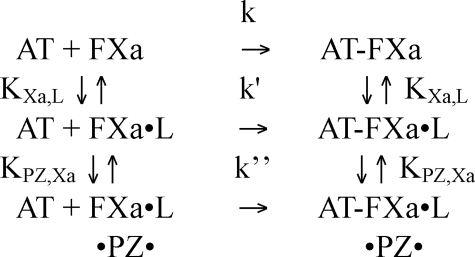Abstract
Protein Z-dependent protease inhibitor (ZPI) is a recently identified member of the serpin superfamily that functions as a cofactor-dependent regulator of blood coagulation factors Xa (FXa) and XIa. Here we show that ZPI and its cofactor, protein Z (PZ), inhibit procoagulant membrane-bound factor Xa by the branched pathway acyl-intermediate trapping mechanism used by other serpins, but with significant variations of this mechanism that are unique to ZPI. Rapid kinetic analyses showed that the reaction proceeded by the initial assembly of a membrane-associated PZ-ZPI-FXa Michaelis complex (KM 53 ± 5 nm) followed by conversion to a stable ZPI-FXa complex (klim 1.2 ± 0.1 s–1). Cofactor premixing experiments together with independent kinetic analyses of ZPI-PZ and factor Xa-PZ-membrane complex formation suggested that assembly of the Michaelis complex through either ZPI-PZ-lipid or factor Xa-PZ-lipid intermediates was rate-limiting. Reaction stoichiometry analyses and native PAGE showed that for every factor Xa molecule inhibited by ZPI, two serpin molecules were cleaved. Native PAGE and immunoblotting showed that PZ dissociated from ZPI once ZPI forms a stable complex with FXa, and kinetic analyses confirmed that PZ acted catalytically to accelerate the membrane-dependent ZPI-factor Xa reaction. The ZPI-FXa complex was only transiently stable and dissociated with a rate constant that showed a bell-shaped pH dependence indicative of participation of factor Xa active-site residues. The complex was detectable by SDS-PAGE when denatured at low pH, consistent with it being a kinetically trapped covalent acyl-intermediate. Together our findings show that ZPI functions like other serpins to regulate the activity of FXa but in a manner uniquely dependent on protein Z, procoagulant membranes, and pH.
Activation of factor X is a focal point for regulation of the blood coagulation cascade. Formation of factor Xa through the extrinsic pathway and its amplification through the intrinsic pathway are regulated by the Kunitz inhibitor, tissue factor pathway inhibitor, and by the serpin, antithrombin, and its cofactor, heparin (1, 2). Whereas tissue factor pathway inhibitor regulates the activity of factor Xa bound in a physiologic complex with membrane-bound tissue factor and factor VIIa, antithrombin and heparin poorly inhibit factor Xa bound in procoagulant membrane-associated physiologic complexes and instead appear to target factor Xa that escapes from its membrane sites of action (3, 4). Broze and co-workers (5, 6) have identified another serpin, protein Z-dependent protease inhibitor or ZPI, that specifically inhibits factor Xa bound to procoagulant membranes, suggesting a potential means for dynamic regulation of factor Xa bound in procoagulant complexes such as prothrombinase and factor Xase.
ZPI2 is a member of the serpin superfamily of protein protease inhibitors and is unusual in requiring protein Z, anionic phospholipids, and calcium as cofactors to rapidly inhibit factor Xa (6). Factor XIa is also inhibited by ZPI, but in a manner that is independent of protein Z, phospholipids, and calcium (7). An unusual feature of this serpin is that the P1 bait residue is a tyrosine, which is unexpected based on the arginine-specific target proteases of ZPI and the fact that most other serpins that inhibit coagulation proteases contain a P1 arginine residue (6). The serpin circulates as a tight stoichiometric complex with protein Z (8), a vitamin K-dependent protein whose domain structure resembles that of factor Xa in consisting of a Gla domain, two epidermal growth factor domains, and a protease domain, but with the protease domain lacking catalytic function (9). Inhibition of free factor Xa in the absence of protein Z is about 1000-fold slower than inhibition of membrane-bound factor Xa by ZPI-protein Z complex (7).
The importance of ZPI as an anticoagulant regulator of factor Xa and factor XIa is suggested by the enhanced thrombosis following arterial injury of mice lacking ZPI or protein Z (10, 11) and the more severe prothrombotic phenotype of mice harboring the factor V Leiden mutation in which the ZPI or protein Z genes have been knocked out. ZPI is consumed when plasma is induced to clot, and this consumption depends on the presence of protein Z and factor X when initiated through the extrinsic pathway and also on factor XI when initiated by the intrinsic pathway (7).
A number of observations have suggested that ZPI inhibition of factor Xa does not conform to the behavior of other well characterized serpin-protease reactions, including the antithrombin-factor Xa reaction. Specifically, the amidolytic activity of factor Xa is not completely inhibitable by a large molar excess of ZPI in the presence of its cofactors (12), even though serpins are known to be irreversible inhibitors of their target proteases (13). Moreover, no SDS-stable ZPI-factor Xa complex can be detected after reaction by SDS-PAGE analysis as with other serpin-protease reactions (7). Finally, ZPI appears to inhibit factor Xa in a temporary fashion based on the facile regeneration of factor Xa activity and concomitant formation of inactive, cleaved ZPI following the reaction (7). Because these unusual properties are difficult to reconcile with the established serpin mechanism of protease inhibition (13), we sought to determine whether ZPI utilized the same branched pathway suicide substrate mechanism of protease inhibition as other serpins and to elucidate the mechanism of instability of the ZPI-protease complex. Our findings suggest that ZPI does in fact inhibit factor Xa by the established serpin inhibitory mechanism in which the serpin conformationally traps the protease at the acyl-intermediate stage of cleavage of an exposed serpin-reactive bond. However, our studies demonstrate that this conformational trapping fails to fully inactivate the catalytic machinery of the protease as it does in other serpin-protease complexes (14, 15), thereby accounting for the apparent anomalous behavior of the ZPI-factor Xa reaction. Our findings further reveal a catalytic role for protein Z in promoting the membrane-associated ZPI-factor Xa reaction, similar to the role of heparin in promoting antithrombin-protease reactions (16).
EXPERIMENTAL PROCEDURES
Materials—SDS, Hepes, Mes, Trizma (Tris base), TAPS, EDTA, polyethylene glycol (Mr, 8,000 and 20,000), and bovine serum albumin were from Sigma. PVDF membranes were from Micron Separations, Inc. (Westborough, MA). Prestained molecular weight standards were purchased from Bio-Rad. Human factor Xa, protein Z, factor XIa, and factor IXaβ were purchased from Enzyme Research Labs (South Bend, IN). Antithrombin and ZPI were purified from human plasma as described (5, 17). High affinity heparin containing ∼50 saccharides was prepared by size and affinity fractionation of commercial heparin as described (17). A mixture of synthetic dioleyl phosphatidylcholine and dioleoyl phosphatidylserine in a weight ratio (and approximate molar ratio) of 7:3 was obtained from Avanti Polar Lipids (Alabaster, AL). Phospholipid vesicles were prepared as described (18), stored at 4 °C, and used within a week. S2366 (l-pyroglutamyl-l-prolyl-l-arginine-p-nitroanilide) was from Diapharma (West Chester, OH), Spectrozyme FXa (SpFXa, MeO-CO-d-cyclohexylglycyl-Gly-Arg-p-nitroaniline) was from American Diagnostica (Greenwich, CT), and the factor Xa fluorogenic substrate, Pefafluor FXa (CH3SO2-(d)-CHA-Gly-Arg-AMC-AcOH), was from Centerchem (Norwalk, CT). Mono S HR 5/5, Sephacryl S-200, and SP-Sepharose resins were purchased from GE Healthcare.
Expression and Purification of Recombinant ZPI—ZPI cDNA, including the N-terminal leader sequence, was cloned into the BamHI and EcoRI sites of pFastbac1vector, and recombinant ZPI was expressed in baculovirus-infected insects cells using the Bac-to-Bac baculovirus expression system (Invitrogen), as in previous studies (19). Viruses were prepared and amplified in Sf9 insect cells, whereas ZPI was expressed in High-Five insect cells. After 2–3 days of expression, the media were collected and centrifuged to remove cells, and then EDTA and phenylmethylsulfonyl fluoride were added to 1 mm.
Recombinant ZPI was purified from the media by successive chromatography steps on SP-Sepharose, Sephacryl S-200, and Mono S (5). 1–2 liters of media containing recombinant ZPI was applied at a flow rate of 70 ml/h to a 3 × 12-cm SP-Sepharose column equilibrated in 0.02 m sodium phosphate, 0.02 m NaCl (pH 6.75) at 4 °C. The column was washed with ∼1 liter of sodium phosphate buffer containing 0.1 m NaCl and then eluted with a 300-ml linear gradient to 0.7 m NaCl in the same buffer. Fractions containing ZPI activity were detected by a factor XIa inhibition assay as follows. Aliquots of fractions were added to 5 nm factor XIa in 20 mm sodium phosphate, 0.1 m NaCl, 0.1% PEG 8000 buffer to a final volume of 50 μl, incubated for 15 min at 25 °C, and then assayed for residual factor XIa activity by adding 950 μl of 100 mm S2366 substrate and measuring the initial rate of substrate hydrolysis at 405 nm relative to an uninhibited control. Active fractions eluting at ∼0.28 m NaCl were combined and concentrated to 5–7 ml. The concentrated sample was applied to a 3 × 100-cm Sephacryl S-200 column equilibrated in 0.02 m sodium phosphate, 0.25 m NaCl (pH 6.75) and eluted at a flow rate of 20 ml/h with the same buffer at 4 °C. Fractions containing ZPI activity were combined, and the pool was concentrated to 7–8 ml. The sample was diluted 4-fold with 0.02 m Mes (pH 6.6) and applied at a flow rate of 0.5 ml/min to a 1-ml Mono S column equilibrated in 0.02 m Mes, 0.1 m NaCl (pH 6.6). The column was washed with 30 ml of equilibrating buffer and then eluted with a 45-ml linear gradient to 0.5 m NaCl in the same buffer. Fractions containing ZPI activity, eluting at ∼0.25 m NaCl, were pooled, and the purified ZPI was stored at –70 °C in small aliquots.
