Abstract
Glut4 storage vesicles (GSVs) represent translocation-competent vesicular carriers in fat and skeletal muscle cells that deliver Glut4 to the plasma membrane in response to insulin stimulation. GSVs include three major cargo proteins: Glut4, insulin-responsive aminopeptidase (IRAP), and sortilin. Previous work has suggested that the lumenal interaction between Glut4 and sortilin and the cytoplasmic interaction between sortilin and GGA adaptors play an important role in recruitment of Glut4 into the GSVs. However, the mechanism of IRAP targeting to this compartment remains unknown. To address this question, we show that in differentiating adipocytes IRAP enters the GSVs from the “donor” membranes on day 3 of differentiation. Forced expression of sortilin in undifferentiated cells does not recruit IRAP into the vesicles. However, double expression of sortilin and Glut4 reconstitutes functional GSVs that incorporate endogenous IRAP. To explain this process, we show by a yeast two-hybrid system and chemical cross-linking that the lumenal domain of IRAP can interact with the lumenal loop of Glut4. IRAP without the lumenal domain is faithfully targeted to the donor membranes but has significantly lower insulin responsiveness than full-length IRAP. We suggest that lumenal interactions between Glut4 and IRAP play an important role in the assembly of the GSVs.
Insulin increases glucose uptake in fat and skeletal muscle tissues by translocating intracellular Glut4-containing vesicles (also called IRVs for insulin-responsive vesicles and GSVs4 for Glut4 storage vesicles) to the plasma membrane (1, 2). One of the major protein components of the GSVs is insulin-responsive aminopeptidase, or IRAP (3-5). Although the biological role of IRAP is not yet clear (6), recent evidence indicates that the presence of IRAP in the GSVs confers insulin responsiveness to this compartment (7). Thus, IRAP has been shown to interact with AS160, a Rab GTPase-activating protein that is phosphorylated by Akt and may couple Glut4 traffic to the insulin signaling pathway (8, 9). IRAP also interacts with other proteins that have been implemented in regulation of Glut4 translocation, such as p115 (10) and tankyrase (7). Interestingly, microinjection into 3T3-L1 adipocytes of the recombinant peptide corresponding to the cytoplasmic tail of IRAP causes translocation of Glut4, presumably by competition with putative GSV anchors (11).
In addition to Glut4 and IRAP, GSVs incorporate a putative sorting receptor, sortilin (12-14), VAMP2 (15), several recycling receptors, and other membrane proteins that may or may not represent essential vesicular components (8). In any case, the presence of Glut4, IRAP, and sortilin as the most prominent components of the GSV membrane is a unique feature of this compartment that differs from other vesicular carriers in the cell. A challenging problem is to determine how these structurally and functionally different proteins become co-localized in the cell and form a unique, highly specialized vesicular carrier. To this end, we (14) and others (16) have recently shown that sortilin plays the key role in the recruitment of Glut4 to the GSVs in differentiating 3T3-L1 cells. According to one model, sortilin interacts with Glut4 via its lumenal Vps10p domain (17) and with GGA adaptors via the cytoplasmic tail (18-20). Thus, sortilin may work as a transmembrane scaffold that couples Glut4 to the cytoplasmic GGA-mediated vesicle budding machinery. However, the mechanism of IRAP targeting to the GSVs remains unknown and is addressed in this study.
We used the developmental approach and analyzed GSV biogenesis in differentiating 3T3-L1 cells. Undifferentiated cells express IRAP (21), whereas sortilin and Glut4 are induced around day 3 of differentiation (13, 14, 22). We have found that in 3T3-L1 cells IRAP is localized in the perinuclear syntaxin 6-positive compartment throughout the differentiation process. IRAP is targeted to this compartment by the cytoplasmic tail. Interestingly, the biochemical nature of IRAP-containing compartment changes dramatically during cell differentiation. In undifferentiated 3T3-L1 pre-adipocytes, IRAP is predominantly localized in heavy membranes that are readily pelleted by low speed centrifugation. Upon cell differentiation, IRAP is re-distributed from the heavy membranes into small vesicles, the GSVs. This transition takes place on day 3 of differentiation, in tandem with the induction of sortilin and Glut4. Sortilin ectopically expressed in undifferentiated 3T3-L1 cells is targeted to small vesicles but has only a minor effect on the recruitment of endogenous IRAP to this compartment. However, double expression of sortilin and Glut4 in undifferentiated cells leads to formation of functional GSVs (14), which as we now show, incorporate endogenous IRAP. Thus, in the process of cell differentiation, sortilin recruits Glut4 into the GSVs, and Glut4 recruits IRAP. To gain insight into the potential mechanism of the latter event, we have determined that the lumenal domain of IRAP interacts with the lumenal loop of Glut4 in the yeast two-hybrid system. Moreover, in 3T3-L1 cells, IRAP can be cross-linked to Glut4 with the help of the membrane-permeable reagent DSP. These results suggest that IRAP and Glut4 may directly interact via lumenal domains. To test the importance of the lumenal domain in IRAP targeting, we have replaced it with the V5 tag. This reporter protein maintains the perinuclear localization in the cell but has significantly lower insulin responsiveness. Thus, protein-protein interactions in the vesicular lumen may play an essential role in protein sorting to and self-assembly of the GSVs.
EXPERIMENTAL PROCEDURES
Antibodies—The monoclonal anti-V5 antibody was purchased from Invitrogen, the monoclonal anti-Myc tag antibody and the polyclonal anti-Myc tag antibody was from Cell Signaling Technology (Danvers, MA), and the monoclonal anti-syntaxin 6 and anti-sortilin (neurotensin receptor 3) antibodies were from BD Biosciences Pharmingen. The monoclonal anti-Glut4 antibody 1F8 (23) and the rabbit polyclonal antibody against cellugyrin (24) were described previously. The rabbit polyclonal antibody against IRAP was a kind gift from Dr. Paul Pilch, Boston University School of Medicine.
cDNA Constructs—The pLenti6/V5 Directional TOPO cloning kit was purchased from Invitrogen. Two oligonucleotides (5′-CACCGAATTCGTTTAAACTGTCGAC-3′ and 5′-GTCGACAGTTTAAACGAATTCGGTG-3′) were annealed and ligated to the pLenti6/V5-D-TOPO vector. This modified vector was named pLenti vector. The other two oligonucleotides (5′-GATCCGTTAACGAATTCTCGCTCGAGCCAGTGTGGTGGTGTTTAAACCTGCAGACTAGTGTCGACAGGGGCCCGC-3′ and 5′-GGGCCCCTGTCGACACTAGTCTGCAGGTTTAAACACCACCACACTGGCTCGAGCGAGAATTCGTTAACG-3′) were annealed and ligated to the BamHI- and SacII-cut pLenti vector. This vector was named pLenti-m3. EYFP was amplified using pEYFP-N1 (a kind gift from Dr. Carmela Abraham, Boston University School of Medicine) as a template and two primers, 5′-ACGACGTCAGGATCCGTCGCCACCATGGTGAGCAA-3′ and 5′-TCGAGTCTGGAATTCCTTGTACAGCTCGTCCATGCC-3′. The PCR product was cut by BamHI and EcoRI and ligated to the correspondingly cut pLenti-m3 vector. The resulting vector was named pLenti-m3-EYFP-C1. Alternatively, EYFP was amplified with the primers 5′-ACGACGACAGTCGACATCGCCACCATGGTGAGCAAG-3′ and 5′-CTGAGTCTGCCGCGGTTACTTGTACAGCTCGTCCATG-3′. The PCR product was digested sequentially by SalI and SacII and ligated into pLenti-m3 to obtain pLenti-m3-EYFP-N3.
