Abstract
Noise exposure is one of the most common causes of hearing loss. There is growing evidence suggesting that noise induced peripheral hearing loss can also induce functional changes in the central auditory system. However, the physiological and biological changes in the central auditory system induced by noise exposure are poorly understood. To address these issues, neurophysiological recordings were made from the auditory cortex (AC) of awake rats using chronically implanted electrodes before and after acoustic overstimulation. In addition, focused gene microarrays and quantitative real-time PCR were used to identify changes in gene expression in the AC. Monaural noise exposure (120 dB SPL, 1 h) significantly elevated hearing threshold on the exposed ear and induced a transient enhancement on the AC response amplitude 4 h after the noise exposure recorded from the unexposed ear. This increase of the cortical neural response amplitude was associated with an upregulation of genes encoding heat shock protein (HSP) 27 kDa and 70 kDa after several hours of the noise exposure. These results suggest that noise exposure can induce a fast physiological change in the AC which may be related to the changes of HSP expressions.
Keywords: auditory cortex (AC), gene expression, heat shock protein (HSP), noise exposure, tinnitus
Introduction
Noise exposure not only results in temporary or permanent hearing loss, but can also induce transient or permanent tinnitus, loudness recruitment and hyperacusis (Axelsson and Hamernik, 1987, Moller, 2007). Previous physiological studies suggest that the reduced neural output from the damaged cochlea alters the central auditory system by increasing its gain and possibly contributing to tinnitus, loudness recruitment and hyperacusis (Salvi et al., 2000, Wang et al., 2002). In addition, human brain imaging studies found changes in neural activity in the AC that were closely linked to changes in the loudness of tinnitus (Lockwood et al., 1998, Reyes et al., 2002). Moreover, sound stimulation evoked greater activity in the AC of patients with tinnitus and cochlear hearing loss (Lockwood et al., 1998). Similarly, in animal studies, a moderate high-frequency noise exposure can induce a significant increase of evoked potential amplitude and spontaneous neural activities (Popelar et al., 1987, Komiya and Eggermont, 2000). However, the molecular mechanisms that give rise to these changes in neural activity of the central auditory system are not clear. In order to elucidate the early molecular mechanisms associated with noise-induced hearing loss and cortical hyperactivity, we used focused gene microarrays and quantitative real time PCR to identify the changes in gene expression that occur in the rats AC following a monaural noise exposure similar to that used in behavioral studies of noise-induced tinnitus used in our and other labs (Brozoski et al., 2007).
Experimental procedures
Animals
Adult Harlan Sprague-Dawley male rats (3–5 months old) weighing from 200–400 g were used for all studies. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo.
Surgery procedures and evoked potential recordings
The rats used for the neurophysiological recordings were implanted with a chronic electrode in the left AC using procedures described in our previous publication (Yang et al., 2007). Rats were anesthetized with 1–2% isoflurane. The left AC was surgical exposed using anatomical location and landmarks on the skull and blood vessels on the surface of the cortex (Sally and Kelly, 1988, Polley et al., 2007). The dura mater was excised and then a silver ball electrode made from Teflon coated wire (0.008’’ diameter, A–M systems) connected to a miniature pin connector was placed on the AC. A stainless steel electrode (0.008’’ diameter, A–M systems) connected to a pin connector was placed on the surface of the frontal lobe and served as ground electrode. The electrode connectors were firmly attached to the skull using the cyanoacrylic glue and the dental cement. Six small stainless steel screws (1/8’’, Small Parts Inc.) were attached to the skull and covered with dental cement; these were used to help anchor a head restraint screw (1/2’’ long with ¼’’ diameter) to the skull using dental cement. The wound was sutured around the electrode connector and then the animal was allowed to recover for 7–10 days before the physiological test.
Sound stimuli were generated with Tucker-Davis Technologies (TDT) hardware and presented through a high frequency speaker (FT28D, Fostex). Tone-bursts (10 ms duration, 1 ms rise/fall time, twice per second) from 4 to 32 kHz were used to elicit the AC local field potential. Responses were amplified and filtered (10–3000 Hz) and digitized (25 kHz sampling rate, sampling duration 300 ms). For the auditory brainstem response (ABR) recording, the implanted stainless steel electrode on the surface of the frontal lobe was used as the non-inverted recorded side, the ipsilateral pinna was used as the inverted reference and contralateral pinna was used as the ground. The sound stimuli used to elicit the AC response were also used for ABR recording. The sound levels were calibrated using a sound level meter (824, Larson Davis, ½”microphone). The electrodes for the AC and ABR recordings were connected to a preamplifier (RA16LA, TDT) using a flexible, low noise cable. The output of the preamplifier was sent to a digital signal processing module (RX5-2, Pentusa Base Station, TDT) and collected by software (BioSigRP version 4.4, TDT).