The molar concentration of ZPI was calculated from the absorbance at 280 nm using a molar absorption coefficient of 31,525 m–1 cm–1, calculated as described (20). The molecular weight of wild type ZPI was calculated to be 48,462.
Experimental Conditions—Most experiments were conducted in either 20 mm sodium phosphate buffer containing 0.1 m NaCl, 0.1% PEG 8000 (pH 7.4) at 25 °C (factor XIa experiments) or 50 mm Hepes buffer containing 0.1 m NaCl, 0.1% PEG 8000 (pH 7.4) (factor Xa) unless specified otherwise.
Stoichiometry of ZPI-Protease Reactions—The stoichiometry of inhibition of proteases by ZPI was determined by reacting a fixed concentration of protease with increasing concentrations of ZPI up to a 3–6-fold molar excess. Reactions with 12 nm factor XIa active sites were done in the absence or presence of 100 nm heparin containing 50 saccharides, and reactions with 5.5 nm factor Xa were done in the presence of 30 nm protein Z, 25 μm phospholipid, and 5 mm calcium chloride. Reactions were performed in polystyrene cuvettes coated with PEG 20,000 to minimize protein adsorption in volumes of 50–100 μl. After allowing sufficient time to reach maximal inhibition based on measured second order association rate constants, remaining proteolytic enzyme activity was measured by diluting the reaction mixture with 900–950 μl of substrate, either 100 μm S2366 for factor XIa reactions or 200 μm Spectrozyme FXa or 30 μm Pefafluor FXa for factor Xa reactions, and the rate of substrate hydrolysis was monitored by absorbance at 405 nm or by fluorescence at λex 380 nm/λem 440 nm. For reactions done in the presence of phospholipids and calcium, substrate solutions contained 10 mm EDTA to quench lipid-dependent reactions. Initial rates of hydrolysis were determined by fitting substrate hydrolysis progress curves to the second order polynomial function shown in Equation 1,
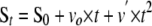 |
(Eq. 1) |
where St and S0 are the absorbance or fluorescence of the product of substrate hydrolysis at time t and time 0, respectively; vo is the initial velocity of substrate hydrolysis, and v′ is the acceleration of the rate of substrate hydrolysis because of dissociation of the ZPI-protease complex. The fraction of residual protease activity was calculated from the ratio of initial hydrolysis rates in the presence and absence of inhibitor. The inhibition stoichiometry was determined from the fitted abscissa intercept of a linear plot of residual protease activity against the molar ratio of inhibitor to protease (17).
Kinetics of ZPI-Protease Reactions—The rate of protease inactivation by ZPI was measured under pseudo-first order conditions in which the inhibitor was in large molar excess over the protease (at least five times greater than the concentration of ZPI required to fully inhibit protease) and in the absence or presence of different cofactors as indicated. Progress curves for the time-dependent inhibition of protease were measured in discontinuous or continuous assays. In the discontinuous assay, samples were withdrawn from reaction mixtures at varying times and diluted into substrate containing 10 mm EDTA for measurement of residual protease activity as in the determinations of inhibition stoichiometry. Initial velocities of substrate hydrolysis were measured by fitting progress curves by the second order polynomial function as described above. For ZPI-factor Xa reactions done in the presence of all cofactors (protein Z, phospholipids, and calcium), observed pseudo-first order rate constants (kobs) were obtained by fitting the loss of protease activity by an exponential decay function with a zero end point or in some cases with a nonzero end point amounting to <5% (21) because of small amounts of degraded protease that were more resistant to inhibition by ZPI. For reactions done in the absence of phospholipids and calcium, progress curves showed incomplete factor Xa inhibition and a progressive return of factor Xa activity. Such curves were fit by a serpin reaction model involving an initial association phase leading to an inhibited ZPI-factor Xa complex (E-I*) and cleaved ZPI (I*) followed by a dissociation phase in which the inhibited complex dissociated to free factor Xa and cleaved ZPI, as shown in Scheme 1. Data were computer fit by numerically integrating the differential equations for the model using Scientist software (Micromath) to yield the second order association rate constant for formation of the common acyl-intermediate for inhibition and substrate pathways (ka), the ratio of rate constants for partitioning of the intermediate between inhibition and substrate pathways (kI/kS), and a first order dissociation rate constant for the conformationally trapped acyl-intermediate (kd).
SCHEME 1.
The continuous assay was used only for ZPI-factor Xa reactions performed in the presence of all cofactors. Reactions were conducted in this case in the presence of a chromogenic or fluorogenic protease substrate, and the decrease in rate of substrate hydrolysis due to inhibition of the protease was continuously monitored under conditions in which substrate consumption was less than 10%, and the rate of hydrolysis remained linear in the absence of inhibitor (17). Reactions were performed with a spectrophotometer for chromogenic substrates or with an SLM 8000 fluorometer (half-lives > 20 s) or an SX-17MV Applied Photophysics stopped-flow fluorometer (half-lives < 20 s) for fluorogenic substrates. Reaction progress curves were fit over 5–10 half-lives by Equation 2 (17),
 |
(Eq. 2) |
where St represents the time-dependent absorbance or fluorescence signal of the product of substrate hydrolysis; ΔS is the amplitude of the exponential term; kobs is the observed pseudo-first order rate constant; and vss is the end point steady-state rate of substrate hydrolysis. Corrections for the competitive effect of substrate on the inhibition reaction were made by dividing the ZPI or ZPI-protein Z complex concentration by the factor, 1 + [S]0/KM,S where [S]0 is the concentration of protease substrate and KM,S is the Michaelis constant for hydrolysis of the substrate by the protease. The latter was determined by measuring the initial rate of substrate hydrolysis at a fixed concentration of protease and with a range of substrate concentrations up to 2–5 × KM,S.
Kinetic studies of the cofactor-dependent ZPI-factor Xa reaction were done either with catalytic or stoichiometric levels of protein Z. In the former experiments, 5 nm factor Xa was preincubated with 0–2 nm protein Z in Hepes buffer containing 5 mm CaCl2 and 25 μm phospholipid prior to initiating the reaction with 200 nm ZPI and following the loss in factor Xa activity by the discontinuous assay method. Reactions with stoichiometric levels of protein Z were all performed using the continuous assay method. Initial experiments evaluated the effect of premixing phospholipid and protein Z cofactors with either ZPI or factor Xa or both reactants in all possible combinations in pH 7.4 Hepes buffer containing 5 mm CaCl2 and 0.1 mg/ml bovine serum albumin prior to mixing equal volumes of the serpin and protease. Final reactant concentrations in this case were 10 nm ZPI, 0.05 nm factor Xa, 1–5 nm protein Z, 25 μm phospholipid, and 40 μm Pefafluor substrate. Based on these studies, a more extensive set of kinetic experiments was performed at a series of fixed concentrations of ZPI as a function of increasing protein Z concentration in Hepes buffer containing 5 mm CaCl2, 25 μm phospholipid, and 0.1 mg/ml bovine serum albumin. In these experiments ZPI and fluorogenic substrate were mixed with an equal volume of factor Xa and protein Z, both in buffer containing phospholipid and calcium. To ensure pseudo-first order conditions, ZPI was maintained minimally at 50 times the factor Xa concentration, and protein Z was present at least 20 times the factor Xa concentration. The dependence of kobs on the ZPI and protein Z concentrations was fit by Equation 3 (22),
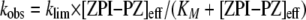 |
(Eq. 3) |
where KM is the Michaelis constant for formation of a membrane-associated ZPI-PZ-FXa ternary complex; klim is the limiting rate constant for transformation of the noncovalent Michaelis complex to a covalent ZPI-FXa complex, and [ZPI-PZ]eff is the effective concentration of ZPI-protein Z complex. [ZPI-PZ]eff is given by Equation 4,
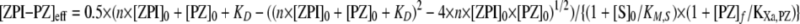 |
(Eq. 4) |
where [ZPI]0 and [PZ]0 are the total concentrations of ZPI and protein Z, respectively; n is the stoichiometry, and KD is the dissociation constant for the ZPI-protein Z interaction; the factor 1 + [S]0/KM,S is the correction for substrate competition as described above; [PZ]f is the uncomplexed protein Z concentration; and KXa,PZ is the apparent dissociation constant for uncomplexed protein Z binding to factor Xa on the membrane. The factor 1 + [PZ]f/KXa,PZ represents a correction for the competitive effect of uncomplexed protein Z on ternary complex formation from ZPI-protein Z complex and factor Xa because of the formation of nonproductive protein Z-factor Xa binary complexes on the membrane. [PZ]f is equal to the difference, [PZ]0 – [ZPI-PZ]. Because the KD value for the ZPI-protein Z interaction was poorly determined by the fitting, the value of 0.6 nm obtained from titrations of protein Z effects on the stoichiometric end point for reactions in the absence of lipid and calcium (Fig. 3C) was assumed, and n, KM, klim, and KXa,PZ were the fitted parameters.
FIGURE 3.
