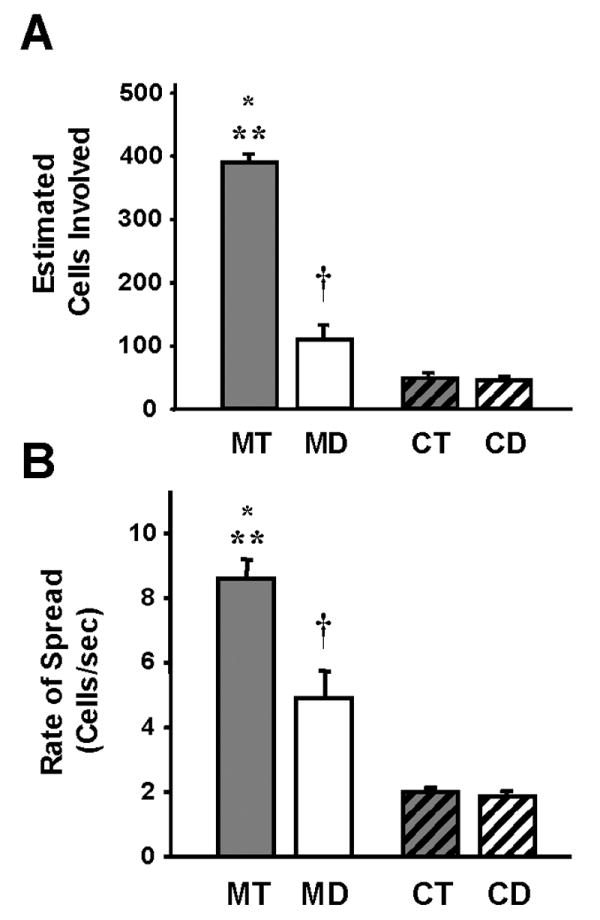
Ca+2 wave propagation differs between mammalian and avian astrocytes. A. The estimated number of cells involved in calcium waves was greater in telencephalic mouse cultures than in the diencephalic mouse cultures (MT vs MD; *, p<0.001, n=10). There were also more cells involved in the mouse telencephalic (MT vs CT and CD; **, p<0.0001, n=10-27) and mouse diencephalic (MD vs CT and CD; †, p<0.003, n=10-27) cultures than in either of the chick astrocyte groups. Chick telencephalic and diencephalic cultures had essentially the same number of cells involved in Ca+2 waves (n=13-27). B. Similar to the number of cells involved in Ca+2 waves, the rate of the spread of the wave was faster in the mouse telencephalic and diencephalic astrocytes than either of the chick astrocyte cultures (MT vs CT and CD; **, p<0.0001, n=10-27; and MD vs CT and CD; †, p<0.01, n=10-27). Mouse telencephalic waves spread faster than mouse diencephalic waves (MT vs MD; *, p<0.003, n=10), whereas the rate of spread was similar between the chick telencephalic and diencephalic waves (n=13-27).
Effects of melatonin on calcium waves
When 10 nM melatonin was administered to the cultures for 30 minutes prior to touch stimulation, the area of the spread of the wave (at 30 seconds after touch) increased 32% over control waves among diencephalic mouse astrocytes (Fig. 5A). However, there was no effect of the melatonin on the telencephalic mouse astrocytes. Melatonin (10 nM) increased the spread of the calcium wave 23% in chick diencephalic cultures (Fig. 5B). As in the mouse cell cultures, telencephalic chick astrocytes were not affected by melatonin, as the area of the spread of the wave in hormone-treated cultures was not significantly different than that of controls (Fig. 5B). Thus, melatonin caused a 20-30% increase in the number of cells involved in a calcium wave, or 10-60 additional diencephalic astrocytes, in chick and mouse cell cultures.
Figure 5.
