Abstract
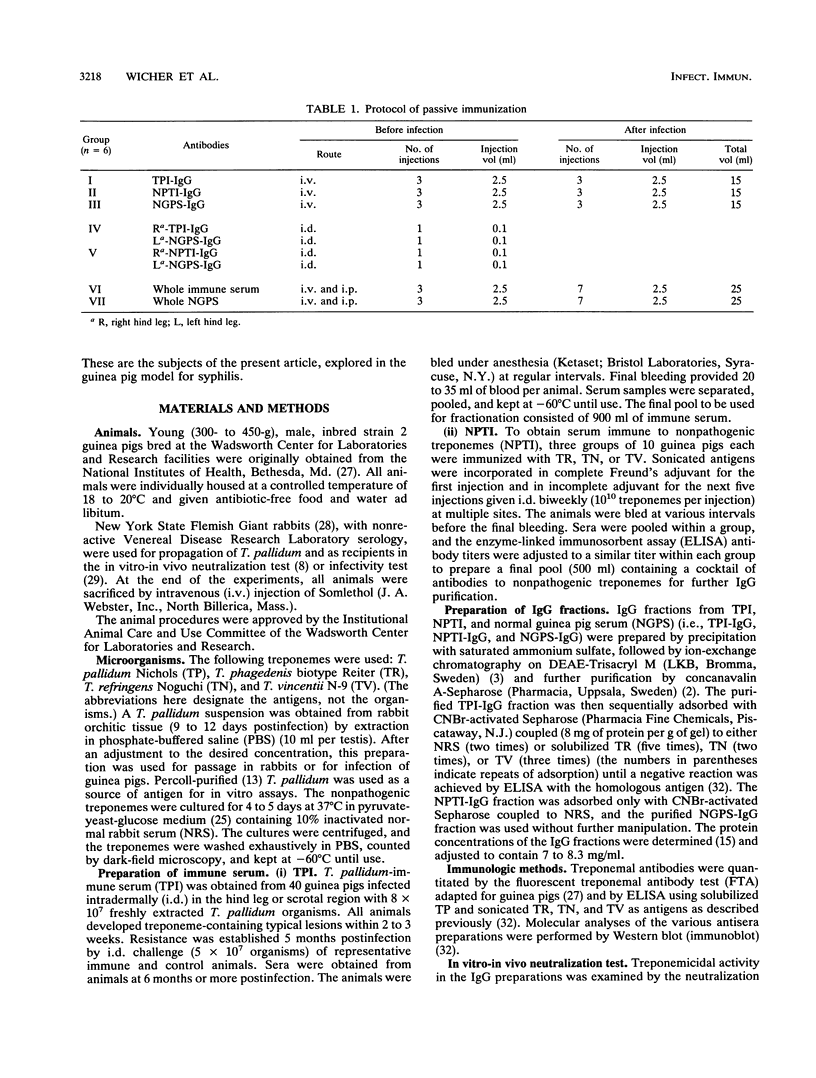
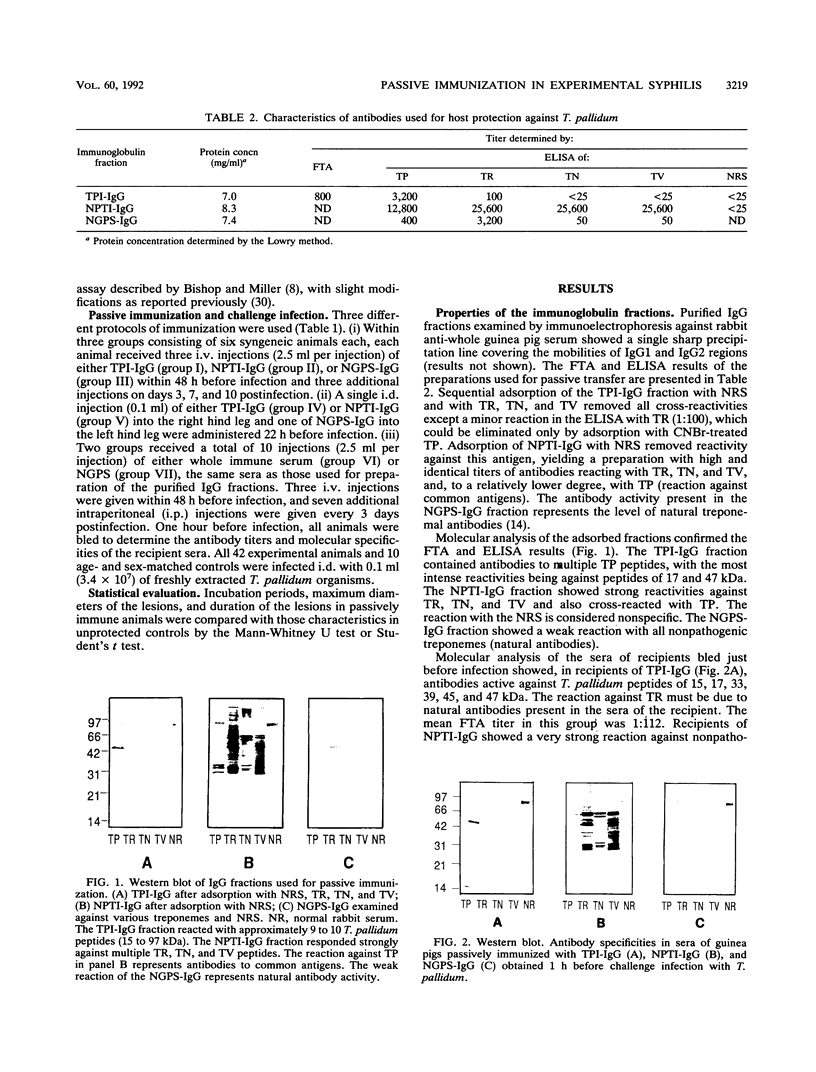
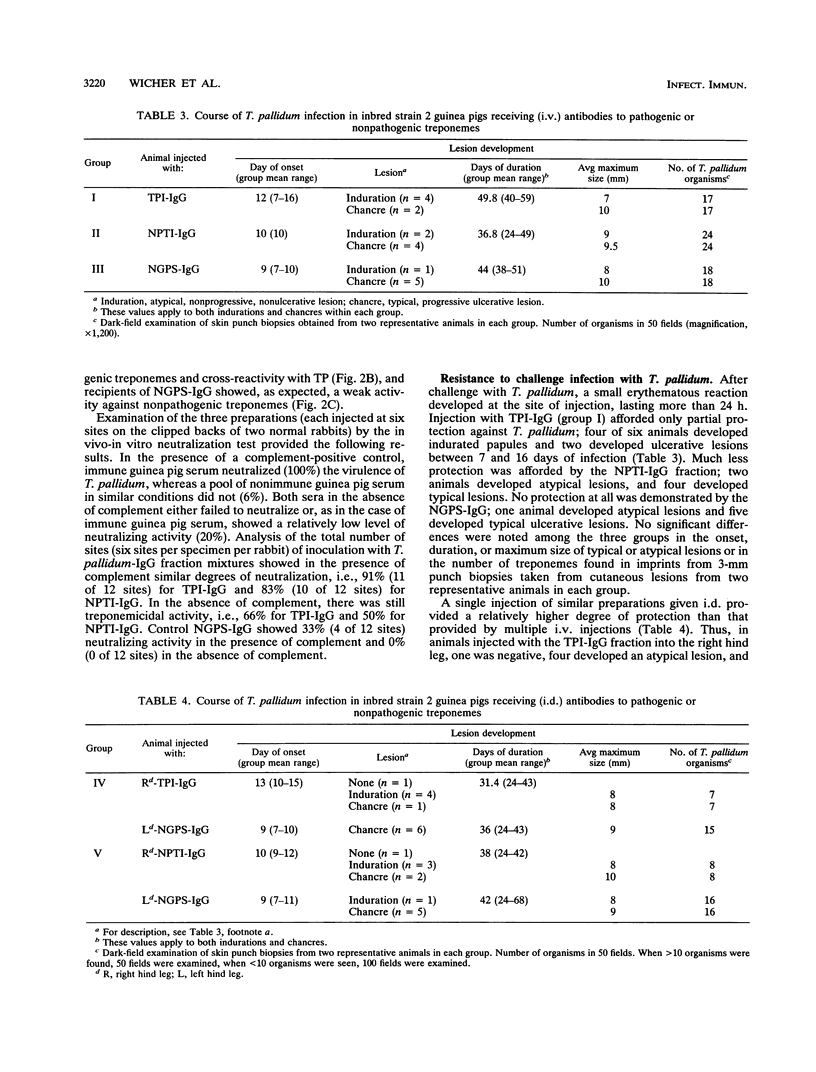
Whole immune serum or highly purified immunoglobulin G (IgG) antibodies to Treponema pallidum exhaustively adsorbed with three strains of nonpathogenic treponemes (TPI-IgG) were used for passive immunization of inbred strain 2 guinea pigs before and after intradermal challenge with 3.4 x 10(7) virulent T. pallidum Nichols organisms. Before challenge, control animals received a similarly purified IgG fraction containing either a cocktail of antibodies against three nonpathogenic treponemes (NPTI-IgG) or IgG prepared from normal guinea pig serum (NGPS-IgG). The purified fractions contained both IgG1 and IgG2 isotypes. The antibody levels (detected by fluorescent treponemal antibody test and enzyme-linked immunosorbent assay) and molecular specificities (immunoblot) of sera obtained from recipient animals before infection reflected those of the purified fractions used for immunization. Three protocols of passive immunization were used. Whole immune serum containing specific and cross-reacting antibodies afforded better protection than TPI-IgG even though asymptomatic animals were not fully protected. A single intradermal injection (0.1 ml) of TPI-IgG or NPTI-IgG into one hind leg 22 h before infection at the same site provided relatively higher protection than multiple intravenous injections (total, 15 ml) of the respective individual preparations. Since purified NGPS-IgG injected in the same animals, into the opposite hind leg, failed to protect against the challenging infection, it is reasonable to assume that specific and