Effect of protein Z on the ZPI-factor Xa reaction in the absence of phospholipid and calcium. A, progress curves for reactions of 50 nm factor Xa with 200 nm ZPI (•, ○) or 300 nm ZPI (▴, ▵) in the absence of protein Z (solid symbols) or in the presence of protein Z equimolar with ZPI (open symbols), all in the absence of phospholipid and calcium. Remaining factor Xa activity was measured relative to a control factor Xa reaction in the absence of ZPI (squares) by discontinuous assay. Progress curves were computer fit by the differential equations for the serpin reaction model given under”Experimental Procedures“(solid line). B, progress curves for reaction of 30 nm ZPI with 2.5 nm factor Xa in the absence (•) or presence (○) of 30 nm protein Z. Control factor Xa incubations performed in the absence of ZPI are also shown (▴, ▵). Curves were fit to a single exponential decay function with a nonzero end point (solid lines). C, titrations of the change in the end point for ZPI reactions with 2.5 nm factor Xa produced by protein Z binding to ZPI. Reaction end points were measured after 150 min of reaction with fixed ZPI concentrations of 30 nm (•) and 60 nm (▴) and varying concentrations of protein Z. Titrations were fit by the quadratic binding equation under”Experimental Procedures“to obtain values for the stoichiometry and KD for the ZPI-protein Z interaction.
Protein Z and Phospholipid Effects on the Kinetics of Factor Xa Inhibition by Antithrombin—Antithrombin (3 μm) was reacted with factor Xa (5 nm) under pseudo-first order conditions in Hepes buffer plus 5 mm CaCl2 in the absence or presence of 25 μm phospholipid or 40 nm protein Z in a total volume of 50 μl. Reactions were quenched after varying times with 950 μl of 200 μm Spectrozyme FXa and the residual factor Xa activity measured from the initial rate of substrate hydrolysis at 405 nm. In a second set of experiments, antithrombin and factor Xa were incubated in Hepes/CaCl2 buffer as above with either 25 or 50 μm phospholipid and variable protein Z concentrations. Reactions were incubated for 5 min and quenched with Spectrozyme FXa substrate and residual factor Xa activity measured as above. Pseudo-first order rate constants were determined by fitting full reaction progress curves to a single exponential decay with zero end point in the first set of experiments or by calculating kobs from the ratio, –ln([E]t/[E0)/t, where [E]t/[E]0 is the fraction of factor Xa activity remaining relative to the uninhibited control, and t is the reaction time. Data were analyzed according to Scheme 2, where k, k′, and k″ represent second order rate constants for the reactions of antithrombin with free factor Xa, lipid-bound factor Xa, and lipid-bound factor Xa complexed with protein Z, respectively; and KXa,L and KPZ,Xa are dissociation constants for the factor Xa-lipid interaction and the interaction of protein Z with the factor Xa-lipid complex, respectively. Implicit in the model is the equilibrium for protein Z binding to lipid with dissociation constant, KPZ,L, as shown in Equation 5.
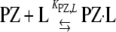 |
(Eq. 5) |
Under conditions (i) that are pseudo-first order, i.e. with antithrombin in large molar excess over factor Xa, and (ii) where the concentrations of lipid and protein Z greatly exceed the factor Xa concentration, the model predicts that antithrombin inhibition of factor Xa will follow an exponential decay with an observed rate constant given by Equation 6,
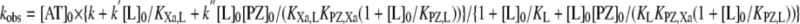 |
(Eq. 6) |
where [AT]0, [L]0, and [PZ]0 are total concentrations of antithrombin, lipid, and protein Z, respectively. At lipid concentrations where essentially all factor Xa and protein Z are bound to lipid (i.e. [L]0 ≫ KXa,L, KPZ,L), the contribution of the free factor Xa reaction can be neglected, and kobs simplifies to that shown in Equation 7,
 |
(Eq. 7) |
where Kapp = KPZ,Xa[L]0/KPZ,L. Measured values of kobs as a function of protein Z concentration at a fixed concentration of lipid were computer fit to the above simplified equation to obtain values for k′, k″, and Kapp.
SCHEME 2.
Kinetic End Point Assay for Determining the Affinity of the ZPI-Protein Z Interaction—Titrations to determine the ZPI-protein Z binding affinity and stoichiometry were done by incubating fixed concentrations of ZPI (30 and 60 nm) with increasing concentrations of protein Z up to a 2-fold molar excess over ZPI, in all cases with a fixed concentration of factor Xa (2.5 nm) in Hepes buffer without calcium or phospholipid. The reaction mixtures were incubated at 25 °C for 150 min and then 200 μm Spectrozyme FXa substrate containing 10 mm EDTA was added, and the residual enzymatic activity was measured from the initial linear rate of change of absorbance at 405 nm. Titrations of the change in factor Xa end point activity as a function of the protein Z concentration were fit by the quadratic binding Equation 8 (17):
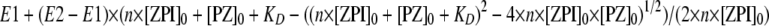 |
(Eq. 8) |
where E1 and E2 represent the factor Xa activity end points in the absence of protein Z and in the presence of saturating protein Z; [ZPI]0 is the total ZPI concentration; [PZ]0 is the total protein Z concentration, and n and KD are the stoichiometry and dissociation constants for the ZPI-protein Z interaction, respectively.
PAGE and Immunoblotting—Nondenaturing native PAGE was performed at 4 °C using the Laemmli buffer system (23) with 5.5 or 8% gels and running times of 2–5 h at 150 V. Protein bands were detected either by Coomassie Blue staining or by immunoblotting with goat anti-factor X (Enzyme Research Laboratories), goat anti-protein Z (Cedarlane Lab, Burlington, Ontario, Canada), or mouse anti-ZPI antibodies (5) (Novus Biological, Littleton, CO) and chemiluminescence detection after electrophoretic transfer of protein bands to PVDF membranes.
SDS-PAGE analysis of proteins was done using the Laemmli discontinuous buffer system (23) with 10% polyacrylamide gels. Protein bands were detected by Coomassie Blue staining or by immunoblotting with anti-factor Xa antibodies or anti-ZPI antibodies after transfer of protein bands to PVDF membranes under renaturing conditions. Experiments to detect ZPI cleavage by factor Xa or factor IXa were done by incubating protease and ZPI at equimolar concentrations (0.9 μm) in the absence or presence of a 10% molar excess of protein Z and 25 μm phospholipid in Hepes buffer plus 5 mm CaCl2. Factor Xa reactions were incubated for 10 s and factor IXa reactions for 5 min; reactions were quenched with 0.5 mm p-amidinophenylmethylsulfonyl fluoride (Sigma) and then denatured in SDS treatment buffer prior to PAGE analysis. For experiments in which the effect of denaturation pH was examined, samples were denatured in Hepes reaction buffer after adding 1% SDS with or without the addition of HCl to reach a pH of 2. After boiling for 5 min, the pH of the acid pH samples was adjusted back to ∼7.0 by adding NaOH and then mixed with sample buffer before electrophoresis.
Kinetics of Dissociation of ZPI-Factor Xa Complexes—First order rate constants for the dissociation of serpin-protease complexes were measured by either discontinuous or continuous assays of the time-dependent appearance of factor Xa activity. In the discontinuous assay, ZPI-factor Xa complexes were prepared by incubating 21.5 nm ZPI and 22 nm PZ, with 2.6 nm FXa in buffers of different pH values (6.0–9.0) containing 25 μm phospholipid, 5 mm CaCl2 at 25 °C. The buffers employed were 50 mm Mes (pH 6.0), 50 mm Hepes (pH 6.5–8.0), and 50 mm Tris (pH 8.4–9.0), all containing 0.1 m NaCl and 0.1% PEG 8000. Incubations were conducted for 5 min at pH 7.4–9.0, 10 min at pH 7.0, 40 min at pH 6.5, and 120 min at pH 6.0 to allow maximal complex formation. Reactions were then quenched by adding 0.1 volume of 250 mm EDTA at the same pH, and 100-μl aliquots were withdrawn at increasing times thereafter and added to 900 μl of 200 μm Spectrozyme FXa in pH 7.4 Hepes buffer for measurement of the factor Xa activity relative to an uninhibited control as in the kinetic assays described earlier.
For the continuous assay, ZPI-factor Xa complexes were prepared by incubating 100–500 nm ZPI and equimolar PZ with 25–125 nm FXa in pH 7.4 Hepes buffer containing 25 μm phospholipid and 5 mm CaCl2 for 2 min to produce maximal ZPI-FXa complex. Aliquots of 10–20 μl were then diluted into 1 ml of pre-warmed buffers at 25 °C containing 200–400 μm Spectrozyme FXa or 40 μm Pefafluor FXa substrate. Buffers employed were 50 mm Mes (pH 5.0–6.5), 50 mm Hepes (pH 6.5–8.5), 50 mm Tris-HCl (pH 9.0), and 50 mm TAPS (pH 9.0–9.5), all containing 0.1 m NaCl, 10 mm EDTA, and 0.1% PEG 8000. Initial rates of complex dissociation (<10% dissociation) were monitored from increases in absorbance or fluorescence for ∼15 min. Progress curves were fit by the parabolic function (14) shown in Equation 9,
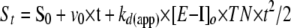 |
(Eq. 9) |
where St and S0 are the absorbance or fluorescence at time t and time 0, respectively; v0 is the initial rate of absorbance/fluorescence change at time 0; kd(app) is the apparent first order rate constant for complex dissociation; TN is the turnover number determined from the slope of a calibration curve relating the initial velocity of substrate cleavage to protease concentration; and [E-I]o is the concentration of ZPI-factor Xa complex. Computer fitting of progress curves by Equation 9 provided the coefficient of the t2 term from which kd(app) was calculated from the complex concentration and the turnover number (14). The linear dependence of the coefficient of the t2 term on complex concentration verified that quenching of reactions with EDTA and dilution of complexes into substrate was sufficient to block any reassociation of unreacted ZPI with dissociated factor Xa. The pH dependence of kd(app) was analyzed according to a double protonation model in which protonation of the catalytic His-57 or deprotonation of the N-terminal Ile-16 of factor Xa blocks deacylation of the ZPI-factor Xa acyl-intermediate complex, i.e. the singly protonated form is solely responsible for the observed rate of deacylation. Equation 10 for this model is shown,
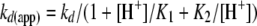 |
(Eq. 10) |
where kd is the intrinsic rate constant for deacylation of the ZPI-factor Xa acyl-intermediate complex in the singly protonated state, and K1 and K2 are the dissociation constants for hydrogen ion binding to His-57 and the N-terminal Ile-16 (14).