Focused gene microarrays
Noise induced changes in gene expression in the AC were evaluated with the Signal Transduction Pathway Finder Array (ORN-014, SuperArray Bioscience Corp.). This gene array contains 112 genes, includes 96 genes associated with common signal transduction pathways, several house keeping genes such as β-actin, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), cyclophilin, ribosomal protein L13a which are relatively abundant and used as a comparison for estimating signal intensity of the target genes, and several negative controls used for quality control (Figure 3). One group of rats (n = 3) was anesthetized with isoflurane (1–2%). The left ear of each rat was exposed for 1 h to a 120 dB SPL narrow band noise (12 kHz, 1000 Hz bandwidth), while the right ear was sealed with cotton and Vaseline. The other group (n = 3) was anesthetized with isoflurane (1–2 %) without noise exposure and served as the control group.
Figure 3.
The gene array results of AC from a control rat (left) and a noise exposure rat (right). On each array, the left top one and six dots on bottom row were different house keeping genes used as the baseline of gene expression. Comparative analysis between the control and noise exposed rats in gene arrays shows the darkness of the right three dots on row 6 which indicates heat shock protein genes (HSP) have strong difference (arrows). Cyclin-dependent kinase inhibitor 1A (CDKN-1A, row three) expression also showed an obvious increase.
Two hours after the noise exposure, rats were sacrificed with an overdose of CO2. The AC tissues were collected for gene array and PCR studies. The total RNA of the AC was extracted using a Total RNA Isolation kit (Z3100, Promega) and mRNA was reverse transcribed to cDNA using a TrueLabeling-Amp Linear RNA Amplification Kit (GA-010, SuperArray). cDNA was labeled with Biotin-16-UTP (#1388908, Roche) and transcript to cRNA, and then purified using the cRNA Cleanup Kit (GA-012, SuperArray). The SuperArray membrane (ORN-014, SuperArray) was rinsed with hybridization solution (H-01, SuperArray) and then incubated with the biotin-labeled cRNA for 1 h in a hybridization oven. The membrane was washed twice with 5 ml washing solution and incubated for 15 min at 60°C with agitation, blocked with GEAblocking solution for 1 h and then treated with chemiluminescence solution (D-01, SuperArray). An image of the array was captured by a Kodak image system and the density of each dot on the membrane was measured using the Kodak Image software.
Quantitative real time PCR
To confirm the findings in the gene array study, quantitative real-time PCR was used to assess the changes in a subset of genes that shows a large change in expression in the gene array. The procedure used for real-time PCR and data analysis were described in our previous publication (Sun et al., 2005). Briefly, after the total RNA was extracted, the mRNA was reverse transcribed to cDNA using a First Strand Synthesis Kit (Cat. 1710, Ambion). Five µl of cDNA solution containing approximately 200 ng of cDNA isolated from the tissue was added to the reaction mixture (10 µl, PA-011, SuperArray) along with 5 µl of forward and reverse primers mixture (concentration 1.25 µM) and pipetted into a well in a 96-well plate (MyIQ, BioRad). Genes analyzed in this experiment included heat shock protein (HSP) 27 kDa, 70 kDa and 90 kDa, Cyclin-dependent kinase inhibitor 1A (CDKN1A) and Cathepsin D (CTSD). GAPDH, an abundantly expressed house-keeping gene which was stably expressed in control and experimental groups, was used as a reference for calculating the change in expression of the target genes. Primers used for real-time PCR tests were ordered from SuperArray Company and had previously been optimized by the vendor. SYBR green fluorescence was measured during amplification and the cycle threshold (CT) was determined when the fluorescence showed a dramatic increase above the background level. The fold changes of the target genes in the experimental group were normalized to GAPDH and the relative changes compared to the control group was calculated for each sample using the equation of 2−ΔΔCT, where ΔΔCT = (CT,Target−CT,GAPDH)experiment−(CT, Target − CT, GAPDH)control (Livak and Schmittgen, 2001). CT, Target represents the cycle threshold of the target gene and CT, GAPDH represents the cycle threshold of GAPDH. The analyses were replicated for each gene.
The graphs and statistic analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) unless otherwise noted. Variability is indicated by the standard error of the mean.