Rat IRAP cDNA in the pPCR-Script Amp SK(+) vector was a kind gift from Dr. Susanna R. Keller (University of Virginia). Full-length IRAP was amplified using two primers, 5′-ACGACGTCACTCGAGAGCTTGGGGCGCTGGGC-3′ and 5′-TCGAGTACGACTAGTCAGCCACAGTGTCAGAGTTT-3′. This PCR product (with no stop codon) was digested by XhoI and SpeI and ligated in-frame into the pLenti-m3-EYFP-C1 vector to obtain pLenti-m3-EYFP-IRAP-V5. The IRAP truncation construct (IRAPtail) comprised of the residual five amino acids in the luminal domain, the transmembrane domain, and the cytoplasmic tail was made by the PCR reaction using two primers, 5′-ACGACGTCACTCGAGAGCTTGGGGCGCTGGGC-3′ and 5′-TCGAGTACGACTAGTAAAGGTACATCTAGGCAGTAG-3′. The PCR product was digested by XhoI and SpeI and ligated into the pLenti-m3-EYFP-C1 vector to obtain pLenti-m3-EYFP-IRAPtail-V5.
ECFP was amplified using two primers, 5′-TCGAGTCTGGAATTCGTCGCCACCATGGTGAGCAA-3′ and 5′-ACGACGTCAGTCGACTTACTTGTACAGCTCGTCCATG-3′. The PCR product was digested with EcoRI and SalI and ligated into the EcoRI- and SalI-cut pBabe-puro vector to obtain the pBabe-ECFP vector. myc7-Glut4 was amplified using two primers, 5′-ACGACGTCAGGATCCGCCGCCACCATGCCGTCGGGTTTCCAGCA-3′ and 5′-ACGACGTCAGAATTCGTCATTCTCATCTGGCCCTAAA-3′. The myc7-Glut4 cDNA was digested by BamHI and EcoRI and ligated into the pBabe-ECFP to obtain pBabe-myc7-Glut4-ECFP.
Stable Cell Lines—3T3-L1 cells stably transfected with mLNCX2-sortilin-myc/His (S cells) as well as 3T3-L1 double-transfected with pBabe-myc7-Glut4 and mLNCX2-sortilin-myc/His (GS cells) were described previously (14). Cells stably transfected with pBabe-myc7-Glut4-ECFP were obtained the same way as G cells. pBabe-myc7-Glut4-ECFP-expressing and wild type 3T3-L1 cells were infected with pLenti-m3-EYFP-N1, pLenti-m3-EYFP-IRAP-V5, and pLenti-m3-EYFP-IRAPtail-V5 according to the Invitrogen protocol. Selection of cells was performed with blasticidin (8 μg/ml) for 2 weeks, and pooled clones were analyzed. All cells were cultured, differentiated, and maintained as described previously (25).
Subcellular Fractionation of 3T3-L1 Cells—Before harvesting, cultured 3T3-L1 adipocytes were washed 3 times with serum-free Dulbecco's modified Eagle's medium heated to 37 °C and incubated in the same media for 3 h. Where indicated, cells were treated with 100 nm insulin or carrier (5 μm HCl) in Dulbecco's modified Eagle's medium for 5 or 15 min at 37 °C. After that, cells were washed three times with HES buffer with protease inhibitors (250 mm sucrose, 20 mm HEPES, 1 mm EDTA, pH 7.4, 1 μm aprotinin, 2 μm leupeptin, 1 μm pepstatin, 5 mm benzamidine, and 1 mm phenylmethylsulfonyl fluoride) at 37 °C. Cells were harvested in the same buffer and homogenized by 10 strokes up and down using a Potter-Elvehjem homogenizer with a Teflon pestle. Alternatively, cells were homogenized with 11 strokes through the ball-bearing cell cracker (Isobiotec) with a 12-μm clearance. In some experiments total homogenate was cleared by centrifugation at 1000 × g for 5 min. Supernatant of this centrifugation (or total homogenate) was centrifuged again at 16,000 × g for 20 min. Plasma membrane, heavy microsomes, light microsomes, and nuclear/mitochondria fractions were obtained by differential centrifugation as described previously (26). Fractions were re-suspended in HES buffer, and protein concentration was determined using a BCA kit (Pierce).
Sucrose Gradient Centrifugation—For velocity gradient centrifugation, light microsomes samples (0.2 ml, 500 μg) were loaded onto a 3.8-ml linear 10-30% (w/v) sucrose gradient in HE buffer (20 mm HEPES, 1 mm EDTA, pH7.4) and centrifuged for 65 min in a Sorvall TST60.4 rotor (Kendro Laboratory Products, Asheville, NC) at 48,000 rpm. Each gradient was separated into 17-25 fractions starting from the bottom of the tube. The protein profile was determined using a BCA kit (Pierce), and the linearity of the gradients was confirmed by measuring the refractive index of fractions. The fractions were further analyzed by gel electrophoresis and Western blotting.
Immunofluorescence—3T3-L1 pre-adipocytes were grown in 60-mm dishes. The night before the experiment cells were reseeded onto Nunc LAB-TEK II 4-well chamber slides. 3T3-L1 adipocytes were grown and differentiated in 60-mm dishes. On day 5 of differentiation, cells were lifted up by trypsin for 10 min at 37 °C and reseeded to 4-well chamber slides. After growing on chamber slides for two more days, adipocytes were used for experiments. Differentiated and undifferentiated cells were washed three times with serum-free Dulbecco's modified Eagle's medium heated to 37 °C and incubated in the same media for 3 h. Then, cells were fixed with 4% paraformaldehyde in PBS for 30 min. Fixed cells were washed with PBS, permeabilized with 0.2% Triton X-100 for 5 min, blocked with PBS with 5% donkey serum and 5% bovine serum albumin and stained with anti-syntaxin 6 monoclonal antibody and Cy3-conjugated donkey antimouse IgG (Jackson ImmunoResearch, West Grove, PA). Incubation with each antibody lasted for 60 min at room temperature and was followed by six quick washes with PBS. A SlowFade-Light Antifade kit (Molecular Probes) was used for mounting cells on slides that were examined with the help of Axiovert 200 m fluorescence microscope (Carl Zeiss Inc., Thornwood, NY). Pictures were taken using the Axio-vision 3.0 program (Carl Zeiss, Inc.). Quantification of images was performed with the help of NIH image analysis software packages, Image J (Version 1.37a) using co-localization threshold plug-in (developed by the Wright Cell Imaging Facility, Toronto, Canada). Zero-zero pixels were included in the threshold calculation. For every data point, at least five different fields were analyzed.