RESULTS
Expression, Purification, and Activity of Recombinant ZPI—Recombinant ZPI was expressed in high yield in insect cells (1–3 mg/liter medium) and could be purified by cation exchange and size-exclusion chromatography steps to >90% homogeneity as judged by SDS-PAGE analysis (not shown). The molecular size of the recombinant protein (∼50 kDa) was lower than that of plasma ZPI (∼72 kDa) and close to the expected molecular weight of the unglycosylated serpin (48 kDa), consistent with the reduced glycosylation observed with other plasma serpins in this expression system (24). Recombinant ZPI rapidly inhibited factor Xa only when protein Z, anionic phospholipid (30% phosphatidylserine, 70% phosphatidylcholine), and calcium ion cofactors were present in the reaction, similar to the behavior of plasma ZPI (5) (Fig. 1A). Indistinguishable rates of factor Xa inactivation were observed when the protease was reacted with equal concentrations of recombinant ZPI or plasma ZPI in the presence of protein Z, lipid, and calcium cofactors. Essentially complete factor Xa inhibition was obtained in either reaction as long as care was taken to determine the initial factor Xa activity immediately after dilution into substrate (Fig. 1, B and C). This precaution was necessary to avoid any contribution of factor Xa that was subsequently released from the transiently stable ZPI-factor Xa complex.
FIGURE 1.
Cofactor dependence of recombinant ZPI inhibition of factor Xa. A, rate of inhibition of 2 nm factor Xa by 34 nm recombinant ZPI in the presence of 36 nm protein Z, 25 μm phospholipids, and 5 mm calcium (•) or in the absence of either protein Z (▴), lipid and calcium (▪), protein Z and lipid (○), or protein Z and calcium (▵) was monitored by discontinuous assays of residual factor Xa enzymatic activity as described under “Experimental Procedures. B and C, reactions of 0.1 nm factor Xa with 2 nm recombinant or plasma ZPI in the presence of 4 nm protein Z, 25 μm phospholipids, and 5 mm calcium. B shows measurements of the initial rates of hydrolysis of factor Xa fluorogenic substrate by residual active factor Xa after the indicated reaction times. Solid lines are fits to a second order polynomial function to obtain the initial hydrolysis rate. C compares plots of the loss of factor Xa activity relative to the uninhibited control activity for reactions of recombinant ZPI and plasma ZPI as a function of time. Solid lines are fits of the data to a single exponential decay. Further details are provided under”Experimental Procedures.“
Recombinant ZPI was also an efficient inhibitor of factor XIa. However, unlike the inhibition of factor Xa by ZPI, this inhibition was not enhanced by protein Z, lipid, and calcium ion cofactors but was accelerated ∼2-fold by heparin (not shown), again confirming the reported behavior of plasma ZPI (7). Similar to the report of Broze and co-workers (6) but contrasting that of Heeb et al. (25), we were unable to detect any inhibition of factor IXa by recombinant ZPI either in the absence or presence of protein Z, phospholipid, and calcium.
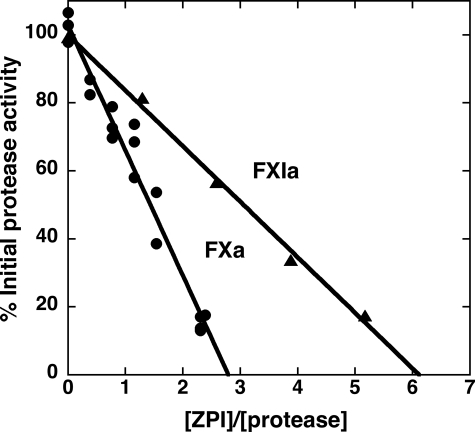
Stoichiometry of ZPI-Protease Reactions—Serpins inhibit their target proteases through a branched pathway suicide substrate mechanism that can result in cleavage of the serpin as a substrate (13). To determine to what extent ZPI might react as a substrate in competition with its reaction as an inhibitor of factor Xa, we measured the stoichiometry of ZPI inhibition (SI) of factor Xa. A fixed concentration of factor Xa was titrated with increasing concentrations of ZPI in the presence of lipid and calcium and a molar excess of protein Z over ZPI, and the inhibition end point was measured after reaction for a time that was sufficient to complete the inhibition phase but to minimize any subsequent dissociation of the inhibited complex (5–10 min). The extent of factor Xa inhibition increased linearly with increasing molar ratios of ZPI to factor Xa, with complete inhibition being reached at 2.8 ± 0.2 mol of ZPI per mol of factor Xa (Fig. 2). This result implied that for every three molecules of ZPI that react with factor Xa, ∼2 molecules of ZPI are cleaved as a substrate, and 1 forms a stable ZPI-factor Xa complex, an expectation borne out by native PAGE analysis of the products of the reaction (see below). The SI was significantly greater for ZPI inhibition of factor XIa, with values of 8.6 ± 0.8 mol of ZPI/mol of factor XIa obtained in the absence of heparin and 6.1 ± 0.4 mol of ZPI/mol of factor XIa in the presence of the polysaccharide (Fig. 2). No evidence for cleavage of ZPI by factor IXa in the absence or presence of cofactors was obtained by SDS-PAGE analysis (not shown), showing that the failure to observe inhibition of factor IXa was not because of a substrate reaction of the serpin with the enzyme.
FIGURE 2.
Stoichiometry of ZPI inhibition of factor Xa and factor XIa in the presence of cofactors. Fixed concentrations of factor Xa (5. 5 nm) or factor XIa (12 nm active sites) were reacted with increasing molar ratios of ZPI to protease in the presence of 30 nm protein Z, 25 μm phospholipids, and 5 mm calcium for factor Xa reactions (•) or in the presence of 100 nm heparin in the case of factor XIa reactions (▴). After 10–15 min of reaction, substrate was added, and the residual protease activity was measured relative to an uninhibited control. Data were fit by linear regression to obtain the stoichiometry of inhibition (SI) from the abscissa intercept. Additional details are provided under”Experimental Procedures.“
Kinetic Analysis of the ZPI-Factor Xa Reaction in the Absence of Lipid and Calcium—ZPI inhibited factor Xa slowly and incompletely in the absence of all cofactors (lipid, calcium, and protein Z). Reaction progress curves conducted with a 4-fold molar excess of ZPI over factor Xa showed a decay of enzyme activity to a minimum value followed by a return of the activity (Fig. 3A). Increasing the ZPI to factor Xa molar ratio to 6 increased the extent of factor Xa inhibition in the initial reaction phase. The enzyme activity recovery phase was substantially delayed under conditions where ZPI was in large molar excess over factor Xa (12-fold) so that only an inhibition phase to a stable end point was observable (Fig. 3B). Fitting of the progress curves to a ZPI-factor Xa association phase to form an inhibited complex and cleaved ZPI followed by a complex dissociation phase indicated that the maximal extent of inhibition can be accounted for by the relative magnitudes of association and dissociation rate constants, i.e. the maximal inhibition end point reflects a steady-state in which the rates of complex formation and complex dissociation have become equal. These fits yielded apparent second order rate constants for the association phase of the reaction, equal to the intrinsic association rate constant divided by the SI (ka/SI) (13), ranging from 6–8 × 103 m–1 s–1 and first order rate constants for the dissociation phase of 1.5–1.6 × 10–4 s–1 (Table 1).
TABLE 1.
Kinetic parameters for the reaction of ZPI with factor Xa in the absence of phospholipid and calcium and in the absence or presence of protein Z in / 0.15 Hepes buffer (pH 7.4) at 25 °C
Reactions contained 50 nm factor Xa and the indicated concentrations of ZPI and protein Z and were followed by the discontinuous assay method. Reaction progress curves, shown in Fig. 3A, were fit by numerical integration of the differential equations for the general serpin model as described under “Experimental Procedures” to obtain kinetic parameters.
| [Protein Z]0 | [ZPI]0 | ka | SI = (ks + kI)/KI | kd |
|---|---|---|---|---|
| nm | m-1 s-1 | s-1 | ||
| 200 | 1.5 ± 0.4 × 104 | 2.4 ± 0.5 | 1.6 ± 0.4 × 10-4 | |
| 300 | 1.8 ± 0.2 × 104 | 3.0 ± 0.3 | 1.5 ± 0.2 × 10-4 | |
| 200 | 200 | 2.9 ± 0.3 × 104 | 7.5 ± 0.3 | 1.3 ± 0.1 × 10-4 |
| 300 | 300 | 2.5 ± 0.2 × 104 | 7.5a | 2.0 ± 0.5 × 10-4 |
Value was not well determined by the fitting procedure, and therefore the value at 200 nm ZPI was assumed in the fitting of this reaction progress curve.
