Abstract
P2X1 receptors belong to a family of cation channels gated by extracellular ATP; they are found inter alia in smooth muscle, platelets, and immune cells. Suramin has been widely used as an antagonist at P2X receptors, and its analog 4,4′,4″,4‴-[carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino))] tetrakis-benzene-1,3-disulfonic acid (NF449) is selective for the P2X1 subtype. Human and mouse P2X1 receptors were expressed in human embryonic kidney cells, and membrane currents evoked by ATP were recorded. ATP (10 nm to 100 μm) was applied only once to each cell, to avoid the profound desensitization exhibited by P2X1 receptors. Suramin (10 μm) and NF449 (3–300 nm) effectively blocked the human receptor. Suramin had little effect on the mouse receptor. Suramin and NF449 are polysulfonates, with six and eight negative charges, respectively. We hypothesized that species differences might result from differences in positive residues presented by the large receptor ectodomain. Four lysines in the human sequence (Lys111, Lys127, Lys138, and Lys148) were changed individually and together to their counterparts in the mouse sequence. The substitution K138E, either alone or together with K111Q, K127Q, and K148N, reduced the sensitivity to block by both suramin and NF449. Conversely, when lysine was introduced into the mouse receptor, the sensitivity to block by suramin and NF449 was much increased for E138K, but not for Q111K, Q127K, or N148K. The results explain the marked species difference in antagonist sensitivity and identify an ectodomain lysine residue that plays a key role in the binding of both suramin and NF449 to P2X1 receptors.
Suramin (8-[(4-methyl-3-{[3-({[3-({2-methyl-5-[(4,6,8-trisulfo-1-naphthyl)carbamoyl]phenyl}carbamoyl)phenyl]carbamoyl}amino)-benzoyl]amino}benzoyl)amino]naphthalene-1,3,5-trisulfonic acid) is an anti-protozoal drug developed by Bayer more than 90 years ago. As an experimental tool, it has been used to block a range of enzymes (1, 2) including lysozyme (3), sarcoplasmic calcium transport (4), plasma membrane ATPase (5), and reverse transcriptase (6). Approximately 20 years ago it was introduced as a blocker of the actions of the sympathetic nerve transmitter released on to vas deferens smooth muscle (7, 8). This action is now known to result from its antagonism at P2X receptors (9). P2X receptors are trimeric membrane proteins, and they assemble into ion channels as homomers or certain heteromers (10). The P2X1 receptor was originally cloned from the vas deferens of the rat (11), and it is widely distributed in smooth muscle, endothelia, platelets, and immune cells. Much of the further pharmacological characterization, as well as extensive studies of structure and function, has been on the human P2X1 receptor (12). Suramin blocks ATP-induced currents at human P2X1 receptors; a concentration of 1 μm causes a shift of almost 10-fold in the ATP concentration-response curve (12).
Most other P2X receptors are also sensitive to suramin, although the P2X4 receptor is much so than the others (13, 14). Several suramin analogs have been developed subsequently with the aim of improving selectivity for P2X1 receptors, because blockers of P2X1 receptors on platelets hold promise as antithrombotic agents (15, 16). One of these is 4,4′,4″,4‴-(carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakis-benzene-1,3-disulfonic acid (NF449),2 which blocks P2X1 receptors in low nanomolar concentrations and has good selectivity over P2X3 receptors (17, 18).
During the course of recent studies on peritoneal macrophages from the mouse, we observed a response to ATP that had all the characteristics of P2X1 receptors (19). It was a rapidly desensitizing inward current, elicited by 1–10 μm ATP, and it was absent in parallel studies on P2X1 knock-out mice (19). However, we were surprised to find that this response was very insensitive to suramin (19). At approximately the same time, responses with several similar properties in mouse megakaryocytes were also reported to be suramin-insensitive (20). We therefore undertook to compare the effects of suramin on mouse and human P2X1 receptors by measuring the blockade of ATP-induced currents after expression of the receptors in HEK 293 cells. In the first part of the present work we confirmed a substantial difference in sensitivity to suramin between the species.