Fluorescence-assisted Cell Sorting—Fluorescence-activated cell sorter analysis was performed as described previously (27) with minor modifications. In brief, 3T3-L1 cells were grown on 6-well dishes. Before the experiment, cells were incubated in serum-free Dulbecco's modified Eagle's medium for at least 3 h, and insulin (100 nm) was administered for 15 min. After that, cells were quickly cooled to 4 °C and washed with cold PBS containing 0.9 mm CaCl2 and 0.5 mm MgCl2 (PBS2+). All subsequent steps were carried out at 4 °C. Cells were incubated with the anti-V5 antibody or nonspecific mouse IgG (1:500) in PBS2+ containing 5% bovine serum albumin and 5% donkey serum (1 ml/well) for 1.5 h and washed twice with PBS2+ for 5 min each time. Cells were then incubated with 5 μg/ml phycoerythrin-conjugated donkey F(ab)2 anti-mouse IgG (Jackson ImmunoResearch) for 1 h. At the end of the incubation, cells were rinsed twice with PBS2+ and additionally washed 3 times with PBS2+ for 10 min each wash. Cells were gently scraped in 1 ml of regular PBS with 5% fetal bovine serum (calcium and magnesium were excluded at this stage to decrease cell adhesion). Alternatively, 3T3-L1 adipocytes were incubated with 1 ml of 0.25% trypsin and 0.5 mg/ml collagenase in PBS at 37 °C for 5-10 min until most cells detached from the plate (28). Detached cells were washed once by centrifugation at 300 × g for 4 min and re-suspended in 1 ml of PBS. Immediately before sorting, cells were passed through a 40-μm filter. All flow cytometric data were acquired using equipment maintained by the Boston University Medical Campus Flow Cytometry Core Facility using a FACScan (Becton Dickinson). Appropriate compensation between FL1 and FL2 channels was adjusted using wild type 3T3-L1 cells, EYFP-expressing cells, and control V5-expressing cells stained with anti-V5 primary antibody and phycoerythrin-conjugated secondary antibody. In each sample, 200,000 cells were counted. Specific phycoerythrin fluorescent signal was determined by subtracting the signal from transfected cells treated under the same conditions as the experimental group and stained with nonspecific IgG and phycoerythrin-conjugated secondary antibody. Specific YFP signal was determined by subtracting background fluorescent signal from non-transfected 3T3-L1 cells. Mean fluorescent intensities were used for quantification.
In Vitro Reconstitution of IRAP-containing Vesicles—Cytosol and the donor membranes were isolated from undifferentiated and differentiated 3T3-L1 cells, and the assay was performed as described earlier (24). The vesicle fraction along with the donor fraction were analyzed by Western blotting. The yield of small vesicles was linear when the heavy membrane fraction was used at a concentration of 0.2-1.6 mg/ml. To compare different experiments, we use arbitrary units defined as (intensity of the IRAP signal in the vesicle fraction - intensity in the “no cytosol” control lane) × (volume of the vesicle fraction)/(intensity of the IRAP signal in the donor fraction) × (volume of the donor fraction). The average of duplicate reactions and the deviation is plotted on the graph.
Cross-linking and Immunoprecipitation—Cross-linking was performed according to Nielsen et al. (29) with minor modifications. Cells were washed twice with PBS and once with KRP buffer (12.5 mm Hepes, 120 mm NaCl, 6 mm KCl, 1.2 mm MgSO4, 1.0 mm CaCl2, 0.6 mm Na2HPO4, 0.4 mm NaH2PO4, 2.5 mm d-glucose, pH 7.4), and dithiobis(succinimidyl propionate) was added to final concentration 2 mm for 30 min at room temperature. Then quenching buffer (50 mm Tris, 10 mm EDTA, 150 mm NaCl, 1 μm aprotinin, 2 μm leupeptin, 1 μm pepstatin, 5 mm benzamidine, and 1 mm phenylmethylsulfonyl fluoride, pH 7.4) was added for 15 min at 4 °C followed by 2 washes with the same buffer. The cells were lysed in 500 μl of quenching buffer with 1% Triton X-100, and cell lysates were cleared by centrifugation at 16,000 × g for 30 min. This material (1.5 mg) was incubated with the monoclonal anti-Myc antibody and nonspecific mouse IgG (2 μg each) along with 30 μl of protein G beads overnight at 4 °C with rotating. The beads were then washed 3 times with 1% Triton X-100 in quenching buffer. Elution was carried out with 30 μl of Laemmli sample buffer with 50 mm dithiothreitol at 37 °C for 30 min.
Yeast Two-hybrid Assay—The MATCHMAKER Gal4 two-hybrid system 3 (Clontech Laboratories, Mountain View, CA) was used for these experiments. The IRAP luminal domain without added tag (IRAP LD) was amplified using two primers, 5′-ACGACGTCACATATGCCTAGATGTACCTTTACCAAAG-3′ and 5′-CTAGACAAGGGATCCCTACAGCCACAGTGTCAGAG-3′. The PCR product was digested by NdeI and BamHI and ligated into the correspondingly cut pGBKT7 vector that encodes the GAL4 DNA binding domain (DNA BD) to obtain pGBKT7-IRAP LD. The first extracellular loop of Glut4 without tag was amplified using two primers, 5′-CTAGACCTGGAATTCAATGCCCCTCAGAAGGTGATT-3′ and 5′-ACGACGTCAGGATCCCCCAGAGGGTGGTGAGGGTG-3′. The PCR product was digested with EcoRI and BamHI and ligated into the pGADT7 vector that encodes GAL4 transcription activation domain (AD) to obtain pGADT7-Loop1. Transformation was done according to the manufacturer's manual. Aliquots of co-transformants (100 μl) were plated on SD/-Leu/-Trp, SD/-His/-Leu/-Trp, SD/-Leu/-Trp/X-α-Gal, and SD/-Ade/-His/-Leu/-Trp plates. Growth was checked after 4 days at 30 °C.
Quantitative Real Time PCR—Total RNA was isolated from wild type 3T3-L1 fibroblasts and GS fibroblasts (three 100-mm dishes per each sample) using Trizol reagent (Invitrogen). Genomic DNA was removed from the RNA samples with the help of TURBO DNA-free (Ambion). RNA samples (1 μg each) were reverse-transcribed using the High Capacity cDNA reverse transcription kit (Applied Biosystems). The resulting cDNA samples were diluted 1:10, and diluted samples (9 μl) were analyzed in triplicate by the TaqMan gene expression assays with 6-carboxyfluorescein-labeled IRAP probe (Applied Biosystems, assay ID Mm00555903_m1) or VIC-labeled glyceraldehyde-3-phosphate dehydrogenase probe (Applied Biosystems, assay ID 4352339E). Quantitative real time PCR was performed in 384-well plates with the Applied Biosystems 7900HT sequence detection system. Data were analyzed with the help of SDS software v2.2 (Applied Biosystems). The level of IRAP mRNA (normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA) in wild type 3T3-L1 fibroblasts was set at 1. The relative amount of IRAP mRNA in GS cells was calculated using the 2-ΔΔCt method.
Gel Electrophoresis and Western Blotting—Proteins were separated in SDS-polyacrylamide gels according to Laemmli with and without reducing agents and transferred to an Immobilon-P membrane (Millipore) in 25 mm Tris, 192 mm glycine. After transfer, the membrane was blocked with 10% nonfat milk in PBS with 0.5% Tween 20 for 1 h at 37°C. The blots were probed with specific antibodies and horseradish peroxidase-conjugated secondary antibodies (Sigma) and detected with an enhanced chemiluminescence substrate kit (PerkinElmer Life Sciences) using a Kodak Image Station 440CF (Eastman Kodak Co.). The signals were quantified using the Kodak 1D image analysis software.