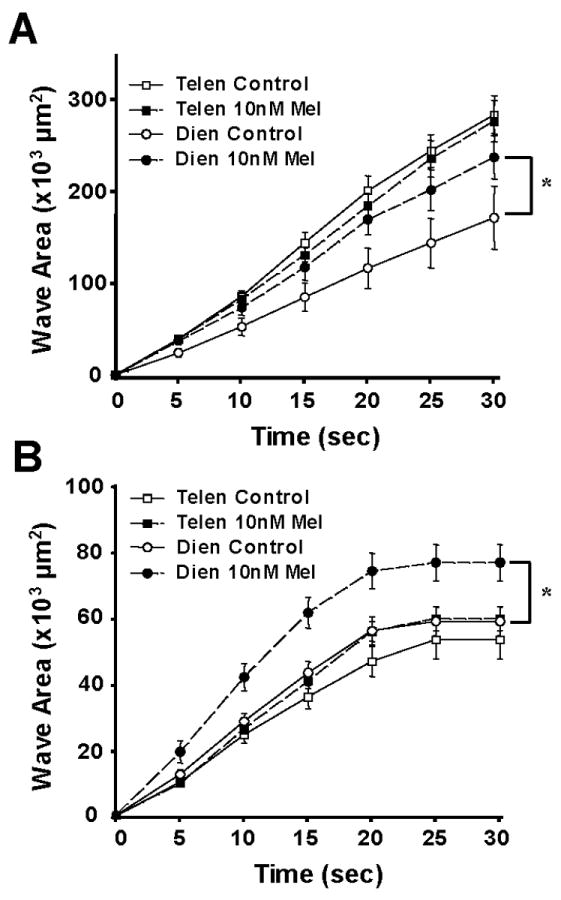
Melatonin enhances intercellular calcium waves in diencephalic, but not telencephalic, glial cultures. A. Mouse astrocyte cultures. Following application of 10 nM melatonin, the area of the Ca+2 wave spread after 30 seconds was increased over control (DMSO-treated) cultures in mouse diencephalic astrocytes (*, p<0.03, n=10), while there was little effect of melatonin on calcium waves among telencephalic glial cells (n=10). B. Chick astrocyte cultures. Nanomolar melatonin also increased the area of the Ca+2 wave spread after 30 seconds in chick diencephalic astrocytes (*, p<0.01, n=27), as compared to DMSO-treated controls, but had no effect on waves of telencephalic cultures (n=10).
Melatonin also significantly increased the rate of wave spread among mammalian diencephalic astroglia as compared to untreated controls (p<0.04; n=10; Fig. 6A), but had no effect on the rate of propagation of telencephalic astrocytes (p=0.21; n=10). A similar facilitation of wave propagation rate was induced by melatonin in chick diencephalic cultures (p<0.01; n=27; Fig. 6B) and, again, melatonin had little effect on the rate of wave spread among chick telencephalic astrocytes (p=0.11; n=13).
Figure 6.
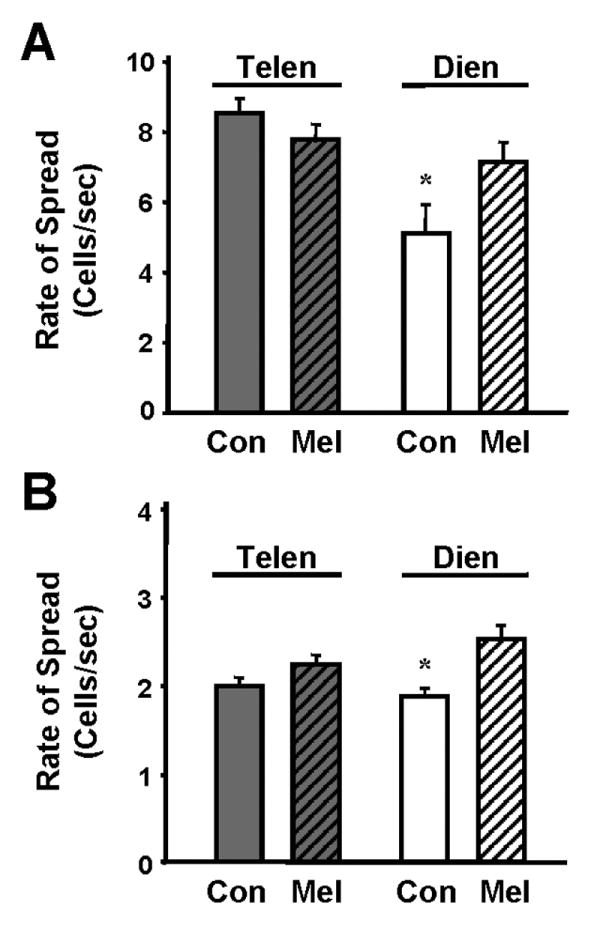
Melatonin increased the rate of calcium wave spread among diencephalic, but not telencephalic, glial cultures. A. Mouse astrocyte cultures. Following application of 10 nM melatonin, the rate of the wave spread in these treated cultures was faster than the rate of wave spread in control (DMSO-treated) cultures in mouse diencephalic astrocytes (*, p<0.04, n=10). There was no effect of melatonin on the rate of Ca+2 wave spread among telencephalic glial cells (n=10). B. Chick astrocyte cultures. Nanomolar melatonin also increased the rate of the Ca+2 wave spread among chick diencephalic astrocytes (*, p<0.01, n=27), as compared to DMSO-treated controls, but had no effect on the rate of Ca+2 wave spread in the telencephalic cultures (n=13).