The inclusion of protein Z in slight molar excess over ZPI reduced both the rate and maximal extent of factor Xa inhibition in the absence of lipid and calcium (Fig. 3, A and B). A similar behavior was previously noted for ZPI inhibition of factor XIa in the absence and presence of protein Z (8), a finding that we verified. Fitting of a similar series of progress curves for the ZPI-factor Xa reaction in the presence of a molar excess of protein Z over ZPI indicated that the protein Z effects resulted from a decreased apparent rate constant for the association phase of the reaction (to 3–4 × 103 m–1 s–1) with no significant effects on the dissociation phase (1.3–2 × 10–4 s–1). Moreover, the decrease in the association phase rate constant was attributable to an increase in the fraction of ZPI that underwent a substrate reaction (SI) and not to any decrease in the intrinsic rate of formation of the ZPI-factor Xa complex (ka) (Table 1).
Titrations of the change in the factor Xa inhibition end point produced by adding protein Z to ZPI-factor Xa reactions showed a progressive decrease in the extent of inhibition to a plateau value after ∼1 mol of protein Z/mol of ZPI had been added (Fig. 3C). Fitting of several titrations assuming that the change in reaction end point is proportional to the fraction of ZPI bound to protein Z allowed us to estimate a KD of 0.6 ± 0.3 nm for formation of the protein Z-ZPI complex.
The formation of a high affinity ZPI-protein Z complex that was independent of phospholipid and calcium cofactors was previously demonstrated by size-exclusion chromatography and native PAGE with purified proteins and in plasma (8). Native PAGE analysis of pure recombinant ZPI and protein Z mixed in varying molar ratios confirmed the appearance of a high affinity complex band with mobility intermediate between that of free ZPI and protein Z bands that was maximally formed when stoichiometric amounts of the two proteins were mixed (Fig. 4).
FIGURE 4.
Native PAGE analysis of the ZPI-protein Z interaction. ZPI and protein Z alone (6–7 μg) or mixed in the indicated molar ratios (6 μg of protein Z and 1–7 μg of ZPI) were subjected to native PAGE followed by detection of protein bands by Coomassie Blue staining as described under”Experimental Procedures.“
Native PAGE and Immunoblotting Analysis of the Products of the Cofactor-dependent ZPI-Factor Xa Reaction—Previous studies showed that a ZPI-factor Xa complex could be detected in the cofactor-dependent reaction of ZPI with factor Xa by native PAGE and immunoblotting with anti-factor Xa antibodies (7). To determine the fate of all proteins in the cofactor-dependent reaction, we analyzed the reaction products by native PAGE and immunoblotting with antibodies against all three reacting proteins, i.e. ZPI, factor Xa, and protein Z (Fig. 5). The immunoblots confirmed the formation of a complex band immunoreactive with both anti-factor Xa and anti-ZPI antibodies and with mobility intermediate between free factor Xa and ZPI bands in the cofactor-dependent reaction (Fig. 5, lane 7). Moreover, anti-ZPI immunoblots showed that significant cleaved ZPI was formed concomitant with the ZPI-factor Xa complex (Fig. 5, lane 7), consistent with the fraction of ZPI that was expected to react as a substrate rather than an inhibitor of factor Xa from the measured SI for the reaction. Most interesting was the finding that although protein Z formed the expected high affinity complex with ZPI in the absence of factor Xa (Fig. 5, lane 5), this complex was no longer detected after ZPI had reacted with factor Xa in the presence of all cofactors (lane 7). There was also no evidence that cleaved ZPI produced in the cofactor-dependent reaction formed a high affinity complex with protein Z. These observations suggested that the affinity of protein Z for ZPI is greatly reduced when ZPI is cleaved in the ZPI-factor Xa complex or in the free serpin, thereby implying that protein Z acts catalytically in the reaction.
FIGURE 5.
Native PAGE and immunoblotting analysis of the products of the cofactor-dependent ZPI-factor Xa reaction. ZPI (28 nm), protein Z (32 nm), and factor Xa (10 nm) were incubated alone or together for ∼1–2 min and then subjected to native PAGE on 5.5 or 8% gels as indicated. Protein bands were transferred to PVDF membranes, and specific proteins were then detected with antibodies against factor Xa, ZPI, or protein Z as indicated. Further details are provided under”Experimental Procedures.“
Kinetic analyses showed that the reaction of ZPI with membrane-associated factor Xa was in fact enhanced by catalytic levels of protein Z that were substoichiometric with respect to both inhibitor and protease, i.e. all factor Xa rather than a fraction stoichiometric with protein Z was inhibited at an accelerated rate through an exponential process (Fig. 6). The association rate constant determined under these multiple turnover conditions (2.8 ± 0.2 × 106 m–1 s–1) was ∼10-fold reduced from that obtained in single turnover kinetic analyses (see below), consistent with dissociation of protein Z from the ZPI-factor Xa complex and rebinding to unreacted ZPI being rate-limiting.
FIGURE 6.
Kinetics of the cofactor-dependent ZPI-factor Xa reaction with catalytic levels of protein Z. Shown are progress curves for the reactions of 200 nm ZPI with 5 nm factor Xa in the presence of 25 μm phospholipids and 5 mm calcium and with the indicated catalytic concentrations of protein Z. Residual factor Xa activity was measured by discontinuous assay relative to an uninhibited control. Progress curves were fit by single exponential decay functions (solid lines) to obtain kobs. The inset shows a plot of kobs as a function of the protein Z concentration and a linear regression fit of the data (solid line).
Rapid Kinetic Analysis of the Cofactor-dependent ZPI-Factor Xa Reaction—The kinetics of ZPI inhibition of factor Xa was analyzed in the presence of lipid and calcium as a function of the protein Z concentration under pseudo-first order conditions yielding a single turnover of protein Z, i.e. with a large molar excess of ZPI and protein Z over factor Xa. Factor Xa inhibition was monitored continuously from the time-dependent decrease in hydrolysis of a reporter fluorogenic substrate either in a conventional fluorometer or a stopped-flow fluorometer. Because of the previous finding that the kinetics of ZPI inhibition of factor Xa in the presence of protein Z, lipid, and calcium cofactors depended on whether protein Z had been preincubated with lipid and calcium (5), we first investigated the effects of different orders of addition of reaction components on the observed second order inhibition rate constant measured at low ZPI-protein Z complex concentrations (Table 2). A maximal rate constant of 1.8 × 107 m–1 s–1 was found when protein Z, factor Xa, and lipid were premixed before mixing with ZPI. A modest but significant ∼25% reduction in the rate constant occurred when only protein Z was premixed with lipid along with ZPI and then mixed with factor Xa with or without lipid. Significantly, a marked ∼75% (4-fold) reduction in the rate constant occurred when protein Z was premixed with either ZPI or factor Xa in the absence of lipid and then mixed with enzyme or serpin, respectively, that had been premixed with lipid. These findings not only confirm previous results suggesting that protein Z binding to lipid is rate-determining in the overall reaction but further suggest that factor Xa binding to lipid with bound protein Z may additionally limit the reaction.
TABLE 2.
Effect of the order of mixing of reaction components on second order rate constants for the cofactor-dependent reaction of ZPI with factor Xa in / 0.15 Hepes buffer (pH 7.4) at 25 °C
Reactions were initiated by adding equal volumes of ZPI and factor Xa that had been premixed with the indicated cofactors in Hepes buffer containing 5 mm CaCl2. The final concentrations were 9.6 nm ZPI, 0.05 nm factor Xa, 25 μm phospholipid, and 1–5 nm protein Z. The factor Xa fluorogenic substrate (40 μm) was premixed with ZPI in all cases. Reactions were followed by continuously monitoring the decrease in the rate of hydrolysis of fluorogenic substrate to an end point, and kobs was obtained by fitting ∼5 half-lives to a single exponential equation. Apparent second order association rate constants (ka(app)) were obtained from the slopes of plots of kobs versus protein Z concentration obtained by linear regression analyses assuming a zero y axis intercept.
|
Reactants
|
ka(app)
|
|
|---|---|---|
| ZPI | FXa | |
| m-1 s-1 | ||
| +PZ, lipid | 1.8 ± 0.1 × 107 | |
| +PZ | +Lipid | 4.6 ± 0.3 × 106 |
| +Lipid | +PZ | 4.8 ± 0.1 × 106 |
| +PZ, lipid | 1.3 ± 0.1 × 107 | |
| +PZ, lipid | +Lipid | 1.3 ± 0.1 × 107 |
| +Lipid | +PZ, lipid | 1.7 ± 0.2 × 107 |
Based on these findings, we undertook a more complete study of the dependence of kobs on protein Z concentration at several fixed concentrations of ZPI under optimal premixing conditions, i.e. reactions were initiated by mixing equal volumes of ZPI/substrate and protein Z/factor Xa, both in buffer containing lipid and calcium. kobs increased in a saturable manner as the protein Z concentration was increased at a fixed ZPI concentration and then decreased at higher protein Z concentrations (Fig. 7A). The maximal values of kobs were reached in all cases at equimolar concentrations of protein Z and ZPI. These values increased proportionally with the ZPI concentration at the lowest concentrations but showed less than proportional increases at higher serpin concentrations, indicating the saturation of a ternary protein Z-ZPI-factor Xa Michaelis complex. The decreases in kobs that occurred following saturation of ZPI with protein Z were ascribable to excess protein Z reducing the ternary complex concentration by competing with ZPI complexed protein Z for the limiting factor Xa. Global fitting of all the data to a model in which the protein Z-ZPI binary complex was the inhibitory species (see “Experimental Procedures”) provided a good fit to the data and indicated values for the Michaelis constant, KM, of 53 ± 5 nm and the limiting rate constant, klim, for conversion of the Michaelis complex to a stable ZPI-factor Xa inhibited complex of 1.2 ± 0.1 s–1 (Fig. 7B). These parameters indicated an overall second order association rate constant (ka) corresponding to the ratio, klim/KM, of 2.3 ± 0.5 × 107 m–1 s–1 which was in good agreement with the value obtained at protein Z-ZPI complex concentrations well below saturation of the Michaelis complex. Comparison with second order rate constants for the reaction in the absence of lipid, calcium, and protein Z showed that the cofactors enhanced ka ∼ 2000-fold.