RESULTS
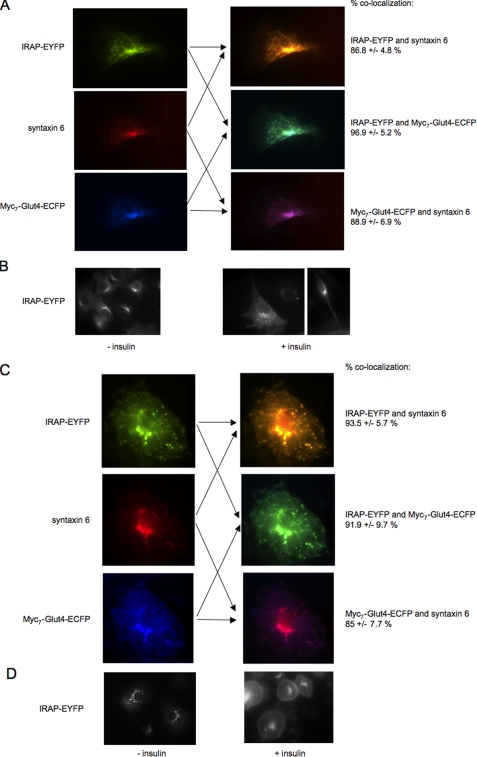
EYFP-conjugated IRAP and ECFP-tagged myc7-Glut4 (Fig. 1) were stably expressed in 3T3-L1 cells. Fig. 2A demonstrates that in 3T3-L1 pre-adipocytes both proteins are predominantly localized in the syntaxin 6-positive perinuclear compartment. Treatment of 3T3-L1 pre-adipocytes with insulin does not induce noticeable changes in the intracellular localization of IRAP and, in particular, does not cause its marked translocation to the plasma membrane (Fig. 2B). In differentiated adipocytes, IRAP-EYFP also shows a significant co-localization with syntaxin 6 in the perinuclear compartment (see also Ref. 30) where it overlaps with myc7-Glut4-ECFP (Fig. 2C). However, with differentiation, IRAP-EYFP gains insulin responsiveness and is clearly translocated to the plasma membrane upon insulin stimulation (Figs. 2D). Previously, we have shown that insulin responsiveness of ectopically expressed Glut4 also dramatically increases with differentiation (14).
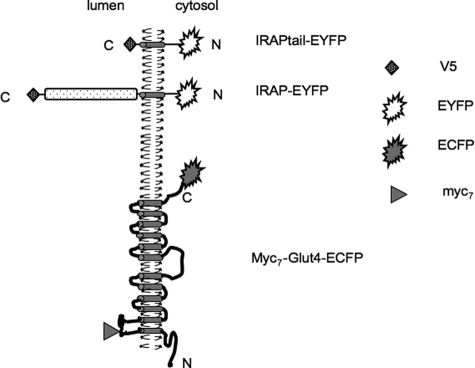
FIGURE 1.
Reporter proteins used in this article. Other constructs were described previously in Refs. 17 and 14.
FIGURE 2.
Intracellular localization and insulin responsiveness of IRAP in 3T3-L1 cells. A and C, undifferentiated (A) and differentiated (C) 3T3-L1 cells stably expressing IRAP-EYFP and myc7-Glut4-ECFP were stained with syntaxin 6 monoclonal antibody and Cy3-conjugated donkey anti-mouse IgG. B and D, undifferentiated (B) and differentiated (D) 3T3-L1 cells stably expressing IRAP-EYFP were treated with 100 nm insulin or carrier (5 μm HCl) for 15 min at 37 °C and analyzed by fluorescence microscopy.
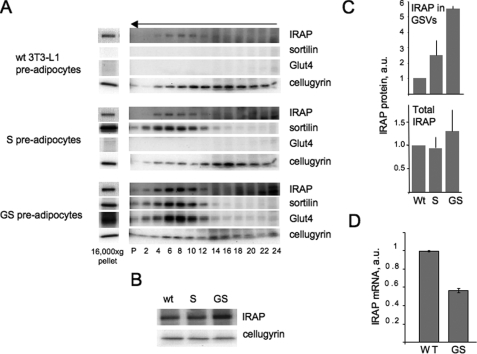
Although immunofluorescence staining does not show significant differentiation-induced differences in the intracellular localization of IRAP (Fig. 2), the biochemical fractionation reveals a dramatic change in the subcellular distribution of this protein upon cell differentiation. Both undifferentiated pre-adipocytes (Fb) and differentiated adipocytes (Ad) were homogenized with 11 strokes through the ball-bearing cell cracker, and total cell homogenates were separated into supernatant and pellet by centrifugation at 16,000 × g for 20 min. The distribution of total (Fig. 3A) and individual (Fig. 3B) proteins between supernatant (S) and pellet (P) of this centrifugation was determined by Ponso Red staining (Fig. 3A) and Western blotting (Fig. 3B). Upon cell differentiation, endogenous IRAP and IRAP-EYFP were re-distributed from the heavy rapidly sedimenting membranes recovered in the pellet to supernatant (Fig. 3B). Further fractionation of supernatant in sucrose velocity gradient (Fig. 3C) demonstrated that IRAP-EYFP co-sedimented with the GSVs marked by the presence of endogenous IRAP, Glut4, and sortilin.
FIGURE 3.
Cell differentiation causes redistribution of IRAP from heavy donor membranes into the GSVs. A, undifferentiated (Fb) and differentiated (Ad) 3T3-L1 cells stably expressing IRAP-EYFP were homogenized and centrifuged at 16,000 × g for 20 min. Supernatants (S, 100 μg/lane) and pellets (P, 100 μg/lane) were separated by PAGE. The panel shows Ponso Red-stained gel. B, individual proteins were analyzed in supernatants (S) and pellets (P) by Western blotting with specific antibodies. C, supernatant obtained from differentiated cells as explained in the legend to panel A was centrifuged in a 10-30% linear sucrose gradient for 65 min in a Sorvall TST60.4 rotor at 48,000 rpm. Gradient fractions, including the pellet of this centrifugation (P), were analyzed by Western blotting. The arrow indicates the direction of sedimentation. D, vesicle reconstitution assay was performed in vitro in duplicate with donor membranes and cytosol isolated from differentiated and undifferentiated wild type 3T3-L1 cells. The lower panel shows the quantification of the Western blot stained with anti-IRAP antibody. The absence of the error signs on the bars indicates that the error is virtually undetectable. A representative result of three independent experiments is shown. a.u., arbitrary units. E, differentiating 3T3-L1 cells were separated into 16,000 × g supernatant and pellet on each day of differentiation as explained in the legend to panel A. Endogenous IRAP in the supernatant (sup) and pellet was analyzed by Western blotting and expressed as the ratios (mean values ± S.E. of three independent experiments) between IRAP content in these fractions on each day of differentiation.
These results together with previously published evidence (14, 31, 32) suggest that formation of the GSVs does not take place in undifferentiated 3T3-L1 cells where IRAP is likely to co-localize with syntaxin 6 and ectopically expressed myc7-Glut4-ECFP in the perinuclear donor membranes from which GSVs originate upon cell differentiation. In differentiated adipocytes, the perinuclear compartment may embody a mixture of the donor membranes and the GSVs that exist in a dynamic equilibrium (33).
To confirm the process of differentiation-related vesicle formation in vitro, we used the GSV reconstitution assay (24). For that, we isolated donor membranes and cytosol from differentiated and undifferentiated cells and used them in different combinations for vesicle budding. Results of the budding reaction were expressed as the ratio between IRAP in the vesicle fraction and in the donor fraction. When donor membranes were isolated from undifferentiated cells, formation of vesicles in vitro was low regardless of the source of cytosol. However, when donor membranes were isolated from differentiated cells, formation of vesicles increased dramatically, also regardless of the source of cytosol (Fig. 3D). Thus, IRAP is recruited to small vesicles by a membrane protein that is induced during cell differentiation.
We then determined at what stage of differentiation IRAP is redistributed from heavy donor membranes to the GSVs. For that, cells were fractionated by 16,000 × g centrifugation on each day of differentiation. As is shown in Fig. 3E, the entry of IRAP from the heavy donor membranes into vesicles takes place on day 3 of differentiation, concurrently with the induction of sortilin and Glut4 (13, 14, 22).