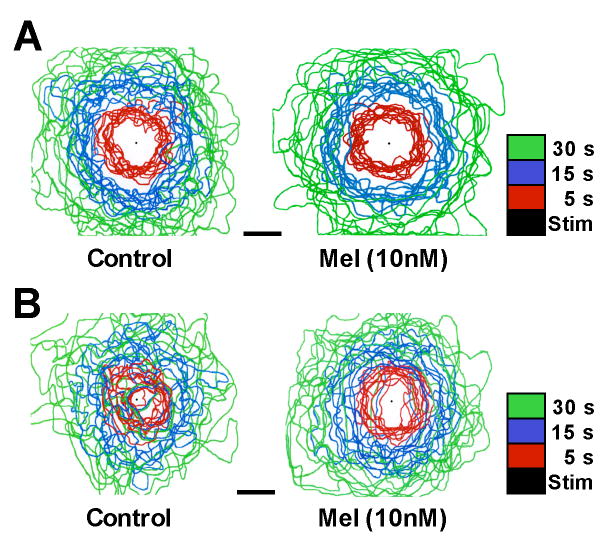
The effects of 10 nM melatonin on the mouse diencephalic astrocytes, not only involved increases in the area and rate of wave spread, but elicited other wave characteristics similar to those observed in telencephalic astrocytes. Telencephalic waves were propagated in a radial and concentric manner, as shown in Figure 7A. The leading edge of the wave at 5, 15, and 30 second time points, rarely overlapped in telencephalic astrocytes demonstrating that they spread in all directions at similar rates. The spread of diencephalic waves in control cultures was more varied with inconsistent rates and directions of propagation (Fig. 7B). Following melatonin treatment, diencephalic waves were similar in their pattern of spread to those of telencephalic waves (Fig. 7B), suggesting that melatonin induced a change in the underlying mechanisms of wave propagation.
Figure 7.
Melatonin affects the dynamic nature of Ca+2 waves spreading among mammalian astrocytes. A. Mouse telencephalic astrocytes. Ca+2 waves were propagated radially at consistent rates, as indicated by the virtual lack of overlap of lines indicating the leading edge of the wave at 3 time points (colored lines). Application of 10 nM melatonin had no effect on the spread of the Ca+2 waves in mouse telencephalic cultures. B. Mouse diencephalic astrocytes. Ca+2 waves were propagated with greater randomness of direction and rate among diencephalic astroglia. Note the extensive overlap of lines depicting the leading edge of the wave at all time points. Application of 10 nM melatonin increased the spread of the Ca+2 waves. Diencephalic Ca+2 wave propagation was more telencephalic-like following melatonin receptor activation, such that the spread of the Ca+2 waves through confluent cultures became more radial and concentric. The leading edge of the Ca+2 wave was outlined at 5 (Red), 15 (Blue), and 30 (Green) seconds. Black dots indicate the site of mechanical stimulation. Each plot shows the overlay of 10 representative waves from each astrocyte group. Bar equals 200 μm.
It should be noted that species-specific differences are not likely due to differences in cell cultures conditions. We employed two different approaches to culture avian diencephalic astrocytes including conventional chick astrocytic protocols (Peters et al., 2005), as well as procedures developed to more closely matched our mammalian culture conditions (see Methods). The spread of calcium waves in chick diencephalic astrocytes was not significantly different between culture approaches (p=0.35; n=10-17). The area of calcium waves, 30 seconds following stimulation, was 53.4 × 103 μm2 and 60.7 × 103 μm2, using conventional and modified culture approaches, respectively. Thus, avian calcium waves do not likely involve fewer cells as a consequence of deficient culture conditions, as compared to mammalian glial cultures, but rather due to inherent properties of chick astrocytes.
Melatonin receptor expression in mouse and chick astrocytes
Immunocytochemical approaches were not sensitive enough to detect differences in receptor expression, presumably due to low antigen-antibody affinity. Therefore, we conducted western blot analyses to test for the presence of the receptor subtypes in protein extracts derived from cell cultures of diencephalic and telencephalic astrocytes from both mouse and chick. Both Mel1a and Mel1c antibodies labeled proteins in whole cell extracts from chick diencephalic and telencephalic cultures (Fig. 8A). Similarly, antibodies to mammalian melatonin receptors, Mel1a and Mel1b, detected proteins in extracts from mouse diencephalic and telencephalic astrocytes (Fig. 8B).