Results
ABR threshold shift
The magnitude of noise induced hearing loss was evaluated using the ABR. The average ABR thresholds on noise exposed ears before and 2 h after the noise exposure are shown in Figure 1A. Before the noise exposure, the average ABR thresholds (Mean±SEM, n = 6) were 45 ± 2 dB, 45 ± 2 dB, 37 ± 1.6 dB, 36 ± 1.6 dB, 39 ± 3.9 dB, 48 ± 1.6 dB and 47 ± 2.5 dB at 4, 6, 8, 12, 16, 24 and 32 kHz respectively. Two hours after the noise exposure, the average ABR thresholds (n = 6) increased to 47 ± 1.7 dB, 46 ± 1 dB, 43 ± 4 dB, 67 ± 4 dB, 58 ± 7 dB, 62 ± 6 dB and 58 ± 5 dB at 4, 6, 8, 12, 16, 24 and 32 kHz respectively. The threshold changes at 12, 16 and 24 kHz in the exposed ears were significant (Paired t-test, p<0.05) (Figure 1A). The unexposed ears showed less than 10 dB threshold shift (Figure 1B). The threshold changes at 4, 6, 16, 24, and 32 kHz were not significant, however, at 8 and 12 kHz, the threshold shifts were significant (Paired t-test, p<0.05) (Figure 1B).
Figure 1.
Noise induced ABR threshold shifts 2 hours after the noise exposure. (A) The narrow band noise (120 dB SPL with 1000 Hz bandwidth for 1 h) induced about 30 dB SPL threshold shift at 12 kHz on the exposed ear, and about 20 dB shift at 12 kHz and no threshold shift at 4 to 6 kHz. (B) There were less than 10 dB threshold shifts on non-exposed ears. (* p<0.05)
AC input/output functions
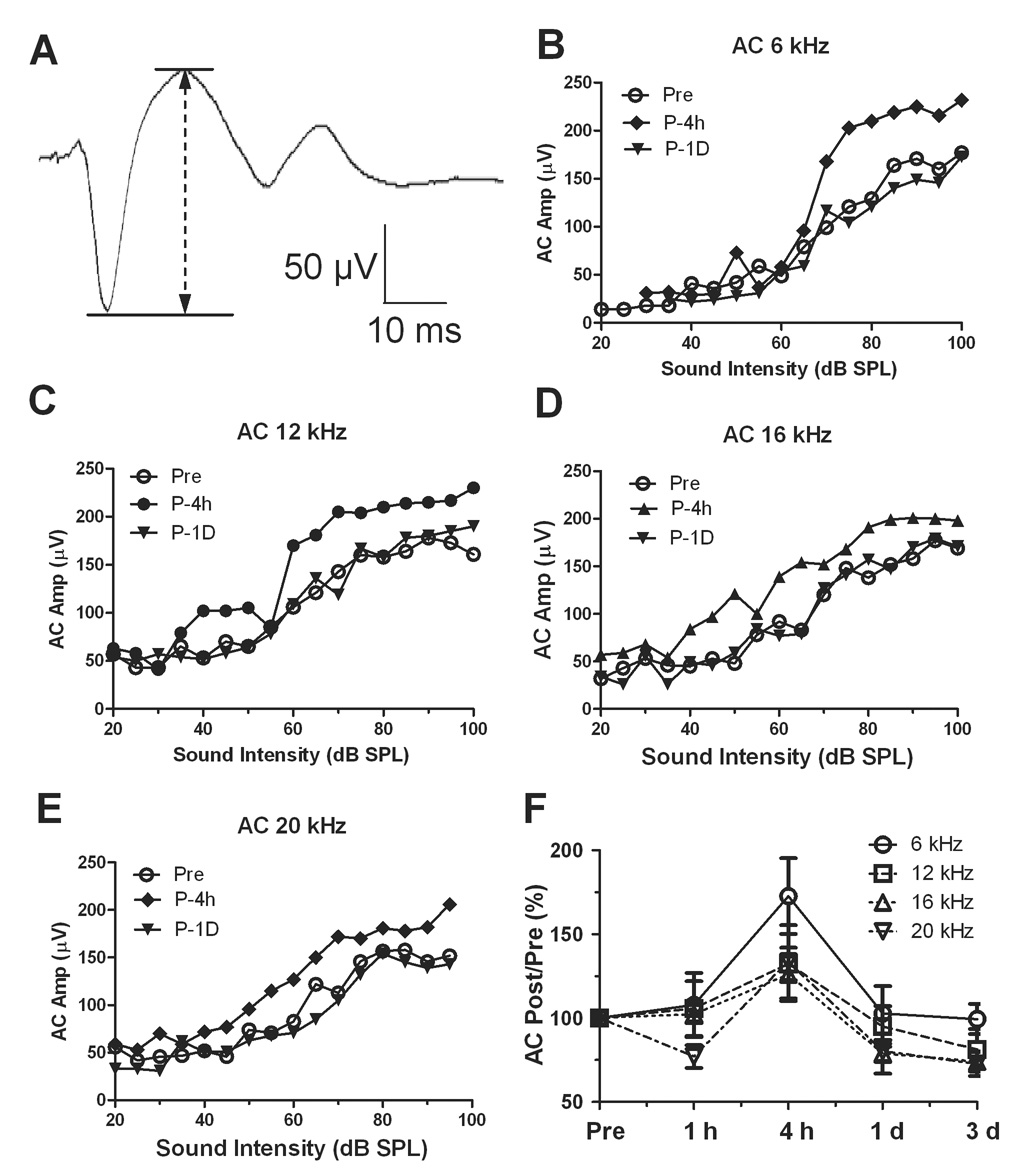
The AC local field potentials were evoked by tone-bursts centered at 6, 12, 16 and 20 kHz from 20 to 100 dB SPL. A typical AC response waveform is shown in Figure 2A. The AC response was recorded from the left AC and sound was delivered to the right ear (none-exposed ear) on each animal. Figure 2B–E showed typical input-output curves of the AC response measured at 6, 12, 16 and 20 kHz before and 4 h and 1 day post-exposure. Before the exposure, the AC amplitude increased monotonically above 40 dB SPL reaching its maximum amplitude around 100 dB SPL. The AC response showed a large increase 4 h after the noise exposure at sound level above 60 to 70 dB SPL; however, by 1 d post-exposure the amplitudes had returned to essentially normal levels.
Figure 2.
Noise exposure (120 dB SPL narrow band noise with 1000 Hz bandwidth for 1 h) induced enhancement of AC response evoked by tone bursts at 90 dB SPL. (A) A typical AC response recorded an awake rat. (B–D) The input-output functions of AC response induced by tone burst at 6, 12 and 16 kHz recorded at 4h after the noise exposure. (E) The ratio of the AC response post-exposure to pre-exposure amplitude showed less change at 1 h after the exposure, but an obvious increase at 4 h which recovered one day after the noise exposure