Because sortilin was previously reported to interact with IRAP (17), we decided to test whether or not expression of this protein is sufficient to recruit IRAP into the vesicles. As is shown in Fig. 4A, wild type 3T3-L1 pre-adipocytes do not express sortilin or Glut4 and have very little IRAP in the GSV fraction (see also Fig. 3B). Sortilin ectopically expressed in undifferentiated 3T3-L1 cells is localized in small vesicles. However, expression of sortilin only minimally increases the presence of endogenous IRAP in the vesicular fraction (Fig. 4, A and C). The distribution of the irrelevant protein, cellugyrin, is shown as a loading control. Next, we examined compartmentalization of IRAP in cells double-transfected with sortilin and myc7-Glut4. We found that the GSV content of IRAP in double-transfected cells is significantly increased (Fig. 4, A and C).
FIGURE 4.
Recruitment of IRAP into the vesicular fraction in undifferentiated 3T3-L1 cells. A, wild type (wt) 3T3-L1 cells as well as 3T3-L1 cells stably expressing sortilin (S cells) and sortilin together with Glut4 (GS cells) were homogenized and centrifuged at 16,000 × g for 20 min. Pellets of this centrifugation (30 μg) were separated by PAGE, and proteins were detected on the same membrane by sequential rounds of Western blotting. Supernatant (200-400 μg in different experiments) was centrifuged in a 10-30% linear sucrose gradient for 65 min in a Sorvall TST60.4 rotor at 48,000 rpm. The arrow indicates the direction of sedimentation. Gradient fractions, including the pellet of this centrifugation (P) were separated by PAGE. IRAP, sortilin, Glut4, and cellugyrin from each cell line were detected on the same membrane by sequential rounds of Western blotting. B, total protein lysates of wild type, G, and GS pre-adipocytes (50 μg each) were analyzed by Western blotting. C, quantification of data shown in panels A and B (mean values ± S.E. of two (S cells) or three (wild type and GS cells) independent experiments). a.u., arbitrary units. D, the levels of IRAP mRNA were determined in wild type and GS pre-adipocytes by quantitative real time PCR and normalized by glyceraldehyde-3-phosphate dehydrogenase mRNA.
It has been previously reported that total IRAP levels may be increased by overexpression of Glut4. In particular, in adipocytes of Glut4-overexpressing mice, IRAP levels are increased by 45%, probably as a result of protein stabilization (34). In agreement with these studies, we also detect a modest (less than 2-fold) increase in total IRAP in GS pre-adipocytes in comparison to wild type cells (Fig. 4, B and C). This effect, however, cannot account for a significant enrichment of IRAP in the vesicular fraction (5.5-6-fold) and is likely to reflect the formation of a novel compartment in GS cells, the GSVs, that represent an additional sink for IRAP. In fact, we have previously described a similar effect of the GSV formation on ectopically expressed Glut4 (14). To test this hypothesis, we have measured the levels of IRAP mRNA in wild type and GS pre-adipocytes with the help of quantitative real time PCR. It turns out that the amount of IRAP mRNA is decreased in GS pre-adipocytes (Fig. 4D) in comparison to wild type cells. Although the explanation of this phenomenon is not immediately clear, it may represent a feedback regulatory connection induced by increased IRAP protein levels. In any case, our results show that Glut4-dependent changes in the amount and intracellular localization of IRAP take place at a post-transcriptional level.
In general, results shown in Fig. 4 suggest that expression of Glut4 is required for the recruitment of IRAP to the GSVs. One possibility to explain this effect is to suggest that IRAP interacts with Glut4 directly. To test this hypothesis, we have subcloned the lumenal domain of IRAP (IRAP LD) into the pGBKT7 vector that encodes the GAL4 DNA binding domain (DNA-BD) and the first luminal loop of Glut4 (Loop 1) into the pGADT7 vector that encodes the GAL4 transcription AD and tested protein interaction in the yeast two-hybrid system. Fig. 5, A and B, shows that cells transformed with either pGBKT7-IRAP LD or pGADT7-Loop1 do not grow on the selection media (left images) and do not secret α-galactosidase when they grow on the permissive media (note the lack of the blue color of colonies on the right images). By the same token, cells double-transformed with empty pGADT7 vector and pGBKT7-IRAP LD (Fig. 5C) or with pGADT7-Loop1 and empty pGBKT7 vector (Fig. 5D) grow on the permissive media (left images), do not grow on the selection media (two middle images), and do not secret α-galactosidase on the permissive media (note a lack of blue staining on the right images). However, cells double-transformed with pGADT7-Loop1 and pGBKT7-IRAP LD grow on the permissive media (Fig. 5E, left) and on the selection media with moderate (-Leu/-Trp/-His) and high (-Leu/-Trp/-His/-Ade) stringency and, in addition, secret GAL4-regulated galactosidase (note blue colonies on Fig. 5E, right). Thus, the lumenal domain of IRAP interacts with the first loop of Glut4 in yeast two-hybrid system.
FIGURE 5.
Interaction of IRAP and Glut4 in the yeast two-hybrid system. The lumenal domain of IRAP interacts with the first lumenal loop of Glut4 in yeast two-hybrid system. Panels A and B show negative and positive controls for single transformed cells. Panels C and D show negative and positive controls for double-transformed cells. Panel E shows that double-transformed cells grow on the permissive media (left) on the regular selection media as well as on the high stringency selection media (two middle images) and secret α-galactosidase (blue colonies on the right image).
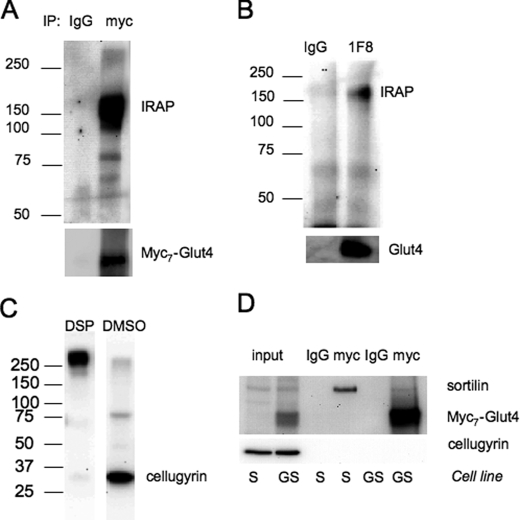
To confirm this result in adipocytes, we used chemical cross-linking with the membrane-permeable reagent DSP. We have found that endogenous IRAP can be cross-linked to myc7-Glut4 in 3T3-L1 adipocytes (Fig. 6A). Moreover, using the similar technique we found that endogenous IRAP can also be cross-linked to endogenous Glut4 in wild type 3T3-L1 adipocytes (Fig. 6B), albeit with a lesser efficiency. We believe that endogenous Glut4 is less cross-linkable because its lumenal domains have only one lysine that is required for the reaction with DSP. Introduction of eight additional lysines in the first lumenal loop of Glut4 (one per each Myc epitope and one in the short linker between myc7 and Glut4 sequence) makes this protein readily accessible for cross-linking with IRAP.
FIGURE 6.