FIGURE 7.
Rapid kinetics of the cofactor-dependent ZPI-factor Xa reaction with stoichiometric levels of protein Z. A, kobs values measured for reactions of factor Xa with fixed concentrations of ZPI and varying concentrations of protein Z under pseudo-first order conditions. Reactions were followed from the decrease in rate of factor Xa hydrolysis of fluorogenic substrate after mixing equal volumes of ZPI and 30 μm fluorogenic substrate with 0.05–0.2 nm factor Xa and protein Z, both in buffer containing 25 μm phospholipid and 5 mm calcium. kobs was determined from exponential fits of reaction progress curves. The dependence of kobs on protein Z concentration was fit by the equation given under”Experimental Procedures“(solid lines). B, kobs values plotted as a function of the effective ZPI-protein Z concentration, calculated from the KD and stoichiometry for the ZPI-protein Z interaction with correction for the competition of the factor Xa substrate and uncomplexed protein Z for binding to factor Xa as described under “Experimental Procedures.” The solid line shows the curve calculated from the fitted parameters of the rectangular hyperbolic equation. The symbols correspond to the different fixed ZPI concentrations used in A.
Role of a Protein Z-Factor Xa Binary Complex in the Assembly of the Michaelis Complex—Broze and co-workers (5) provided evidence that protein Z forms a complex with factor Xa on the lipid surface that protects factor Xa from antithrombin inhibition. We confirmed that the pseudo-first order rate constant (kobs) for antithrombin inhibition of factor Xa was unaffected by protein Z alone, modestly reduced 35% by lipid and calcium alone, but significantly reduced by 52% when protein Z, lipid, and calcium were all present (Fig. 8A). To further characterize the protein Z-factor Xa interaction, the protective effect was analyzed as a function of protein Z and lipid concentration (Fig. 8B). Protein Z reduced kobs for antithrombin inhibition of factor Xa in the presence of lipid and calcium in a saturable manner by 1.8-fold with an apparent KD of 36 ± 4 nm. Doubling the lipid concentration increased the apparent KD by 2-fold to 72 ± 14 nm but yielded an indistinguishable decrease in the inhibition rate constant. Such data in conjunction with reported KD values for protein-lipid interactions (26) support a model in which factor Xa and protein Z are mostly bound to lipid at the concentrations employed and form a complex on the lipid surface with an apparent KD value that increases in proportion to the lipid concentration because of the statistical redistribution of factor Xa and protein Z to separate lipid vesicles. That the lipid concentrations employed were in fact saturating with respect to the interaction with factor Xa was confirmed by the observation that the effect of lipid alone on the antithrombin-factor Xa inhibition rate constant was already saturated at 10 μm lipid. Together, these findings suggest that the KD value for the lipid-associated factor Xa-protein Z interaction is well below the observed apparent value of 35 nm.
FIGURE 8.
Titration of a phospholipid-dependent factor Xa-protein Z interaction. A, progress curves for the inactivation of 5 nm factor Xa by 3 μm antithrombin in the absence (•, ○) or presence (▴, ▵) of 25 μm phospholipid and 5 mm calcium either without protein Z (open symbols) or with 40 nm protein Z (closed symbols). Progress curves were fit by a single exponential function to obtain kobs. B, dependence of kobs for the reaction of 3 μm antithrombin with 5 nm factor Xa in the presence of 5 mm calcium and either 25 μm phospholipid (○) or 50 μm phospholipid (•) as a function of the protein Z concentration. Solid lines are fits by a model in which protein Z binding to factor Xa on the membrane produces a saturable decrease in kobs for antithrombin inhibition of the protease. Fits of the data with 25μm phospholipid yielded second order rate constants of 1.6 ± 0.1 × 103 m–1 s–1 for the antithrombin-factor Xa reaction without protein Z and 0.88 ± 0.08 × 103 m–1 s–1 for the reaction with saturating protein Z. Because the end point was not as well determined for the 50 μm lipid data, these values were fixed in the fit of this data. Further details are provided under”Experimental Procedures.“
pH-dependent Stability of the ZPI-Factor Xa Complex—The ZPI-factor Xa complex was previously shown to be significantly less stable than other serpin-protease complexes under physiologic conditions (7). To determine whether this greater complex lability was because of the failure to suppress factor Xa catalytic activity in the ZPI-factor Xa complex (14), we analyzed the pH dependence of complex dissociation. Factor Xa was reacted with ZPI at pH 7.4 in the presence of protein Z, lipid, and calcium cofactors with a sufficient molar excess of the serpin to fully complex the protease. The reaction was then quenched with EDTA, diluted in buffer of the desired pH and recovery of factor Xa activity measured in samples withdrawn at increasing times. Essentially complete recovery of factor Xa activity was obtained between pH 7 and 9 in a first order exponential process over a time frame of 2–6 h (Fig. 9A). The dissociation rate constant increased as the pH was increased over this range with evidence of an approach to a limiting value between pH 7.8 and 8.4. Formation of the complex in the absence of cofactors and monitoring dissociation in the presence of 5 mm calcium without EDTA showed that calcium had no significant effect on the dissociation rate (not shown).
FIGURE 9.
pH dependence of the rate of dissociation of the ZPI-factor Xa complex. A, progress curves of the recovery of factor Xa activity from preformed ZPI-factor Xa complex as a function of pH. Complex was formed rapidly in the presence of cofactors and then dissociation-initiated by dilution into buffer of the desired pH. The reaction was followed by discontinuous assay of factor Xa activity relative to an uninhibited control. Solid lines are fits to single exponential functions. B, pH dependence of first order rate constants for ZPI-factor Xa complex dissociation measured from full progress curves in A (○ or from initial rates of dissociation (•) as described under “Experimental Procedures.” Data were fit by an equation that assumes two acidic groups control the dissociation rate constant as described in the text. Error bars represent S.E. from three to six determinations.
The rate of complex dissociation was analyzed over a broader pH range by measuring the initial rate of complex dissociation. In these experiments, preformed complex was extensively diluted into a solution of chromogenic or fluorogenic substrate at the desired pH, and the acceleration of substrate hydrolysis due to factor Xa dissociation from the ZPI-factor Xa complex was continuously monitored over a time frame in which ∼10% or less complex dissociation had occurred (14). Fitting of the complex dissociation curves by the expected parabolic function yielded a dissociation rate constant that was independent of the concentration of complex analyzed and in agreement with values obtained by analyzing complete progress curves. This analysis revealed that the pH dependence of the dissociation rate constant was bell-shaped with a sigmoidal increase to a maximum value in the range 5.7–8.3 followed by a sigmoidal decrease at higher pH (Fig. 9B).
Such a dependence is diagnostic of the participation of two key residues that regulate catalysis by chymotrypsin family serine proteases, His-57 and Ile-16 (chymotrypsin numbering) (14, 15). His-57 is part of the catalytic triad whose function depends on the residue being in the unprotonated basic form, whereas Ile-16 is the N-terminal residue formed upon activation of the zymogen whose positively charged amine group in the protonated state forms an activating salt bridge with Asp-194 at the active site (27). The data were satisfactorily fit by a model in which catalysis of ZPI-factor Xa complex dissociation requires an unprotonated His-57 with pKa of 7.4 ± 0.1 and protonated Ile-16 with pKa 9.1 ± 0.1. The fit provided an intrinsic deacylation rate constant of 4.4 ± 0.5 × 10–4 s–1 for the singly protonated form of the complex. Notably, the fitted pKa values are perturbed from the values of 6.4 and >10 characterizing the deacylation of normal serine protease acyl-intermediates (14, 27), consistent with factor Xa being distorted in the complex but not to the extent observed with other serpin-protease complexes (14).
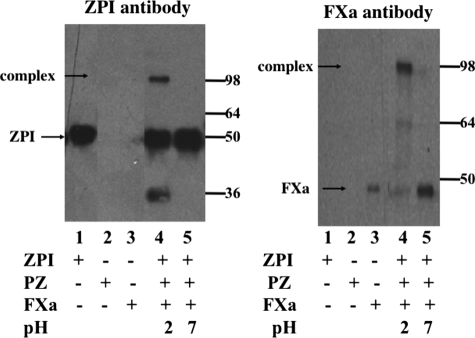
The propensity of the ZPI-factor Xa complex to dissociate at physiologic pH most likely explains the inability of previous studies to observe an SDS-stable complex by SDS-PAGE (7). Because the stability of the complex is greatly increased below pH 7, we attempted to trap an SDS-stable ZPI-factor Xa complex by quenching the reaction and denaturing the complex at pH 2 followed by SDS-PAGE analysis and detection of complex bands by immunoblotting (Fig. 10). As is evident from the blot, a higher molecular weight complex band containing both factor Xa and ZPI and with a molecular weight consistent with a 1:1 ZPI-factor Xa complex was clearly detected when the complex was quenched at low pH but not at neutral pH. These data support the view that ZPI forms an SDS-stable acyl-intermediate complex with factor Xa like other serpins, but the stability of this complex is reduced at neutral pH because of incomplete distortion of the protease.
FIGURE 10.
SDS-PAGE and immunoblotting of the products of the cofactor-dependent ZPI-factor Xa reaction at neutral and acid pH values. Reactions of 40 nm ZPI, 40 nm protein Z, and 10 nm factor Xa were done in pH 7.4 Hepes buffer containing 25 μm phospholipid and 5 mm calcium for 10 min at 25 °C. HCl or reaction buffer was added to yield pH values of 2 or 7.4, followed by addition of SDS and boiling for 5 min. The pH of the acid sample was returned to neutral by addition of NaOH, and 30-μl samples were then electrophoresed along with unreacted protein controls on 10% gels. Protein bands were transferred to PVDF membranes, and specific proteins were then detected either with anti-factor Xa or anti-ZPI antibodies. Molecular weight markers are indicated for each immunoblot. The lower molecular weight band in the anti-ZPI immunoblot represents a cleavage artifact of low pH denaturation.