cross-reacting antitreponemal antibodies of the IgG1 subclass, which in guinea pigs are homocytotropic, play a relevant role in local protection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder J. D., Daugherty N., Harris O. N., Liu H., Steiner B. M., Schell R. F. Phagocytosis of Treponema pallidum pertenue by hamster macrophages on membrane filters. J Infect Dis. 1989 Aug;160(2):289–297. doi: 10.1093/infdis/160.2.289. [DOI] [PubMed] [Google Scholar]
- Azadegan A. A., Schell R. F., LeFrock J. L. Immune serum confers protection against syphilitic infection on hamsters. Infect Immun. 1983 Oct;42(1):42–47. doi: 10.1128/iai.42.1.42-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadegan A. A., Schell R. F., Steiner B. M., Coe J. E., Chan J. K. Effect of immune serum and its immunoglobulin fractions on hamsters challenged with Treponema pallidum ssp. pertenue. J Infect Dis. 1986 Jun;153(6):1007–1013. doi: 10.1093/infdis/153.6.1007. [DOI] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Chan J. K., Schell R. F., LeFrock J. L. Ability of enriched immune T cells to confer resistance in hamsters to infection with Treponema pertenue. Infect Immun. 1979 Nov;26(2):448–452. doi: 10.1128/iai.26.2.448-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Alden J. Limited protection of rabbits against infection with Treponema pallidum by immune rabbit sera. Br J Vener Dis. 1979 Dec;55(6):399–403. doi: 10.1136/sti.55.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Jakubowski A., Wicher V., Gruhn R., Wicher K. Natural antibodies to treponemal antigens in four strains of guinea-pigs. Immunology. 1987 Feb;60(2):281–285. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- MILLER J. N., WHANG S. J., FAZZAN F. P. STUDIES ON IMMUNITY IN EXPERIMENTAL SYPHILIS. I. IMMUNOLOGIC RESPONSE OF RABBITS IMMUNIZED WITH REITER PROTEIN ANTIGEN AND CHALLENGED WITH VIRULENT TREPONEMA PALLIDUM. Br J Vener Dis. 1963 Sep;39:195–198. doi: 10.1136/sti.39.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Acquired resistance and expression of a protective humoral immune response in guinea pigs infected with Treponema pallidum Nichols. Infect Immun. 1985 Oct;50(1):66–72. doi: 10.1128/iai.50.1.66-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J., Saunders J. Antibody-mediated protection of guinea-pigs against infection with Treponema pallidum. Immunology. 1985 Oct;56(2):195–202. [PMC free article] [PubMed] [Google Scholar]
- Sepetjian M., Salussola D., Thivolet J. Attempt to protect rabbits against experimental syphilis by passive immunization. Br J Vener Dis. 1973 Aug;49(4):335–337. doi: 10.1136/sti.49.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- Turner T. B., Hardy P. H., Jr, Newman B., Nell E. E. Effects of passive immunization on experimental syphilis in the rabbit. Johns Hopkins Med J. 1973 Nov;133(5):241–251. [PubMed] [Google Scholar]
- Van Horn K. G., Smibert R. M. Fatty acid requirement of Treponema denticola and Treponema vincentii. Can J Microbiol. 1982 Mar;28(3):344–350. doi: 10.1139/m82-051. [DOI] [PubMed] [Google Scholar]
- Weiser R. S., Erickson D., Perine P. L., Pearsall N. N. Immunity to syphilis: passive transfer in rabbits using serial doses of immune serum. Infect Immun. 