IRAP can be cross-linked to Glut4 in 3T3-L1 cells. A, differentiated G adipocytes stably expressing myc7-Glut4 were incubated with DSP, and cell lysate (1.5 mg) was immunoprecipitated (IP) the anti-Myc antibody or nonspecific IgG and protein G. Proteins were eluted with Laemmli sample buffer with 50 mm dithiothreitol at 37 °C for 30 min. B, wild type 3T3-L1 adipocytes were incubated with DSP, and cell lysate (1.5 mg) was immunoprecipitated with the anti-Glut4 monoclonal antibody 1F8 or nonspecific IgG. C, wild type 3T3-L1 adipocytes were incubated with DSP or DMSO as described under “Experimental Procedures,” and cell lysate (50 μg) was analyzed by PAGE (without reducing agents) and Western blotting with the polyclonal antibody against cellugyrin. D, S and GS pre-adipocytes were incubated with DSP, and cell lysates (0.15 mg each) were immunoprecipitated with the anti-Myc antibody or nonspecific IgG and protein G. Input lanes show Western blot analysis of 15 μg of total lysates.
As a control for these experiments, we decided to determine whether or not myc7-Glut4 can be cross-linked to cellugyrin. Previously, we have shown that a significant fraction (up to 50%) of Glut4, IRAP, and sortilin are co-localized with cellugyrin in intracellular transport vesicles that are not insulin-responsive (35, 36).5 As is shown in Fig. 6C, incubation of cells with DSP results in a virtually 100% cross-linking of cellugyrin to some unidentified cellular component(s). However, cellugyrin is not cross-linked to either sortilin or myc7-Glut4 (Fig. 6D). This suggests that co-localization in the same vesicular population per se is not sufficient for cross-linking and that physical interaction between proteins may be required for this reaction.
In general, our results suggest that IRAP may be recruited to the insulin-responsive vesicles via the lumenal interactions with Glut4. Therefore, to test the importance of the IRAP lumenal domain in targeting to the insulin-responsive compartment, we have replaced the entire lumenal domain with the V5 tag (Fig. 1).
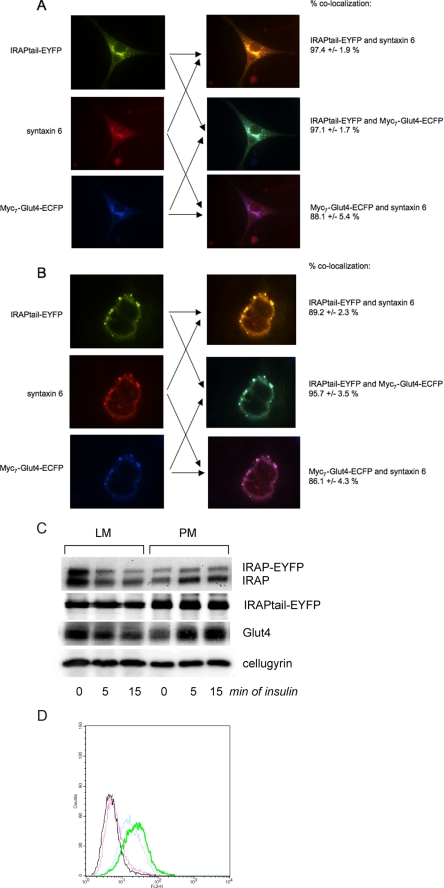
The intracellular localization of IRAPtail-EYFP in both undifferentiated and differentiated cells is similar to the full-length chimera protein (compare Fig. 7, A and B, and Fig. 2, A and D), suggesting that the cytoplasmic tail of IRAP is responsible for protein targeting to the syntaxin 6-positive perinuclear compartment.
FIGURE 7.
IRAP without the lumenal domain is targeted to the donor membranes but has low insulin responsiveness. A and B, undifferentiated (A) and differentiated (B) 3T3-L1 cells stably expressing IRAPtail-EYFP and myc7-Glut4-ECFP were stained with syntaxin 6 monoclonal antibody and Cy3-conjugated donkey anti-mouse IgG. C, differentiated 3T3-L1 cells stably expressing IRAP-EYFP and IRAPtail-EYFP were treated with 100 nm insulin or carrier (5 μm HCl) for 5 and 15 min at 37 °C and fractionated by differential centrifugation into the plasma membrane (PM) and light microsomal (LM) fractions, which were analyzed by Western blotting. A representative result of five independent experiments is shown. D, differentiated 3T3-L1 cells stably expressing IRAPtail-EYFP and IRAP-EYFP were treated with 100 nm insulin or carrier (5 μm HCl) for 15 min at 37 °C and analyzed by fluorescence-activated cell sorter. Black, IRAP-EYFP (-) insulin; green, IRAP-EYFP (+) insulin; red, IRAPtail-EYFP (-) insulin; blue, IRAPtail-EYFP (+) insulin. A representative result of five independent experiments is shown.
When we compared the cell surface translocation of IRAP-EYFP and IRAPtail-EYFP, we found a large difference in the insulin responsiveness between these two reporter molecules. Using the biochemical fractionation according to Simpson et al. (26), we can detect insulin-dependent plasma membrane translocation of endogenous Glut4 and IRAP as well as ectopically expressed IRAP-EYFP but not IRAPtail-EYFP (Fig. 7C). As a loading control for these experiments, we use cellugyrin that does not show any insulin responsiveness (35, 36).
We then decided to study translocation of our reporters with the help of fluorescence-activated cell sorting (Fig. 7D). Because IRAP-EYFP and IRAPtail-EYFP are tagged with the same V5 epitope in the extracellular domain and with EYFP in the cytoplasmic tail (Fig. 1), we can quantitatively compare the extent of translocation of these proteins by fluorescence-activated cell sorter. Importantly, we only use the cells with the same levels of expression of IRAP-EYFP and IRAPtail-EYFP. We have determined in 5 independent experiments that IRAPtail-EYFP is translocated 3.54±0.93-fold less than IRAP-EYFP. In other words, full-length IRAP is ∼3.5 times more insulin-responsive than its truncated mutant without the luminal domain. This result may explain why we were not able to determine translocation of IRAPtail-EYFP using less sensitive biochemical fractionation. We believe that our data are consistent with previously published results of the McGraw and co-workers (37), who worked with an analogous reporter molecule (the cytoplasmic tail of IRAP conjugated to the extracellular domain of the transferring receptor) and found that insulin caused a moderate increase in its surface expression which was more than the insulin effect on the transferring receptor but less than the normal effect of insulin on Glut4 and IRAP.
DISCUSSION
In undifferentiated 3T3-L1 pre-adipocytes, IRAP is localized mostly in the perinuclear syntaxin 6-positive compartment. Subcellular fractionation shows that this compartment is represented not by small vesicles but, rather, by heavy rapidly sedimenting membranes (Fig. 3A). The biological nature of the IRAP-containing perinuclear compartment is not totally clear as it contains markers of both trans-Golgi network and recycling endosomes (14, 32, 38-40). Importantly, upon ectopic expression in undifferentiated 3T3-L1 pre-adipocytes, Glut4 (Fig. 2A) is targeted to the same perinuclear compartment, suggesting that the later may serve as the donor compartment for the formation of the GSVs upon cell differentiation. In agreement with this hypothesis, biochemical fractionation of differentiated adipocytes shows that both IRAP (Fig. 3A) and ectopically expressed myc7-Glut4 (14) re-distribute from heavy donor membranes to much lighter GSVs.
Previously, Ross et al. (21) demonstrated that in undifferentiated 3T3-L1 cells IRAP is readily biotinylated by membrane-impermeable sulfo-NHS-LC-biotin and that the efficiency of biotinylation is decreased with cell differentiation. Based on these results, they concluded that IRAP is present at the plasma membrane of undifferentiated 3T3-L1 cells and undergoes intracellular sequestration upon cell differentiation. On the other hand, Johnson et al. (40) showed that in Chinese hamster ovary cells a reporter protein consisting of the cytoplasmic tail of IRAP and the luminal domain of the transferrin receptor is localized primarily in the perinuclear compartment and recycles between this compartment and the plasma membrane. Although our data are more consistent with the results of Johnson et al. (40), we believe that the experimental data of Ross et al. (21) are also in line with our conclusions as it implies that, in undifferentiated 3T3-L1 cells, IRAP can recycle between the perinuclear compartment and the plasma membrane. At the same time, the rate of GSV recycling to the plasma membrane is extremely slow (33, 41). Therefore, massive redistribution of IRAP to this compartment upon cell differentiation should slow down recycling of IRAP and decrease the efficiency of its biotinylation.