In order to measure the average amplitude enhancement across the entire group of animals, the post-exposure amplitude at 90 dB SPL was normalized to the pre–exposure amplitude measured at the highest intensity (100 dB SP) at each frequency. Figure 2F shows the post-exposure amplitude at 90 dB SPL expressed in percent of the Post/Pre-exposure ratio. The mean (n = 3) Post/Pre amplitude ratio at 6, 12, 16 and 20 kHz showed relatively little change at 1 h post-exposure, but at 4 h post-exposure the mean AC amplitude had increased by 72% ± 39%, 32% ± 16%, 26% ± 27% and 33% ± 37% at 6, 12, 16 and 20 kHz respectively. The changes at 6 and 12 kHz were statistically significant compared to 1 h after noise exposure (Student’s t-test, p<0.05). The AC amplitudes recovered to normal levels by 1 day post-exposure. Despite the noise-induced elevation in the hearing threshold, the AC response showed a transient increase and was most prominent at 6 and 12 kHz.
Gene expression changes induced by the noise exposure
To gain insights into the mechanisms that may contribute to the AC amplitude enhancement, a focused gene microarray was used to identify potential genes of interest in different signal transduction pathway after the noise exposure. Figure 3A–B shows an example of typical membranes from Signal Transduction Pathway Finder Microarrays obtained from the AC harvested from rats with and without noise exposure. The mean intensities of the six housekeeping genes at the bottom of these arrays were used to normalize the expression of target genes on each array. To quantify the gene expression changes, the average signal intensity of the target gene was normalized to the signal intensity for the housekeeping gene on each of the arrays obtained from noise exposed samples (n = 3) versus control samples (n = 3). Genes that showed an increase or decrease of 50% (≥1.5 fold, ≤0.5 fold) or more were considered to be biologically significant. Four genes on the signal transduction pathway array showed increases of 50% ore more. Compared to the control group, the average gene expression in the noise group showed an approximate increase of 1.8 fold (80%), 1.85 fold (85%), 1.85 fold (85%) and 1.87 fold (87%) on HSP-1, HSP-86, CDKN-1A and CTSD respectively. One gene in the noise-exposed array showed a large decease in relative intensity compared to the control array. The average normalized intensity of ei24 gene, involved in growth suppression and apoptosis in the p53-dependent pathway (Gu et al., 2000), showed a 0.58 fold change (42% decrease).