Figure 8.
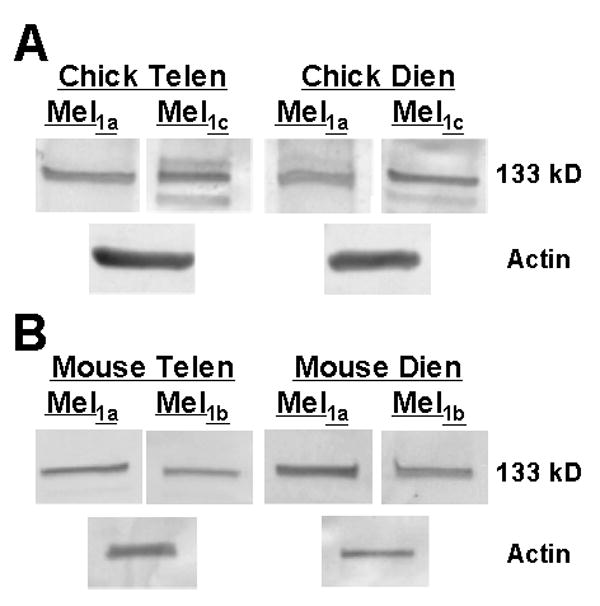
Protein expression of melatonin receptor subtypes in astrocytes. A. Western blot analysis of cell culture extracts demonstrated the expression of both the Mel1a and Mel1c receptor proteins in chick diencephalic and chick telencephalic astrocytes. B. Protein extracts of mouse diencephalic and telencephalic glia cells expressed both the Mel1a (MT1) and Mel1b (MT2) receptor subtypes. Anti-MelR probes detected multiple proteins, especially in chick extracts. However, a consistent protein band with a molecular weight of 133 kD was present in all samples. Anti-actin immunostaining was used as a protein loading control. These gels represent one of three replicate experiments that produced similar results.
Discussion
Astrocytes have many functions, ranging from buffering ions and neurotransmitters to regulating the transport of metabolites between the blood vessels and neurons (Tsacopoulos and Magistretti, 1996; Magistretti, 2000; Nedergaard et al., 2003; Simard and Nedergaard, 2004). Astrocytes are closely apposed to neurons and synapses of the central nervous system are typically ensheathed by glial processes (Schikorski and Stevens, 1997; Ventura and Harris, 1999). Recently, a role for astrocytes in the regulation of neural communication and synaptic function has been discovered (Haydon, 2001; Volterra et al., 2002; Auld and Robitaille, 2003; Braet et al., 2004). In the light of this emerging integrative function of astrocytes, it is important to determine whether astrocyte-astrocyte and astrocyte-neuron interactions are different among diverse brain regions and how modulators of neural function affect these interactions. For example, in the hypothalamus, astrocyte-neuron signaling is thought to regulate gonadotropin-releasing hormone (GnRH) secretion and production (Dhandapani et al., 2003). Hypothalamic astrocytes modulate synaptic plasticity associated with GnRH neurons, perhaps involving dynamic glial ensheathment, the extension and retraction of glial processes (Witkin et al., 1995; Witkin et al., 1997). Glial cells modulate synaptic activity of hippocampal neurons by releasing glutamate in a calcium-dependent manner (Parpura and Haydon, 2000) and glial glutamate release synchronizes neural activity in hippocampal slices (Angulo et al., 2004).
Astrocytes are abundant in the hypothalamus (Morin et al., 1989). Diencephalic structures, including the hypothalamus, express several types of G-protein-coupled melatonin receptors (Weaver et al., 1989; Brooks & Cassone, 1992; Cassone et al., 1995; Reppert et al., 1995; Reppert et al., 1996; von Gall et al., 2002) and calcium signaling and gap junctional communication among diencephalic astrocytes is modulated by melatonin (Peters et al., 2005). Using western blot analyses, we have demonstrated that Mel1a (MT1) and Mel1b (MT2) proteins are present in both hypothalamic and cortical astrocytes of mice, while Mel1a (MT1) and Mel1c receptor proteins are present in chick diencephalic and telencephalic astrocytes. Melatonin caused robust enhancement in the spread of intercellular calcium waves among diencephalic astrocytes of both birds and mammals, but had no effect on calcium waves among telencephalic astrocytes in either species. Thus, besides its canonical roles in the regulation of vertebrate visual and circadian pathways, melatonin regulates calcium signal communication among hypothalamic glial cells.