The question of whether or not IRAP recycling in pre-adipocytes is regulated by insulin remains controversial. Using immunofluorescence staining (Fig. 2B) and fluorescence-activated cell sorter analysis (not shown), we do not register a massive insulin-dependent translocation of IRAP to the plasma membrane in undifferentiated cells. It is possible that a more sensitive technique based on 125I-labeled transferrin binding (40) allows one to visualize modest insulin-dependent translocation of IRAP in pre-adipocytes, which, however, is clearly increased upon cell differentiation (compare Fig. 2, B-D; see also Ref. 21). Importantly, the effect of insulin on IRAP recycling in fibroblasts is likely to be explained by stimulation of budding of transport vesicles from the donor compartment (42), which is different from massive translocation of preformed vesicles triggered by insulin in differentiated adipocytes.
The reporter molecule, which has the cytoplasmic tail of IRAP (IRAPtail-EYFP), mimics full-length IRAP in targeting to the syntaxin 6-positive perinuclear compartment but has a much lower insulin responsiveness, suggesting that the cytoplasmic tail targets IRAP to the GSV donor membranes (with zero or low insulin responsiveness) but may not be sufficient for the efficient protein entry into the highly insulin sensitive GSVs. To explain the role of the IRAP luminal domain in the process of GSV formation, we propose the following model.
In pre-adipocytes, endogenous IRAP is targeted to the perinuclear syntaxin 6-positive donor membranes by its cytoplasmic tail. Sortilin, which is induced around day 3 of differentiation, is targeted to the same perinuclear compartment by its cytoplasmic tail, where it interacts with IRAP (and, possibly, other proteins) in the membrane lumen (14, 17). This interaction seems essential for the correct intracellular localization of sortilin as the truncated sortilin molecule without the luminal domain is spread out from the perinuclear compartment to random intracellular loci upon cell differentiation (17). Finally, Glut4 is induced on day 3-4 of differentiation and is targeted to the same perinuclear donor membranes by its C terminus.6
Thus, three major component proteins of the GSVs, IRAP, Glut4, and sortilin, may be independently targeted to the same perinuclear compartment by their cytoplasmic tails. Upon arriving into this compartment, all three proteins may interact with each other via their lumenal domains. The heteromeric complex consisting of IRAP, Glut4, and sortilin may then re-distribute from the perinuclear donor membranes to the GSVs as a single entity using the C terminus of sortilin to recruit GGA adaptors (14, 18) and the central loop of Glut4 to recruit ACAP-1 (43).
As we and others have previously reported, myc7-Glut4, ectopically expressed in undifferentiated 3T3-L1 cells, is not targeted to small vesicles and is rapidly degraded by lysosomes (14, 44). However, if myc7-Glut4 is expressed together with sortilin, it is effectively targeted to the GSVs, which increases its stability and through that, intracellular content (14).
Interestingly, the interaction with sortilin is required for the GSV localization of Glut4 but has a lesser effect on IRAP. The latter protein is likely to be recruited to the GSVs by Glut4 itself (or by Glut4 and sortilin together). This mechanism may explain a well known phenomenon that knock-out of Glut4 decreases intracellular levels and/or sorting of IRAP at a post-transcriptional level (7, 34, 45, 46).
An important difference between these previous experiments and our studies is that we are using a “gain-of-function” approach that allows us to create an insulin-responsive vesicular compartment in undifferentiated cells. Note, that vesicles formed in GS pre-adipocytes upon ectopic expression of sortilin and Glut4 are indistinguishable from the GSVs present in differentiated wild type 3T3-L1 adipocytes by sucrose velocity centrifugation (compare Figs. 4A and 3C), equilibrium density centrifugation, and by immunoadsorption.7 In addition, these vesicles translocate ectopically expressed Glut4 to the plasma membrane and confer insulin-stimulated glucose uptake to undifferentiated 3T3-L1 cells to the same level as is found in differentiated adipocytes (14). Last but not least, they are built of the same major proteins. These data suggest that expression of sortilin and Glut4 creates authentic GSVs in 3T3-L1 pre-adipocytes.
Interestingly, Gross et al. (47) recently reported that some translocation of IRAP takes place in NIH-PPARγ cells that express little Glut4. However, translocation of IRAP in this study does not exceed 2.5-fold, suggesting that it may not be mediated by specialized GSVs.
If luminal interactions between vesicular proteins play such an important role in vesicle biogenesis, why then does IRAPtail-EYFP also show some insulin responsiveness (Fig. 7D)? We suggest that because this molecule is significantly enriched in the GSV donor membranes, some of it can be recruited into budding GSVs simply by mass action. Thus, deletion of the lumenal domain may decrease the efficiency of IRAP sorting into the GSVs but not eliminate it completely. Interestingly, the dileucine motif at positions 76 and 77 is required for the targeting IRAP to the perinuclear syntaxin 6-positive compartment (30). Moreover, this effect is GGA-dependent, indicating that the cytoplasmic tail of IRAP may also be involved in its targeting to the GSVs. Further studies should shed more light on the molecular mechanism of this process.
Acknowledgments
We thank Dr. Pilch for general gifts of antibodies and helpful discussions, Drs. Keller and McGraw for the IRAP cDNA, and Yanhui Deng for help with cell sorting.
This work was supported, in whole or in part, by National Institutes of Health Grant DK52057 and DK56736. This work was also supported by a Research Award from the American Diabetes Association (to K. V. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GSV, glucose transporter isoform 4 (Glut4) storage vesicle; IRAP, insulin-responsive aminopeptidase; DSP, dithiobis(succinimidyl propionate); AD, activation domain; EYFP, enhanced yellow fluorescent protein; ECFP, enhanced cyan fluorescent protein; PBS, phosphate-buffered saline.