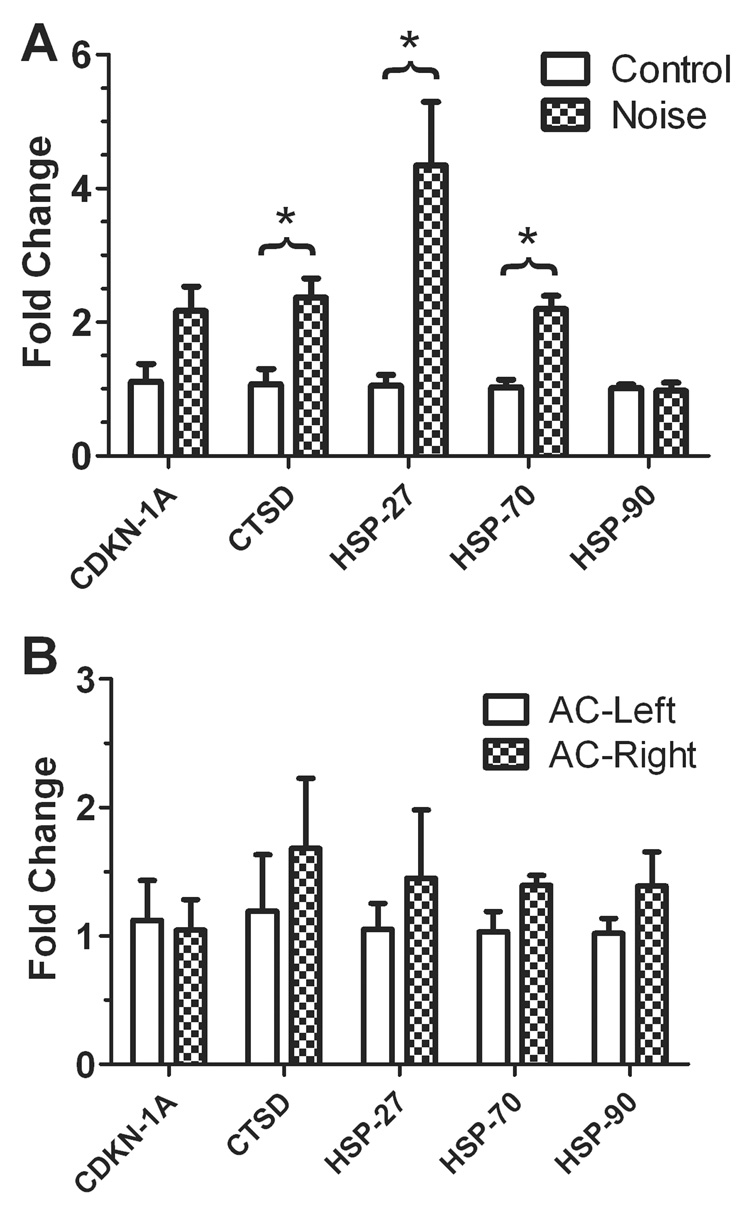
Quantitative RT-PCR was used to confirm the gene array studies using AC tissues from two control rats and four noise-exposed rats. The relative expression of HSP-27, HSP-70, HSP-90, CDKN-1A and CTSD genes were compared in the AC collected from noise-exposed rats compared to the AC collected from control animals. GAPDH, a house keeping gene used in the gene array, was used as a reference. Compared to the control group, the expression level of HSP-27, HSP-70 and CTSD showed a significant increase after the noise exposure (Student’s t-test, p<0.05, Figure 4A), whereas the changes in HSP-90 and CDKN-1A were not statistically significant. The expression of these genes in the left side AC and the right AC were also compared in the noise exposure group. None of these genes showed significant changes compared to the right AC (contralateral to the exposed ear) to the left AC (ipsilateral to the exposed ear)(Figure 4B).
Figure 4.
The relative gene expression changes of HSP-27, HSP-70, HSP-90, CDKN-1A and CTSD in the AC induced by noise exposure. (A) The expression of HSP-27, HSP-70 and CTSD showed significant changes in the AC collected from noise exposed group compared to the control group (*, p<0.05). The expression change of HSP-90 and CDKN-1A were not significant. (B) Comparison of the gene expression in the left AC (ipsilateral to the exposed ear) to the right AC (contralateral to the exposed ear) in the noise exposed rats. The expression of CTSD, HSP-27, HSP-70 and HSP-90 was slightly higher in the right AC compared to the left AC, but not significant.