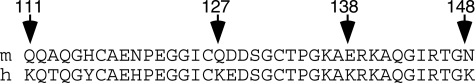
In the amino acid sequences of the human and mouse P2X1 receptors (SwissProt: mouse P51576 and human P51575), there are 40 differences in 399 residues, 33 of which are in the ectodomain. In four cases, there are lysine residues in the human sequence that correspond in position to neutral or negatively charged residues in the mouse sequence. These are clustered in a part of the protein ectodomain that begins some 60 amino acids after the end of the first transmembrane domain (positions 111, 127, 138, and 148; numbering is the same for mouse and human) (Fig. 1). Suramin and NF449 bear fixed negative charges by virtue of their polysulfonates (six in suramin and eight in NF449). We therefore hypothesized that the difference in suramin sensitivity between the two species resulted from the different presentation of positively charged residues by the two receptors and elected to test this in the first instance by focusing on the region Lys111 to Lys148. We tested this hypothesis by systematically substituting each of the four lysines in the human sequence by its counterpart in the mouse and vice versa.
FIGURE 1.
Four lysines in the human P2X1 receptor are missing in the mouse ortholog. Comparison of sequences of the human and mouse receptors in the ectodomain segment from residues 111 to 148 (numbering is the same in both species) shows four positions where lysine is replaced by uncharged polar or negative amino acid.
EXPERIMENTAL PROCEDURES
Molecular and Cell Biology—HEK 293 cells were maintained at 37 °C and 5% CO2 in growth medium (HEK-GM) containing Dulbecco's modified Eagle's medium/F-12, 10% fetal calf serum, and 2 mm l-glutamine (Invitrogen). Human or mouse P2X1 subunits with a C-terminal EMYPME epitope tag subcloned into pcDNA3.1(+) were used as the template for all mutagenesis reactions. The mutations were introduced using QuikChange site-directed mutagenesis (Stratagene), and the entire coding region was confirmed by sequencing (Seqman II; DNASTAR). HEK 293 cells were plated out onto 35-mm Petri dishes and allowed to attain cell density of 105 cells/cm2 before transfection. The cells were transfected for 4–6 h using Lipofectamine (Invitrogen; see Ref. 12), with 0.5 μg/ml receptor DNA and 0.05 μg/ml of pEGFP-N1 for subsequent cell visualization. The cells were then trypsinized, washed, reconstituted in HEK-GM, and plated out on glass coverslips at 1500 cells/cm2. Transfection efficiency with this approach was >60% as judged with epifluorescence microscopy. The coverslips were maintained in 35-mm dishes containing 2 ml of HEK-GM at 37 °C and 5% CO2 for 24–48 h before recordings were made.
Whole Cell Recording and Application of Agonists and Antagonists—All of the cells were pretreated with apyrase (2 units/ml, Type VII; Sigma) for at least 2 h before the recordings were commenced. The coverslips with attached cells were placed in a recording chamber mounted on the stage of an Axiovert microscope (Carl Zeiss). Extracellular recording solution containing 147 mm NaCl, 3 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, and 13 mm d-glucose, 13 (pH adjusted to 7.4 with NaOH) was superfused at a rate of 5.5 ml/min. Whole cell recordings were made at room temperature (20–23 °C) using an EPC9 amplifier, and data were collected using Pulse software (HEKA). The membrane potential was held at –60 mV. Patch electrodes and “puffer” electrodes (for ATP application) were pulled from glass pipettes (Harvard Apparatus, Edenbridge, UK) on a vertical puller (HEKA) and ranged from 6 to 9 mΩ in resistance when filled with an intracellular solution containing 147 mm NaCl, 10 mm HEPES, 10 mm EGTA (pH adjusted to 7.3 with NaOH). ATP solutions were prepared on the day of recording by diluting 100 mm frozen stock solution (pH adjusted to 7.3) in the external recording solution. Suramin and NF449 were prepared as 10 mm frozen stock and diluted to the required concentration on the day of recording currents. ATP was applied via a glass puffer pipette (1-μm tip diameter, ≈10 p.s.i., 69 kPa) using a pneumatic PicoPump (PV830; World Precision Instruments). The tip of the puffer pipette was positioned downstream from the cell with respect to the direction of flow of the superfusing solution and temporarily repositioned to a point ∼15 μm from the cell only for the period of application. With a concentration of ATP >1 μm, second applications 2 min after the first evoked a current that was less than 20% of the first response. Therefore, all concentration-response relations shown in this study were constructed from pooled data, in which ATP (0.001–100 μm) was applied only once to each cell on each coverslip. Accordingly, the same data points for control concentration-response curves appear more than once in the panels of Figs. 2, 3, 4, 5. Suramin and NF449 were applied in the superfusing solution for 5–10 min prior to the application from the puffer pipette of a solution containing both ATP and the appropriate antagonist. The currents evoked were then compared with those observed in other cells with no antagonist pretreatment.