DISCUSSION
A thorough kinetic characterization of the cofactor-dependent reaction of the serpin, ZPI, with factor Xa in this study has served to illuminate those features of the mechanism of this reaction that are shared with other serpin-protease reactions and those that are novel. Our central findings show that ZPI behaves in many respects like a normal serpin in its inhibitory reaction with factor Xa, but they more importantly highlight how the cofactors, protein Z, lipid, and calcium, play unique roles in enforcing the specificity of this reaction, in agreement with previous proposals (6, 7, 28).
We chose to study a recombinant ZPI because of the low plasma concentration of the serpin (5) and consequent difficulty in purifying the protein from plasma in good yield. Although the recombinant protein expressed in insect cells appears to be underglycosylated relative to the plasma protein, its requirement for protein Z, lipid, and calcium cofactors for rapid inhibition of factor Xa, and cofactor-independent inhibition of factor XIa appears to be indistinguishable from what has been reported for plasma ZPI (5–7). Direct comparison of recombinant and plasma ZPIs verified that the two serpin forms inhibited factor Xa with equivalent rates in the presence of cofactors. Contrasting a recent report (25), we found no evidence that ZPI could inhibit factor IXa in the absence or presence of protein Z and procoagulant membranes. Our finding that ZPI was not cleaved by factor IXa under these conditions suggests that the ZPI reactive loop sequence is not recognized by free or membrane-bound factor IXa, and therefore not likely to be a physiologic target of ZPI, consistent with the initial report of Broze and co-workers (6).
Our studies have clarified several puzzling features of ZPI noted in previous studies that appeared to set ZPI apart from other serpins. These include the inability of ZPI to completely inhibit factor Xa amidolytic activity or to form an SDS-stable ZPI-factor Xa complex (5, 7, 12). Such features can be accounted for by the instability of the ZPI-factor Xa complex that was evident in prior studies (7). Notably, the complex was found to be least stable at the pH previously used for amidolytic assay of residual factor Xa activity in monitoring ZPI inhibition of factor Xa (half-life of ∼30 min at pH 8.3, 25 °C) (5). The incomplete inhibition observed may thus be due to factor Xa dissociated from the complex during the several minutes required to measure the amidolytic activity. This explanation is supported by our finding that the fitting of residual factor Xa amidolytic activity curves by a polynomial function was necessary to measure an accurate initial velocity even at pH 7.4. and by the previous finding that factor Xa clotting activity was more completely inhibited under conditions in which the inhibited complex had less opportunity to dissociate (5).
The facile dissociation of the ZPI-factor Xa complex at physiologic pH and ionic strength contrasts with the resistance of most other serpin-protease complexes, including the antithrombin-factor Xa complex to dissociate under these conditions (14). Analysis of the pH dependence of this dissociation provided evidence that dissociation was mediated by His-57 and Ile-16 catalytic residues of the protease, consistent with factor Xa retaining residual function in the conformationally trapped acyl-intermediate complex to catalyze its deacylation. This contrasts with the pH dependence of dissociation of the antithrombin-factor Xa complex and other serpin-protease acyl-intermediate complexes that show no evidence for catalytic residue involvement in the dissociation (14, 15). Dissociation rate constants for such complexes are thus >100-fold lower than that found for the ZPI-factor Xa complex and increase ∼10-fold per unit increase in pH without bound, characteristic of an uncatalyzed hydroxide-mediated hydrolysis of the acyl bond (14). An interesting exception is the neuroserpin-tPA complex that shows an instability like that of the ZPI-factor Xa complex (29).
Notably, the ZPI-factor Xa complex dissociation rate constant at physiologic pH is comparable with that observed for serpin-trypsin complexes in the presence of calcium, a ligand whose binding to the protease in the complex was shown to partially restore catalytic function (14). The latter observation has suggested that serpin-protease complexes exist in an equilibrium between a state in which the protease active site is partially distorted with residual catalytic function to one with complete active-site distortion and absence of catalytic function. Moreover, this equilibrium can be modulated by protease ligands that stabilize the active state (30). It would thus appear that factor Xa does not undergo the full active-site distortion that usually occurs after the protease is translocated to the opposite end of the serpin by reactive loop insertion into β-sheet A (13). This distortion likely occurs after the loosely tethered protease arrives at the distal end of sheet A at which point it is induced to form an intimate association with the serpin at the edge of sheet A. The distortion results from the reactive loop tether tightening and forcing the active-site serine out of a catalytically competent alignment (31–33). The incomplete distortion of the ZPI-factor Xa complex may thus reflect a looser tethering of factor Xa to ZPI in the final complex or a poor fit between the protease and the distal end of the serpin, either of which may fail to induce the full protease distortion.
The instability of the ZPI-factor Xa complex explains why previous studies did not detect an SDS-stable ZPI-factor Xa complex. That the ZPI-factor Xa complex is a conformationally trapped acyl-intermediate like that of other serpin-protease complexes was demonstrated by trapping the intermediate at low pH where the complex is stable and detecting it on SDS-PAGE. Once denatured at low pH, the complex does not dissociate under the slightly alkaline conditions of SDS-PAGE because the protease has unfolded (34). Heat denaturation of the complex at neutral pH presumably allows the protease enough time to catalyze complex dissociation before it has unfolded.
Our studies have shown that ZPI forms a conformationally trapped acyl-intermediate by the characteristic branched pathway suicide substrate mechanism of serpin-protease reactions that concomitantly produces cleaved ZPI (13). Inhibition stoichiometries of ∼3 and ∼8 mol of ZPI per mol of factor Xa for the protein Z-dependent reaction of ZPI with membrane-bound factor Xa and free factor Xa, respectively, and values of ∼6 and 9 mol of ZPI per mol of factor XIa active-sites for heparin-accelerated and unaccelerated ZPI-factor XIa reactions, respectively, were shown to reflect concomitant formation of cleaved ZPI in the reaction by native PAGE analysis and immunoblotting. Deacylation of the acyl-intermediate formed in the reactions of ZPI with factor Xa and XIa thus effectively competes with conformational trapping of the intermediate and contributes to a reduced efficiency of inhibition that is reflected in the association rate constant for inhibition (13). These findings suggest that the differential reactivities of ZPI as an inhibitor and substrate of factor Xa and factor XIa provide a carefully balanced regulation of factor Xa and factor XIa activity in the coagulation response.
Our studies confirm and expand the proposed roles for protein Z in regulating ZPI anticoagulant function. Native PAGE confirmed that protein Z forms a high affinity stoichiometric complex with ZPI that is independent of lipid or calcium. The KD value for this association was estimated to be subnanomolar from titrations of an antagonistic effect of protein Z on the extent of ZPI inhibition of factor Xa in the absence of lipid and calcium and from kinetic titrations of the agonist effect of protein Z on ZPI inhibition of factor Xa in the presence of lipid and calcium cofactors. The antagonistic effect of protein Z appeared to reflect an enhanced substrate reactivity of ZPI with factor Xa in the absence of lipid and calcium and not a decreased rate of association of ZPI and factor Xa. Protein Z binding to ZPI may thus impede the trapping serpin conformational change when the ZPI-factor Xa acyl-intermediate is formed in the absence of a membrane surface. Protein Z had a similar protective effect on ZPI inhibition of factor XIa.
A significant new finding of our study is that the high affinity interaction between ZPI and protein Z is disrupted following reaction of ZPI with factor Xa in the presence of lipid and calcium as was evident by native PAGE and immunoblotting of the products of the reaction. Thus protein Z was not found to associate with either the ZPI-factor Xa complex or cleaved ZPI products of the reaction. This observation implies that the conformational change induced by cleavage of ZPI both in the ZPI-factor Xa acyl-intermediate complex and in the free serpin reduces the affinity of protein Z for ZPI sufficiently to promote protein Z dissociation. Such dissociation suggests that the released protein Z can rebind to unreacted ZPI and thereby act as a catalyst in the reaction. Kinetic studies affirmed that protein Z acts catalytically in enhancing the rate at which ZPI inhibits membrane-bound factor Xa. This behavior is analogous to that of heparin promotion of the reactions of antithrombin with clotting proteases (16). Heparin thus binds antithrombin with high affinity, but it is dissociated once antithrombin forms a stable complex with proteases because of an ∼1000-fold decrease in heparin affinity. Heparin dissociation from the acyl-intermediate in fact is required for efficient conformational trapping of the intermediate and thus controls the partitioning between deacylation and conformational trapping of the serpin-protease acyl-intermediate (35, 36). The rate of dissociation of protein Z from ZPI may as well limit the ability to conformationally trap the ZPI-factor Xa acyl-intermediate if bound protein Z impedes the serpin conformational change.