Discussion
AC amplitude enhancement
Acoustic overstimulation that damages the cochlea can lead to significant functional changes in the AC. One of the most striking examples of this is the tonotopic map reorganization seen after selective cochlear damage. Significant tonotopic map reorganization has been seen in primary AC 7–16 weeks after intense noise exposure (126 dB SPL) in kittens; the region of the AC normally tuned to 10–40 kHz was re-tuned to 10 kHz after the noise exposure (Komiya and Eggermont, 2000). The spontaneous firing rates were much higher in the reorganized area compared to the un-reorganized region. These changes, however, occur over weeks or months unlike the rapid functional changes seen here.
Our results show that high intensity noise exposure that damages the cochlea can increase the amplitude of the AC evoked response to sounds presented at suprathreshold levels. Such an increase is counterintuitive since the noise exposure reduces the neural output from the cochlea (Salvi et al., 1979). The AC amplitude enhancement observed here is similar to previous reports showing an increase in AC sound evoked activity following cochlear damage from ototoxic drugs and acoustic overstimulation (Popelar et al., 1987, Popelar et al., 1994, Qiu et al., 2000, Salvi et al., 2000). In the case of bilateral noise exposure, a significant reduction was observed in AC potentials immediately after the exposure which was followed by enhanced AC responses a week after the exposure (Syka and Rybalko, 2000). Others, however, have reported enhanced AC amplitudes 1 and 24 h after bilateral noise exposure. Importantly, the AC amplitude was enhanced despite large reductions in neural responses from the cochlea and inferior colliculus (IC) (Popelar et al., 1987). Importantly, the AC amplitude enhancement was abolished when recordings were made under anesthesia. Thus the reduced AC responses seen in some studies following acoustic over-stimulation may be due to the effects of anesthesia (Tan et al., 2007).
Gene expression
The AC amplitude enhancement, which presumably arises from an increase in the gain of the central auditory system, has been observed after several different types of cochlear trauma. The sound evoked hyperactivity seen in the AC 4 h after the exposure may result in an excitotoxic response which could induce an acute stress response (Helfferich and Palkovits, 2003). The recovery of the AC response to normal levels 1 d post-exposure suggests that the system reacts to counter this hyperactivity and returns to a homeostatic state. The biological basis underlying this form of neural hyperactivity and recovery is poorly understood. To gain insights into its biological bases, a pathway finder gene array was used to screen for potential candidate genes and RT-PCR was used to verify the results. After impulse noise exposure, a slight-to-moderate increase of c-Fos immunolabeling was seen in the AC mainly at 1 h post-exposure with lesser labeling at longer survival times (Campeau et al., 2002, Wallhausser- Franke et al., 2003, Zhang et al., 2003). In contrast, one recent gene expression study reported a significant decrease in the AC of Arg3.1/arc and c-Fos genes after binaural exposure to a traumatic noise suggesting a putative plasticity responses in the auditory system (Tan et al., 2007). The different c-Fos trends observed in these two studies could be due to difference in protein versus gene expression changes as well as differences in noise exposure conditions. Although the c-Fos gene was included in our array, we did not observe a significant change in its expression in AC following acoustic overstimulation. The lack of change in c-Fos expression could be due to the fact that we used a monaural exposure as opposed to earlier studies that employed binaural exposures.
Noise exposure and other toxic agents have long been known to induce cell death in the cochlea; however, effects on the central auditory system are less clear. A few recent studies have found evidence of apoptotic cell death in the central auditory pathway following acoustic overstimulation possibly due to excitotoxcity or loss of neurotrophic support (Aarnisalo et al., 2000, Saljo et al., 2002, Basta et al., 2005). HSP which promotes cell survival has been shown to be upregulated in the cochlea following intense noise exposure and other ototraumatic agents (Altschuler et al., 2002). An increase of constitutive HSP was also observed in the IC (Helfert et al., 2002) or other brain tissues (Samson et al., 2007) following exposure to an intense noise. One of the important findings of this study is that both HSP-70 and HSP-27 stress genes significantly increased in the AC after the noise exposure (Figure 3– Figure 4). The activation of HSP genes may involve an acute protective response to prevent excitotoxic cell death in the central auditory pathway. For example, HSP were found to protect against excess glutamatergic synaptic transmission that can give rise to glutamate exicitoxicity (Mokrushin et al., 2005), the major excitatory neurotransmitter in the peripheral (Puel, 1995) and central auditory systems (Parks, 2000). The activation of HSP by a thermal preconditioning can reduce the increase of neurotransmitter currents, such as GABA and glutamate, induced by hyperthermia or hypoxia and preserve the physiological function of the central nervous system (Kelty et al., 2002). In addition, HSP, which are upregulated after neurotrophin withdrawal, promote neuron survival (Dodge et al., 2006). These results suggest that the elevation of HSP may protect AC neurons from excitotoxicity caused by excess glutamate release.