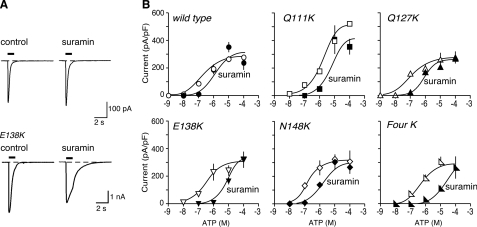
FIGURE 2.
Introduction of lysine residues into the mouse receptor can increase sensitivity to suramin. A, representative traces of membrane currents evoked by ATP (10 μm), applied for 1 s as indicated by the horizontal bars. Left panels, control. Right panels, in suramin (10 μm). B, ATP concentration-response curves for wild type mouse P2X1 receptors and for receptors with one or four lysines introduced at the positions indicated. Open symbols, control. Solid symbols, in presence of suramin (10 μm). Note the increased effectiveness of suramin as an antagonist in the case of mouse P2X1[E138K] and of mouse P2X1[4K] receptors.
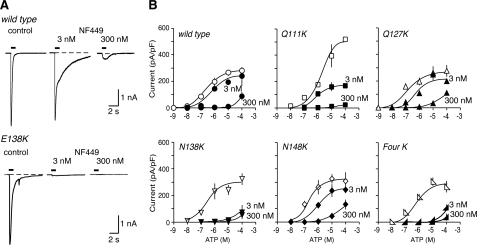
FIGURE 3.
Introduction of lysine residues into the mouse receptor can increase sensitivity to NF449. A, representative traces of membrane currents evoked by ATP (10 μm), applied for 1 s as indicated by the horizontal bars. Left panels, control. Center and right panels, in NF449 (3 and 300 nm). B, ATP concentration-response curves for wild type mouse P2X1 receptors and for receptors with one or four lysines introduced at the positions indicated. Open symbols, control. Solid symbols, in presence of NF449 (3 and 300 nm). Note the increased effectiveness of NF449 (3 nm) as an antagonist in the case of mouse P2X1[E138K] and of mouse P2X1[4K] receptors.
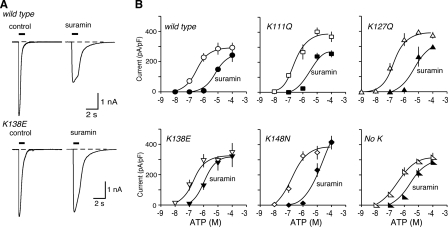
FIGURE 4.
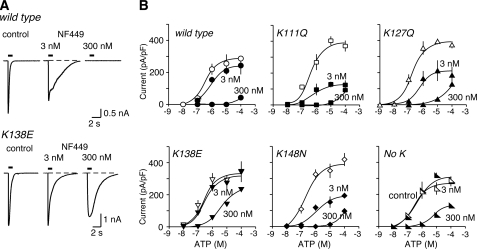
Removal of lysine residues from the human mouse receptor can reduce sensitivity to suramin. A, representative traces of membrane currents evoked by ATP (10 μm), applied for 1 s as indicated by the horizontal bar. Left panels, control. Right panels, in suramin (10 μm). B, ATP concentration-response curves for wild type mouse P2X1 receptors and for receptors with one or four lysines removed at the positions indicated. Open symbols, control. Solid symbols, in presence of suramin (10 μm). Note the decreased effectiveness of suramin as an antagonist in the case of human P2X1[K138E] and also for human P2X1[No K] receptors.
FIGURE 5.
Removal of lysine residues from the human can reduce sensitivity to NF449. A, representative traces of membrane currents evoked by ATP (10 μm), applied for 1 s as indicated by the horizontal bar. Left panels, control. Center and right panels, in NF449 (3 and 300 nm). B, ATP concentration-response curves for wild type human P2X1 receptors and for receptors with one or four lysines removed at the positions indicated. Open symbols, control. Solid symbols, in presence of NF449 (3 and 300 nm). Note the decreased effectiveness of NF449 (300 nm) as an antagonist in the case of human P2X1[K138E] and also for human P2X1[No K] receptors.