Our kinetic studies of the cofactor-dependent ZPI-factor Xa reaction have confirmed earlier studies showing that the reaction rate depends on the order of addition of reaction components (5) and have shown that the reason for this is an ∼5-fold faster association rate constant when protein Z is preincubated with lipid and calcium. We further confirmed that factor Xa bound to a membrane shows enhanced protection from antithrombin inhibition in the presence of protein Z in accordance with factor Xa forming a high affinity complex with protein Z on the membrane (5). Rapid kinetic studies provided evidence that the reaction of ZPI with preformed factor Xa-protein Z-lipid complex proceeded by the initial assembly of a ternary protein Z-ZPI-factor Xa Michaelis complex intermediate on the membrane followed by conversion of the Michaelis complex to the conformationally trapped ZPI-factor Xa acyl-intermediate complex. The observed KM for forming the Michaelis complex and limiting rate constant for its transformation to the trapped ZPI-factor Xa acyl-intermediate complex were compatible with an overall diffusion-limited association rate constant for inhibition of membrane-bound factor Xa comparable with that of antithrombin-heparin complex inhibition of free factor Xa (36). These findings are in keeping with the previous proposal that protein Z binding to the membrane may be rate-limiting in the assembly of the Michaelis complex because of the unusually slow off-rate for the protein Z-membrane interaction compared with the factor Xa-membrane interaction (26, 28). Our studies additionally suggest that formation of a factor Xa-protein Z complex on the membrane may be partly rate-determining in Michaelis complex formation.
In summary, our kinetic studies have shown that ZPI inhibits factor Xa like a normal serpin by a branched pathway suicide substrate mechanism that leads to a conformationally trapped acyl-intermediate complex. The complex is surprisingly less stable than other serpin-protease complexes, the physiologic significance of which is presently unclear. Interestingly, the cofactor function of protein Z resembles that of heparin in antithrombin-protease reactions with respect to the dissociation and recycling of the cofactor following formation of the serpinprotease complex. Previous studies have shown that prothrombinase-bound factor Xa is efficiently inhibited by the ZPI-protein Z complex but that prothrombin effectively competes with ZPI for prothrombinase-bound factor Xa. Membrane-associated factor Xa may thus be a target of ZPI either prior to factor V activation or after significant consumption of prothrombin to account for the protein Z-dependent dampening of the procoagulant response by ZPI as suggested previously (7).
A remaining question is how the ZPI-protein Z complex promotes the assembly of a high affinity Michaelis complex with factor Xa on the membrane. The unusual P1 Tyr of ZPI would seem to be incompatible with a high affinity interaction of the ZPI reactive loop with the active site of factor Xa (6). The specific factor Xa inhibitor, TAP, also has a P1 Tyr instead of the usual P1 Arg bait, and yet it is able to form a strong lock-and-key interaction with factor Xa principally through exosite interactions involving the TAP N terminus (37, 38). Exosites similarly mediate the interaction of the serpin, heparin cofactor II, with thrombin in the presence of glycosaminoglycan cofactors to overcome a less favorable interaction of the protease with a P1 Leu. These unfavorable P1 residues thus appear to be critical for preventing interaction of the protein protease inhibitor with other coagulation proteases that recognize P1 Arg and thus for enforcing specificity. Whether exosite determinants exist on ZPI or protein Z to mediate the high affinity Michaelis complex interaction with factor Xa seems likely and consistent with recent mutagenesis studies showing the importance of factor Xa surface loops in promoting the ZPI-factor Xa interaction (12).
Acknowledgments
We thank Dr. Peter Gettins of the University of Illinois at Chicago for helpful comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-78827 (to S. T. O.) and HL-060782 (to G. J. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ZPI, protein Z-dependent protease inhibitor; PVDF, polyvinylidene difluoride; PZ, protein Z; Mes, 2-(N-morpholino)ethanesulfonic acid; TAPS, 3-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl] amino}-1-propanesulfonic acid; FXa, factor Xa; SI, stoichiometry of inhibition.
References
- 1.Broze, G. J., Jr., Girard, T. J., and Novotny, W. F. (1990) Biochemistry 29 7539–7546 [DOI] [PubMed] [Google Scholar]
- 2.Lu, G., Broze, G. J., and Krishnaswamy, S. (2004) J. Biol. Chem. 279 17241–17249 [DOI] [PubMed] [Google Scholar]
- 3.Brufatto, N., and Nesheim, M. (2001) J. Biol. Chem. 276 17663–17671 [DOI] [PubMed] [Google Scholar]
- 4.Rezaie, A. R. (2001) Blood 97 2308–2313 [DOI] [PubMed] [Google Scholar]
- 5.Han, X., Fiehler, R., and Broze, G. J., Jr. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9250–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han, X., Huang, Z. F., Fiehler, R., and Broze, G. J., Jr. (1999) Biochemistry 38 11073–11078 [DOI] [PubMed] [Google Scholar]
- 7.Han, X., Fiehler, R., and Broze, G. J., Jr. (2000) Blood 1 3049–3055 [PubMed] [Google Scholar]
- 8.Tabatabai, A., Fiehler, R., and Broze, G. J., Jr. (2001) Thromb. Haemostasis 85 655–660 [PubMed] [Google Scholar]
- 9.Broze, G. J., Jr., and Miletich, J. P. (1984) J. Clin. Investig. 73 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin, Z., Huang, Z., Cui, J., Fiehler, R., Lasky, N., Ginsburg, D., and Broze, G. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6734–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, J., Tu, Y., Lu, L., Lasky, N., and Broze, G. J., Jr. (2008) Blood 111 4973–4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezaie, A. R., Manithody, C., and Yang, L. (2005) J. Biol. Chem. 280 32722–32728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettins, P. (2002) Chem. Rev. 102 4751–4803 [DOI] [PubMed] [Google Scholar]
- 14.Calugaru, S. V., Swanson, R., and Olson, S. T. (2001) J. Biol. Chem. 276 32446–32455 [DOI] [PubMed] [Google Scholar]
- 15.Plotnick, M. I., Samakur, M., Wang, Z. M., Liu, X., Rubin, H., Schechter, N. M., and Selwood, T. (2002) Biochemistry 41 334–342 [DOI] [PubMed] [Google Scholar]
- 16.Björk, I., and Olson, S. T. (1997) in Chemistry and Biology of Serpins (Church, F. C., Cunningham, D. D., Ginsburg, D., Hoffman, M., Stone, S. R., and Tollefsen, D. M., eds) pp. 17–33, Plenum Publishing Corp., New York
- 17.Olson, S. T., Björk, I., and Shore, J. D. (1993) Methods Enzymol. 222 525–560 [DOI] [PubMed] [Google Scholar]
- 18.Heeb, M. J., Mesters, R. M., Tans, G., Rosing, J., and Griffin, J. H. (1993) J. Biol. Chem. 268 2872–2877 [PubMed] [Google Scholar]
- 19.Izaguirre, G., Zhang, W., Swanson, R., Bedsted, T., and Olson, S. T. (2003) J. Biol. Chem. 278 51433–51440 [DOI] [PubMed] [Google Scholar]
- 20.Gill, S. C., and von Hippel, P. H. (1989) Anal. Biochem. 182 319–326 [DOI] [PubMed] [Google Scholar]
- 21.Bedsted, T., Swanson, R., Chuang, Y.-J., Bock, P. E., Björk, I., and Olson, S. T. (2003) Biochemistry 42 8143–8152 [DOI] [PubMed] [Google Scholar]
- 22.Olson, S. T., Swanson, R., Verhamme, I., Kvassman, J., and Shore, J. D. (2001) Biochemistry 40 11742–11756 [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. (1970) Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- 24.Ersdal-Badju, E., Lu, A., Peng, X., Picard, V., Zenderouh, P., Turk, B., Björk, I., Olson, S. T., and Bock, S. C. (1995) Biochem. J. 310 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeb, M. J., Cabral, K. M., and Ruan, L. J. (2005) J. Biol. Chem. 280 33819–33825 [DOI] [PubMed] [Google Scholar]
- 26.McDonald, J. F., Shah, A., Schwalbe, R. A., Kisiel, W., Dahlback, B., and Nelsestuen, G. L. (1997) Biochemistry 36 5120–5127 [DOI] [PubMed] [Google Scholar]
- 27.Hedstrom, L., Lin, T.-Y., and Fast, W. (1996) Biochemistry 35 4515–4523 [DOI] [PubMed] [Google Scholar]
- 28.Broze, G. J., Jr. (2001) Thromb. Haemostasis 86 8–13 [PubMed] [Google Scholar]
- 29.Barker-Carlson, K., Lawrence, D., and Schwartz, B. (2002) J. Biol. Chem. 277 26852–32768 [DOI] [PubMed] [Google Scholar]
- 30.Han, J.-H., and Tollefsen, D. M. (1998) Biochemistry 37 3203–3209 [DOI] [PubMed] [Google Scholar]
- 31.Dementiev, A., Dobó, J., and Gettins, P. G. W. (2006) J. Biol. Chem. 281 3452–3457 [DOI] [PubMed] [Google Scholar]
- 32.Huntington, J. A., Read, R. J., and Carrell, R. W. (2000) Nature 407 923–926 [DOI] [PubMed] [Google Scholar]
- 33.Zhou, A., Carrell, R. W., and Huntington, J. A. (2001) J. Biol. Chem. 276 27541–27547 [DOI] [PubMed] [Google Scholar]
- 34.Kvassman, J.-O., Verhamme, I., and Shore, J. D. (1998) Biochemistry 37 15491–15502 [DOI] [PubMed] [Google Scholar]
- 35.Olson, S. T. (1985) J. Biol. Chem. 260 10153–10160 [PubMed] [Google Scholar]
- 36.Izaguirre, G., Swanson, R., Raja, S. M., Rezaie, A. R., and Olson, S. T. (2007) J. Biol. Chem. 282 33609–33622 [DOI] [PubMed] [Google Scholar]
- 37.Jordan, S. P., Mao, S.-S., Lewis, S. D., and Shafer, J. A. (1992) Biochemistry 31 5374–5380 [DOI] [PubMed] [Google Scholar]
- 38.Mao, S.-S., Huang, J., Welebob, C., Neeper, M. P., Garsky, V. M., and Shafer, J. A. (1995) Biochemistry 34 5098–5103 [DOI] [PubMed] [Google Scholar]