Why does melatonin affect calcium signaling only in diencephalic astrocytes, when telencephalic glial cells also expressed melatonin receptors? Calcium waves propagating among mouse telencephalic astrocytes were robust and pervasive, involving many more cells on average than control diencephalic cultures. Following melatonin-induced facilitation, mouse diencephalic calcium waves were indistinguishable from those of telencephalic cultures, including the number of cells involved, the rate of wave spread, and the dynamic manner of propagation (i.e., radial rather than multidirectional). It is possible that melatonin had little effect on calcium signaling in mouse cortical astrocytes because these cultures already possessed maximal propagation dynamics prior to melatonin receptor activation; however, it is more likely that region-specific astrocytes have intrinsically difference cellular responses to melatonin receptor activation. In fact, the extent of astroglial calcium wave propagation varies across brain regions, with cortical and hippocampal waves often spreading twice that of those in astrocytes from hypothalamus and brain stem (Blomstrand et al., 1999). Interestingly, serotonin affects calcium signaling in a manner that is completely opposite that of melatonin. Serotonin decreases the spread of calcium waves in cortical and hippocampal astrocytes, but has no effect on hypothalamic astroglial waves and inhibits waves among brain stem astrocytes (Blomstrand et al., 1999).
Astrocytes respond to increases in brain activity, via neuronal glutamate release, by increasing consumption of glucose and production of lactate (Pellerin and Magistretti, 1994). Neurons then preferentially utilize this glia-supplied lactate to meet their energy demands, a process called the astrocyte-neuron lactate shuttle (Kasischke et al., 2004). Melatonin modulates glucose uptake in astrocytes and their production and release of pyruvate and lactate (Adachi et al., 2002). Sodium-dependent glucose uptake by glial cells is also influenced by intercellular calcium waves propagating among mouse cortical astrocytes (Bernardinelli et al., 2004) and astrocytic calcium signals influence endothelial and smooth muscle cells of brain vasculature, affecting blood flow (Braet et al., 2004). Astrocytes, therefore, function as a cellular network for metabolic coupling in the brain and this coupling can be regulated by intercellular calcium signal communication. Melatonin-mediated regulation of glial calcium signaling or glucose uptake, we predict, would have a significant homeostatic impact on melatonin-sensitive brain regions, particularly the hypothalamus, but also astrocyte-neuron networks in telencephalic structures. Interestingly, cortical mouse astrocytes have recently been showed to express genes involved in the regulation of circadian rhythmicity (Prolo et al., 2005). Thus, it is likely that the biological clock impacts cortical function. Whether or not melatonin might be involved in this regulation, even if not affecting calcium signaling, remains to be determined.
Although calcium waves among diencephalic, but not telencephalic, astrocytes were facilitated by melatonin in both mouse and chick, fundamental differences were observed between these chick and mouse intercellular waves. Calcium waves among chick astrocytes involved many fewer cells than did those in mouse cultures. Why do intercellular calcium waves spread better among mouse astrocytes than among chick astrocytes? These species differences are not likely due to cell cultures conditions as several were compared, including conventional chick astrocytic protocols and approaches that more closely match mammalian culture conditions. More likely, these differences reflect species-specific heterogeneity of astrocytes (e.g., receptor phenotypes, signaling mechanisms) in the glial cultures.