M. Jedrychowski, K. V. Kandror, and P. Pilch, unpublished observations.
L. V. Li and K. V. Kandror, manuscript in preparation.
J. Shi and K. V. Kandror, unpublished observations.
References
- 1.Hou, J. C., and Pessin, J. E. (2007) Curr. Opin. Cell Biol. 19 466-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang, S., and Czech, M. P. (2007) Cell Metab. 5 237-252 [DOI] [PubMed] [Google Scholar]
- 3.Kandror, K. V., L. Yu, and Pilch, P. F. (1994) J. Biol. Chem. 269 30777-30780 [PubMed] [Google Scholar]
- 4.Kandror, K. V., and Pilch, P. F. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 8017-8021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller, S. R., Scott, H. M., Mastick, C. C., Aebersold, R., and Lienhard, G. (1995) J. Biol. Chem. 270 23612-23618 [DOI] [PubMed] [Google Scholar]
- 6.Keller, S. R., Davis, A. C., and Clairmont, K. B. (2002) J. Biol. Chem. 277 17677-17686 [DOI] [PubMed] [Google Scholar]
- 7.Yeh, T. Y., Sbodio, J. I., Tsun, Z. Y., Luo, B., and Chi, N. W. (2007) Biochem. J. 402 279-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larance, M., Ramm, G., Stockli, J., van Dam, E. M., Winata, S., Wasinger, V., Simpson, F., Graham, M., Junutula, J. R., Guilhaus, M., and James, D. E. (2005) J. Biol. Chem. 280 37803-37813 [DOI] [PubMed] [Google Scholar]
- 9.Peck, G. R., Ye, S., Pham, V., Fernando, R. N., Macaulay, S. L., Chai, S. Y., and Albiston, A. L. (2006) Mol. Endocrinol. 20 2576-2583 [DOI] [PubMed] [Google Scholar]
- 10.Hosaka, T., Brooks, C. C., Presman, E., Kim, S. K., Zhang, Z., Breen, M., Sztul, E., and Pilch, P. F. (2005) Mol. Biol. Cell 16 2882-2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters, S. B., D'Auria, M., Martin, S. S., Nguyen, C., Kozma, L. M., and Luskey, K. L. (1997) J. Biol. Chem. 272 23323-23327 [DOI] [PubMed] [Google Scholar]
- 12.Lin, B.-Z., Pilch, P. F., and Kandror, K. V. (1997) J. Biol. Chem. 272 24145-24147 [DOI] [PubMed] [Google Scholar]
- 13.Morris, N. J., Ross, S. A., Lane, W. S., Moestrup, S. K., Petersen, C. M., Keller, S. R., and Lienhard, G. E. (1998) J. Biol. Chem. 273 3582-3587 [DOI] [PubMed] [Google Scholar]
- 14.Shi, J., and Kandror, K. V. (2005) Dev. Cell 9 99-108 [DOI] [PubMed] [Google Scholar]
- 15.Cain, C. C., Trimble, W. S., and Lienhard, G. E. (1992) J. Biol. Chem. 267 11681-11684 [PubMed] [Google Scholar]
- 16.Ariga, M., Nedachi, T., Katagiri, H., and Kanzaki, M. (2008) J. Biol. Chem. 283 10208-10220 [DOI] [PubMed] [Google Scholar]
- 17.Shi, J., and Kandror, K. V. (2007) J. Biol. Chem. 282 9008-9016 [DOI] [PubMed] [Google Scholar]
- 18.Li, L. V., and Kandror, K. V. (2005) Mol. Endocrinol. 19 2145-2153 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen, M. S., Madsen, P., Christensen, E. I., Nykjaer, A., Gliemann, J., Kasper, D., Pohlmann, R., and Petersen, C. M. (2001) EMBO J. 20 2180-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson, R. T., Khan, A. H., Furukawa, M., Hou, J. C., Li, L., Kanzaki, M., Okada, S., Kandror, K. V., and Pessin, J. E. (2004) EMBO J. 23 2059-2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross, S. A., Keller, S. R., and Lienhard, G. E. (1998) Biochem. J. 330 1003-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, Z., Xie, Y., Morrison, R. F., Bucher, N. L. R., and Farmer, S. R. (1998) J. Clin. Investig. 101 22-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James, D. E., Brown, R., Navarro, J., and Pilch, P. F. (1988) Nature 333 183-185 [DOI] [PubMed] [Google Scholar]
- 24.Xu, Z., and Kandror, K. V. (2002) J. Biol. Chem. 277 47972-47975 [DOI] [PubMed] [Google Scholar]
- 25.Stephens, J. M., Lee, J., and Pilch, P. F. (1997) J. Biol. Chem. 272 971-976 [DOI] [PubMed] [Google Scholar]
- 26.Simpson, I. A., Yver, D. R., Hissin, P. J., Wardzala, L. J., Karnieli, E., Salans, L. B., and Cushman, S. W. (1983) Biochim. Biophys. Acta 763 393-407 [DOI] [PubMed] [Google Scholar]
- 27.Bogan, J. S., McKee, A. E., and Lodish, H. F. (2001) Mol. Cell. Biol. 21 4785-4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose, A., Cherniack, A. D., Langille, S. E., Nicoloro, S. M., Buxton, J. M., Park, J. G., Chawla, A., and Czech, M. P. (2001) Mol. Cell. Biol. 21 5262-5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen, M. S., Jacobsen, C., Olivecrona, G., Gliemann, J., and Petersen, C. M. (1999) J. Biol. Chem. 274 8832-8836 [DOI] [PubMed] [Google Scholar]
- 30.Hou, J. C., Suzuki, N., Pessin, J. E., and Watson, R. T. (2006) J. Biol. Chem. 281 33457-33466 [DOI] [PubMed] [Google Scholar]
- 31.ElJack, A., Kandror, K. V., and Pilch, P. F. (1999) Mol. Biol. Cell 10 1581-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeigerer, A., Lampson, M. A., Karylowski, O., Sabatini, D. D., Adesnik, M., Ren, M., and McGraw, T. E. (2002) Mol. Biol. Cell 13 2421-2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karylowski, O., Zeigerer, A., Cohen, A., and McGraw, T. E. (2004) Mol. Biol. Cell 15 870-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho, E., Schellhorn, S. E., Zabolotny, J. M., Martin, S., Tozzo, E., Peroni, O. D., Houseknecht, K. L., Mundt, A., James, D. E., and Kahn, B. B. (2004) J. Biol. Chem. 279 21598-21605 [DOI] [PubMed] [Google Scholar]
- 35.Kupriyanova, T. A., and Kandror, K. V. (2000) J. Biol. Chem. 275 36263-36268 [DOI] [PubMed] [Google Scholar]
- 36.Kupriyanova, T. A., Kandror, V., and Kandror, K. V. (2002) J. Biol. Chem. 277 9133-9138 [DOI] [PubMed] [Google Scholar]
- 37.Subtil, A., Lampson, M. A., Keller, S. R., and McGraw, T. E. (2000) J. Biol. Chem. 275 4787-4795 [DOI] [PubMed] [Google Scholar]
- 38.Hudson, A. W., Ruiz, M. L., and Birnbaum, M. J. (1992) J. Cell Biol. 116 785-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shewan, A. M., van Dam, E. M., Martin, S., Luen, T. B., Hong, W., Bryant, N. J., and James, D. E. (2003) Mol. Biol. Cell 14 973-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, A. O., Lampson, M. A., and McGraw, T. E. (2001) Mol. Biol. Cell 12 367-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandror, K. V. (1999) J. Biol. Chem. 274 25210-25217 [DOI] [PubMed] [Google Scholar]
- 42.Lampson, M. A., Schmoranzer, J., Zeigerer, A., Simon, S. M., and McGraw, T. E. (2001) Mol. Biol. Cell 12 3489-3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, J., Peters, P. J., Bai, M., Dai, J., Bos, E., Kirchhausen, T., Kandror, K. V., and Hsu, V. W. (2007) J. Cell Biol. 178 453-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, L. B., Omata, W., Kojima, I., and Shibata, H. (2007) Diabetes 56 1977-1985 [DOI] [PubMed] [Google Scholar]
- 45.Abel, E. D., Betuing, S., Pham, M., Reay, P., Kandror, V., Haney, N., Kupriyanova, T. A., Xu, Z., and Kandror, K. V. (2004) Mol. Endocrinol. 18 2491-2501 [DOI] [PubMed] [Google Scholar]
- 46.Jiang, H., Li, J., Katz, E. B., and Charron, M. J. (2001) Biochem. Biophys. Res. Commun. 284 519-525 [DOI] [PubMed] [Google Scholar]
- 47.Gross, D. N., Farmer, S. R., and Pilch, P. F. (2004) Mol. Cell. Biol. 24 7151-7162 [DOI] [PMC free article] [PubMed] [Google Scholar]