Increased expression of the CTSD gene also occurred a few hours after the noise exposure. CTSD or cathepsin D encodes for a peptidase that belongs to a family of lysosomal aspartyl proteases which has been implicated in diverse biological processes. The CTSD gene is involved in caspase-independent apoptosis (Steinfeld et al., 2006). Increased concentrations of CTSD have been observed during ischemic, inflammatory, and regenerative processes. Inhibition of CTSD prevented hydrogen peroxide-induced cell death (Castino et al., 2007). Noise induced over expression of CTSD suggests that the noise exposure may trigger an apoptotic signal in the central auditory system; however, it is unclear if an increase in this signaling pathway is sufficient to induce cell death in the AC.
The CDKN1A gene, which codes for the cyclin-dependent kinase inhibitor p21Waf1/Cip1, is involved in p53 and TGFβ signaling pathways. Similar to CTSD, CDKN1A is involved in modulation of cell cycles, apoptosis, senescence and differentiation. The CDKN1A gene is rapidly upregulated in the brain, kidney, liver and heart following inflammation induced by intraperitoneal injection of bacterial lipopolysaccharides, the immunogenic component of gram-negative bacteria (Ring et al., 2003). Increased expression of mRNA for CDKN1A (p21) along with HSP 70 has also been observed in the cortex and white matter after brain injury. The increase in CDKN1A could conceivably protect cells by preventing cell cycle progression and facilitating DNA repair (Jakob et al., 2000, Katano et al., 2000). High intensity noise exposure has been reported to reduce neuron density in the AC of p21 mice whose brains are still developing (Basta et al., 2005) and neuronal cell death has been observed in the temporal cortex and hippocampus of adult animals after extremely high level impulse noise exposure (Saljo et al., 2002).
In summary, we found that the monaural noise exposure induced a transient amplitude increase in the AC associated with an upregulation of HSP-27, HSP-70, CTSD and CDKN1A gene expression. This suggests AC neurons underline a fast protective change to compensate the peripheral damage induced by noise exposure. Further research is underway to find the physiological and biological changes in the AC that may be related to tinnitus and hyperacusis in the animal model.
Acknowledgement
We want to thank Dr. James Samson and Dr. Kari Suzanne Kraus for reading the manuscript and providing very suggestive comments. We also want to thank Dr. Brazeau and Diane Letina in Department of Pharmaceutical Sciences of University at Buffalo for providing technical supports on this study. This project is supported by grants from National Institute of Health (DC008685-01 to W.S., DC009091 and DC009219 to R.S.).
List of abbreviations
- ABR
auditory brainstem response
- AC
auditory cortex
- CDKN1A
cyclin-dependent kinase inhibitor 1A
- CT
cycle threshold
- CTSD
cathepsin D
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HSP
heat shock protein
- IC
inferior colliculus
- PCR
polymerase chain reaction
- SPL
sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnisalo AA, Pirvola U, Liang XQ, Miller J, Ylikoski J. Apoptosis in auditory brainstem neurons after a severe noise trauma of the organ of Corti: intracochlear GDNF treatment reduces the number of apoptotic cells. ORL J Otorhinolaryngol Relat Spec. 2000;62:330–334. doi: 10.1159/000027764. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Fairfield D, Cho Y, Leonova E, Benjamin IJ, Miller JM, Lomax MI. Stress pathways in the rat cochlea and potential for protection from acquired deafness. Audiol Neurootol. 2002;7:152–156. doi: 10.1159/000058301. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Hamernik RP. Acute acoustic trauma. Acta Oto-Laryngologica. 1987;104:225–233. doi: 10.3109/00016488709107322. [DOI] [PubMed] [Google Scholar]