The mechanisms underlying mammalian calcium waves have been studied in much greater detail than avian calcium waves. Purinergic extracellular signaling via ATP mediates calcium waves among mammalian astrocytes (Guthrie et al. 1999), as well as other diffusible signals such as nitric oxide and prostaglandins (Charles, 1998). In addition, cytoplasmic signaling via gap junctions likely mediates calcium waves among some glia (Charles, 1998; Scemes, 2000; Bennett et al., 2003; Braet et al., 2004). It is likely that both avian and mammalian astrocytes employ combinations of signaling mechanisms for wave propagation. In fact, downregulation of connexin43 alters expression of the P2Y purinergic receptor subtype in spinal cord astrocytes, suggesting a complex interaction between gap junctional and extracellular modes of glial communication (Suadicani et al., 2003). The irregular, multidirectional and restricted spread of chick calcium waves indicates a greater role for gap junctions in these avian astrocytes. While in mouse astrocytes, the radial, pulsating and pervasive spread of calcium waves suggests a diffusible and less constrained paracrine mechanism. This idea is supported by calcium wave studies of chick diencephalic astrocytes that indicate substantial dye coupling among astrocytes and minimal effects of paracrine signaling antagonists (Peters et al., 2005) and studies of mouse telencephalic astrocytes where paracrine signaling via ATP is a key mechanism in wave propagation (Guthrie et al., 1999). Rat astrocyte cultures derived from the hypothalamus possess greater dye coupling and levels of connexin43 expression than cortical glial cell cultures (Blomstrand et al., 1999). In chick astrocytes, melatonin induces a fundamental shift in cell-cell communication, facilitating calcium waves, while suppressing gap junctional coupling and connexin43 expression (Peters et al., 2005). Interestingly, a potential role for connexins in both gap junctional and paracrine (i.e., functioning as hemichannels) signaling is emerging (Goodenough and Paul, 2003; Bennett et al., 2003). Thus, we hypothesize that those disparate mechanisms of astrocytic signaling account for the differences in propagation of intercellular calcium waves between birds and mammals. In support of this interpretation, disruption of extracellular signaling pathways known to mediate calcium waves among mammalian astrocytes, namely ATP, nitric oxide, and prostaglandins (Charles, 1998; Guthrie et al., 1999; Fam et al., 2000), have little effect on waves among chick diencephalic astrocytes (Peters et al., 2005).
Nocturnal versus diurnal activity patterns
Rodents are generally nocturnal animals (i.e., more active at night), whereas most birds are diurnal (i.e., more active in the day). One might expect pineal melatonin levels to be phase-linked with activity patterns across species; that is, melatonin should be highest in all species when they sleep. On the contrary, melatonin levels peak in the circulation of both diurnal birds and nocturnal rodents at night (Pang and Ralph, 1975; Cassone and Menaker, 1983; Cassone et al., 1986b). Therefore, the link between melatonin rhythms and activity rhythms must be found in the nature of the cellular and molecular mechanisms of melatonin action. In part, this will be answered as more becomes known about the signaling pathways involved in melatonin transduction in diverse species. As mentioned above, mammals and birds, express Mel1a (MT1) and Mel1b (MT2) receptors; however, birds, but not mammals, express the Mel1c receptor, particularly on astrocytes (Weaver et al., 1989; Cassone et al., 1995; Reppert et al., 1996; von Gall et al., 2002; Adachi et al., 2002). Nevertheless, with regard to intercellular calcium signaling, melatonin facilitated both avian and mammalian astrocytic waves.
Why is the effect of melatonin on calcium waves the same in nocturnal mice and diurnal chicks? In fact, although the melatonin-induced response was facilitatory in both species, the astrocytic responses were not identical. Melatonin facilitated avian diencephalic calcium signaling such that waves became less telencephalic in their propagation. In fact, chick diencephalic astrocytes exhibit a fundamental shift in intercellular coupling following activation of their melatonin receptors (Peters et al., 2005). This switch in mode of coupling involves a facilitation of paracrine signaling (i.e., intercellular calcium waves as reported here) and a suppression of cytoplasmic signaling (i.e., gap junctional coupling). In comparison, melatonin facilitated calcium waves in mouse diencephalic astrocytes such that their spread attained more telencephalic-like features, including a radial and concentric wave, rather than the meandering one present prior to melatonin receptor activation. Like chick astrocytes, melatonin may induce a shift in the mode of mammalian astroglial cell coupling that is detectable as alterations in the nature of wave transmission. Until more is known about the transduction pathways mediating melatonin receptor and calcium signaling in nocturnal and diurnal species, and how these cellular pathways mediate circadian behavior, the role of melatonin and glial calcium waves in these processes will remain unclear.
Glial calcium signaling and brain function
The similarity and disparity of calcium wave propagation between mouse and chick astrocytes are only meaningful if such waves impact brain function and animal behavior. Recently, a role for glia in modulating neuronal signaling in the brain and retina has been determined and has given rise to the tripartite synapse hypothesis (Araque et al., 1999; Volterra et al., 2002; Nedergaard et al., 2003; Newman, 2003). This idea implicates astrocytes in neural integration at synaptic contacts. Specifically, glial calcium signaling regulates neurotransmitter release from glia cells in cell culture (Parpura et al., 1994; Araque et al., 1999), modulates retinal activity (Newman and Zahs, 1998), and likely mediates glutamate release from astrocytes, which in turn synchronizes neuronal activity in hippocampal slices (Angulo et al., 2004).