1976 May;13(5):1402–1407. doi: 10.1128/iai.13.5.1402-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Wicher V., Gruhn R. F. Differences in susceptibility to infection with Treponema pallidum (Nichols) between five strains of guinea pig. Genitourin Med. 1985 Feb;61(1):21–26. doi: 10.1136/sti.61.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Wicher V., Nakeeb S. M., Dubiski S. Studies of rabbit testes infected with Treponema pallidum. I Immunopathology. Br J Vener Dis. 1983 Dec;59(6):349–358. doi: 10.1136/sti.59.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K., Jakubowski A., Nakeeb S. M. Adoptive transfer of immunity to Treponema pallidum Nichols infection in inbred strain 2 and C4D guinea pigs. Infect Immun. 1987 Oct;55(10):2502–2508. doi: 10.1128/iai.55.10.2502-2508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K., Rudofsky U., Zabek J., Jakubowski A., Nakeeb S. Experimental neonatal syphilis in a susceptible (C4D) and a resistant (Albany) strain of guinea pig. Clin Immunol Immunopathol. 1990 Apr;55(1):23–40. doi: 10.1016/0090-1229(90)90066-y. [DOI] [PubMed] [Google Scholar]
- Wicher V., Zabek J., Wicher K. Kinetics of pathogen-specific humoral response in Treponema pallidum-infected young and old inbred strain 2 guinea pigs. Clin Exp Immunol. 1989 Jul;77(1):144–150. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Zabek J., Wicher K. Pathogen-specific humoral response in Treponema pallidum-infected humans, rabbits, and guinea pigs. J Infect Dis. 1991 Apr;163(4):830–836. [PubMed] [Google Scholar]
- Wos S. M., Wicher K. Extensive cross reactivity between Treponema pallidum and cultivable treponemes demonstrated by sequential immunoadsorption. Int Arch Allergy Appl Immunol. 1986;79(3):282–285. doi: 10.1159/000233987. [DOI] [PubMed] [Google Scholar]