- Basta D, Tzschentke B, Ernst A. Noise-induced cell death in the mouse medial geniculate body and primary auditory cortex. Neurosci Lett. 2005;381:199–204. doi: 10.1016/j.neulet.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8:105–118. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castino R, Bellio N, Nicotra G, Follo C, Trincheri NF, Isidoro C. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radic Biol Med. 2007;42:1305–1316. doi: 10.1016/j.freeradbiomed.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Dodge ME, Wang J, Guy C, Rankin S, Rahimtula M, Mearow KM. Stress-induced heat shock protein 27 expression and its role in dorsal root ganglion neuronal survival. Brain Res. 2006;1068:34–48. doi: 10.1016/j.brainres.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Gu Z, Flemington C, Chittenden T, Zambetti GP. ei24, a p53 response gene involved in growth suppression and apoptosis. Mol Cell Biol. 2000;20:233–241. doi: 10.1128/mcb.20.1.233-241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfert RH, Glatz FR, 3rd, Wilson TS, Ramkumar V, Hughes LF. Hsp70 in the inferior colliculus of Fischer-344 rats: effects of age and acoustic stress. Hear Res. 2002;170:155–165. doi: 10.1016/s0378-5955(02)00487-2. [DOI] [PubMed] [Google Scholar]
- Helfferich F, Palkovits M. Acute audiogenic stress-induced activation of CRH neurons in the hypothalamic paraventricular nucleus and catecholaminergic neurons in the medulla oblongata. Brain Res. 2003;975:1–9. doi: 10.1016/s0006-8993(03)02509-5. [DOI] [PubMed] [Google Scholar]
- Jakob B, Scholz M, Taucher-Scholz G. Immediate localized CDKN1A (p21) radiation response after damage produced by heavy-ion tracks. Radiat Res. 2000;154:398–405. doi: 10.1667/0033-7587(2000)154[0398:ilcprr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Katano H, Masago A, Taki H, Nakatsuka M, Fuse T, Yamada K. p53-independent transient p21(WAF1/CIP1) mRNA induction in the rat brain following experimental traumatic injury. Neuroreport. 2000;11:2073–2078. doi: 10.1097/00001756-200007140-00003. [DOI] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Mokrushin AA, Pavlinova LI, Plekhanov AY. Heat shock protein HSP70 increases the resistance of cortical cells to glutamate excitotoxicity. Bull Exp Biol Med. 2005;140:1–5. doi: 10.1007/s10517-005-0396-x. [DOI] [PubMed] [Google Scholar]
- Moller AR. Tinnitus: presence and future. Prog Brain Res. 2007;166:3–16. doi: 10.1016/S0079-6123(07)66001-4. [DOI] [PubMed] [Google Scholar]
- Parks TN. The AMPA receptors of auditory neurons. Hear Res. 2000;147:77–91. doi: 10.1016/s0378-5955(00)00122-2. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Popelar J, Erre JP, Aran JM, Cazals Y. Plastic changes in ipsi-contralateral differences of auditory cortex and inferior colliculus evoked potentials after injury to one ear in the adult guinea pig. Hear Res. 1994;72:125–134. doi: 10.1016/0378-5955(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Popelar J, Syka J, Berndt H. Effect of noise on auditory evoked responses in awake guinea pigs. Hear Res. 1987;26:239–247. doi: 10.1016/0378-5955(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995;47:449–476. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139:153–171. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171:43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- Ring RH, Valo Z, Gao C, Barish ME, Singer-Sam J. The Cdkn1a gene (p21Waf1/Cip1) is an inflammatory response gene in the mouse central nervous system. Neurosci Lett. 2003;350:73–76. doi: 10.1016/s0304-3940(03)00883-8. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Jingshan S, Hamberger A, Hansson HA, Haglid KG. Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. J Neurotrauma. 2002;19:985–991. doi: 10.1089/089771502320317131. [DOI] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Auditory nerve activity and cochlear morphology after noise exposure. Archives of Oto-Rhino-Laryngology. 1979;224:111–116. doi: 10.1007/BF00455233. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Samson J, Sheeladevi R, Ravindran R, Senthilvelan M. Stress response in rat brain after different durations of noise exposure. Neurosci Res. 2007;57:143–147. doi: 10.1016/j.neures.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, Saftig P, Gartner J. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet. 2006;78:988–998. doi: 10.1086/504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Mercado E, 3rd, Wang P, Shan X, Lee TC, Salvi RJ. Changes in NMDA receptor expression in auditory cortex after learning. Neurosci Lett. 2005;374:63–68. doi: 10.1016/j.neulet.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hear Res. 2000;139:59–68. doi: 10.1016/s0378-5955(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Wallhausser-Frank E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA, Wang J, Kim SA. Fos-like immunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp Brain Res. 2003;153:655–660. doi: 10.1007/s00221-003-1612-4. [DOI] [PubMed] [Google Scholar]