Viewed in the context of the tripartite synapse hypothesis, a melatonin-mediated change in hypothalamic calcium waves would impart fundamental and far-reaching changes in an animal's physiology. Hypothalamic nuclei mediate vast regulatory influences over autonomic physiology, endocrine signaling, and behavioral states of vertebrate animals (Aston-Jones et al., 2001; Saper et al., 2001), including blood pressure, body temperature, sleep/wake cycles, food intake, and energy metabolism. It is, in fact, neurons of hypothalamic nuclei (i.e., the SCN) in mammals and birds that set the pace of biological rhythms (Ebihara and Kawamura, 1981; Takahashi and Menaker, 1982; Moore, 1983; Klein et al., 1997), including pineal production and release of melatonin (Cassone and Menaker, 1983; Klein et al., 1997; Moore & Silver 1998). Since high affinity melatonin receptors are expressed in neural cells of the hypothalamus, a regulatory feedback loop has been suggested involving the hypothalamus and the pineal gland (Cassone and Menaker, 1984; Cassone et al., 1986a; Cagampang et al, 1994). Melatonin alters diencephalic glial physiology in many ways, both in terms of metabolic activity (Adachi et al., 2002) and in cell-to-cell communication (Peters et al., 2005). The modulation of glial calcium waves reported here, defines a feedback mechanism by which melatonin could profoundly alter the synchrony of neuronal activities in the hypothalamus. Thus, the daily rise and fall of melatonin levels in the brain would profoundly impact the function of the same neural structures that drive the ebb and flow of this neurohormone's production.
Acknowledgments
This research was supported by NINDS Grant PO1 NS-39546 (VMC and MJZ). Ratiometric image analysis was conducted in Cell Physiology and Molecular Image Core Facility in the Department of Biology at Texas A&M University.
Contributor Information
Jennifer L. Peters, Email: jpeters@mail.bio.tamu.edu.
Barbara J. Earnest, Email: bearnest@mail.bio.tamu.edu.
Ronald B. Tjalkens, Email: Ron.Tjalkens@colostate.edu.
Vincent M. Cassone, Email: vmc@mail.bio.tamu.edu.
References
- Adachi A, Natesan AK, Whitfield-Rucker MG, Weigum SE, Cassone VM. Functional melatonin receptors and metabolic coupling in cultured chick astrocytes. Glia. 2002;39:268–278. doi: 10.1002/glia.10109. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgirl RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- Braet K, Cabooter L, Paemeleire K, Leybaert L. Calcium signal communication in the central nervous system. Biol Cell. 2004;96:79–91. doi: 10.1016/j.biolcel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Brooks DS, Cassone VM. Daily and circadian regulation of 2-[125I]iodomelatonin binding in the chick brain. Endocrinology. 1992;131:1297–1304. doi: 10.1210/endo.131.3.1324157. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Okamura H, Inouye S. Circadian rhythms of norepinephrine in the rat suprachiasmatic nucleus. Neurosci Lett. 1994;173:185–188. doi: 10.1016/0304-3940(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Cantwell EL, Cassone VM. Daily and circadian fluctuation in 2-deoxy[14C]-glucose uptake in circadian and visual system structures of the chick brain: effects of exogenous melatonin. Brain Res Bull. 2002;57:603–611. doi: 10.1016/s0361-9230(01)00753-5. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Menaker M. Sympathetic regulation of chicken pineal rhythms. Brain Res. 1983;272:311–317. doi: 10.1016/0006-8993(83)90578-4. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Menaker M. Is the avian circadian system a neuroendocrine loop? J Exp Zool. 1984;232:539–549. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Chesworth MJ, Armstrong SM. Entrainment of rat circadian rhythms by daily injection of melatonin depends upon the hypothalamic suprachiasmatic nuclei. Physiol Behav. 1986a;36:1111–1121. doi: 10.1016/0031-9384(86)90488-9. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Lane RF, Menaker M. Melatonin-induced increases in serotonin concentrations in specific regions of the chicken brain. Neuroendocrinology. 1986b;42:38–43. doi: 10.1159/000124246. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Roberts MH, Moore RY. Effects of melatonin on 2-deoxy-[1-14C]glucose uptake within rat suprachiasmatic nucleus. Am J Physiol. 1988;255:R332–337. doi: 10.1152/ajpregu.1988.255.2.R332. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Forsyth AM, Woodlee GL. Hypothalamic regulation of circadian noradrenergic input to the chick pineal gland. J Comp Physiol [A] 1990;167:187–192. doi: 10.1007/BF00188110. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Brooks DS, Kelm TA. Comparative distribution of 2-[125I]iodomelatonin binding in the brains of diurnal birds: outgroup analysis with turtles. Brain Behav Evol. 1995;45:241–256. doi: 10.1159/000113553. [DOI] [PubMed] [Google Scholar]
- Charles A. Intercellular calcium waves in glia. Glia. 1998;24:39–49. doi: 10.1002/(sici)1098-1136(199809)24:1<39::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47:284–289. doi: 10.1002/glia.20086. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Mahesh VB, Brann DW. Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 2003;228:253–260. doi: 10.1177/153537020322800303. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of Mammalian melatonin receptors. Front Biosci. 2003;8:D1093–1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Kawamura H. The role of pineal melatonin and the suprachiasmatic nucleus in the control of circadian locomotor rhythms in the Java sparrow, Padda orizivora. J Comp Physiol [A] 1981;131:207–214. [Google Scholar]
- Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res. 1996;73:135–139. doi: 10.1016/0166-4328(96)00085-x. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, Falcon J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. [PubMed] [Google Scholar]
- Lu J, Cassone VM. Daily melatonin administration synchronizes circadian patterns of brain metabolism and behavior in pinealectomized house sparrows, Passer domesticus. J Comp Physiol [A] 1993;173:775–782. [Google Scholar]
- Magistretti PJ. Cellular bases of functional brain imaging: insights from neuronglia metabolic coupling. Brain Res. 2000;886:108–112. doi: 10.1016/s0006-8993(00)02945-0. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- Morin LP, Johnson RF, Moore RY. Two brain nuclei controlling circadian rhythms are identified by GFAP immunoreactivity in hamsters and rats. Neurosci Lett. 1989;99:55–60. doi: 10.1016/0304-3940(89)90264-4. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman S. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–74. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang SF, Ralph CL. Pineal and serum melatonin at midday and midnight following pinealectomy or castration in male rats. J Exp Zool. 1975;193:275–280. doi: 10.1002/jez.1401930304. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Cassone VM, Zoran MJ. Melatonin modulates intercellular communication among cultured chick astrocytes. Brain Res. 2005;1031:10–19. doi: 10.1016/j.brainres.2004.09.064. [DOI] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. 2003;23:9888–9896. doi: 10.1523/JNEUROSCI.23-30-09888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Perlow MJ, Ungerleider LG, Mishkin M, Tamarkin L, Orloff DG, Hoffman HJ, Klein DC. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J Neurosci. 1981;1:1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Cassone VM, Godson C, Kolakowski LF., Jr Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron. 1995;15:1003–1015. doi: 10.1016/0896-6273(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17:100–102. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Cassone VM, Weaver DR, Reppert SM. Melatonin receptors in chick brain: characterization and localization. Endocrinology. 1989;125:363–368. doi: 10.1210/endo-125-1-363. [DOI] [PubMed] [Google Scholar]
- Rusak B. Neural mechanisms for entrainment and generation of mammalian circadian rhythms. Fed Proc. 1979;38:2589–2595. [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Scemes E. Components of astrocytic intercellular calcium signaling. Mol Neurobiol. 2000;22:167–179. doi: 10.1385/MN:22:1-3:167. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Cassone VM, Moore RY. Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1989;97:140–144. doi: 10.1016/0304-3940(89)90153-5. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–96. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, De Pina-Benabou MH, Urban-Maldonado M, Spray DC, Scemes E. Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes. Glia. 2003;42:160–171. doi: 10.1002/glia.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Menaker M. Role of the suprachiasmatic nuclei in the circadian system of the house sparrow, Passer domesticus. J Neurosci. 1982;2:815–828. doi: 10.1523/JNEUROSCI.02-06-00815.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Magistretti PJ, Haydon PG. The Tripartite Synapse: Glia in Synaptic Transmission. Oxford: Oxford UP; 2002. [Google Scholar]
- Volterra A, Steinhauser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–57. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J Neurosci. 1989;9:2581–2590. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Weissman T, Riquelme P, Ivic L, Flint A, Kriegstein A. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2005;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Witkin JW, O'Sullivan H, Ferin M. Glial ensheathment of GnRH neurons in pubertal female rhesus macaques. J Neuroendocrinol. 1995;7:665–671. doi: 10.1111/j.1365-2826.1995.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Witkin JW, O'Sullivan H, Miller R, Ferin M. GnRH perikarya in medial basal hypothalamus of pubertal female rhesus macaque are ensheathed with glia. J Neuroendocrinol. 1997;9:881–885. doi: 10.1046/j.1365-2826.1997.00649.x. [DOI] [PubMed] [Google Scholar]