Data Analysis—Numerical data are the means ± S.E. for the number of macrophages tested. The current traces were obtained using Axograph (Molecular Devices), Kaleidagraph (Synergy Software, Reading, PA), and Canvas (ACD Systems) software. Concentration-response curves were fitted using the nonlinear regression program from Prism 4 (GraphPad, San Diego, CA) to the mean values of the currents evoked at each concentration. The agonist concentration required for the half-maximal responses (EC50) is expressed as its negative logarithm (pEC50) ± S.E. Statistical significance between data were determined using unpaired t tests and analysis of variance (InStat, GraphPad Software Inc., San Diego, CA), and differences were considered significant at the level of p < 0.05.
Chemicals—ATP, apyrase, and suramin were purchased from Sigma. NF449 was purchased from Tocris. All other chemicals used in the present study were purchased from Sigma or VWR International Ltd.(Poole, UK).
RESULTS
Mouse Receptors: Effect of Suramin and NF449—ATP applied for 1 s evoked concentration-dependent inward currents at wild type mouse P2X1 (mP2X1) receptors (Fig. 2). The pEC50 was 6.5 ± 0.12 (n = 66), which is similar to values reported previously in HEK 293 cells for human (12) and rat receptors (21, 22) (Fig. 2 and Table 1). Similar currents were also evoked by αβ-methylene-ATP, with pEC50 of 5.4 ± 0.4 (n = 16). Suramin (10 μm) had little or no effect on the response evoked by 10 μm ATP (Fig. 2); neither suramin nor NF449 changed the holding current in the absence of applied ATP. The pEC50 for ATP in the presence of suramin was 6.2 ± 0.53 (n = 40), which is not different (p > 0.05) from that in its absence (Table 1). The effectiveness of ATP was not much altered by introduction of single lysine residues at Gln111, Gln127, Glu138, or Asn148 or at all four positions, except in the case of mP2X1[Q111K], which was less sensitive to ATP (pEC50 5.7 ± 0.22, n = 27) (Table 1 and Fig. 2B). Likewise, these mutations did not cause any marked change in the peak amplitude of the current evoked by ATP (100 μm), except in the case of mP2X1[Q111K] (Fig. 2B), where the maximal peak response was ∼2-fold greater. In the presence of suramin (10 μm), the ATP concentration-response curve was significantly (∼40-fold) shifted to the right for mP2X1[E138K] and mP2X1[Q111K,Q127K,E138K,N148K] (mP2X1[Four K]). It was much less shifted (∼7-fold) for mP2X1[N148K] and unaffected in the other two cases (mP2X1-[Q111K] and mP2X1[Q127K]). We tested two concentrations of NF449 (3 and 300 nm) most extensively. When applied against currents evoked by 10 μm ATP, NF449 had a very small effect at 3 nm but gave almost complete inhibition at 300 nm (Fig. 3). Concentration-response curves of ATP evoked in the presence of 3 nm NF449 showed a pEC50 of 6.1 ± 0.22 (n = 20) (control was 6.5 ± 0.12, n = 66; Table 1). The corresponding value for 300 nm NF449 was considerably less than 4, indicating very complete antagonism (Fig. 3B). mP2X1[N138K] and mP2X1[Four K] were much more sensitive to antagonism by NF449 (3 nm) than the wild type receptor (Fig. 3B). In contrast, mP2X1[Q127K] and mP2X1[N148K] showed only minor changes in blockade by 3 or 300 nm NF449 (Fig. 3B). The effect on mP2X1[Q111K] was complicated by the change in peak amplitude of the ATP current that resulted from the point mutation (Fig. 3B); however, there was no marked increase in the effectiveness of NF449. Experiments with 30 nm NF449 gave intermediate results (data not shown). Replacement of the glutamic acid at position 138 with aspartic acid (mP2X1-[E138D]) resulted in a receptor that was similar to the wild type receptor with respect to its sensitivity to suramin (Table 1).
TABLE 1.
Summary pEC50 data for wild type and mutants of mouse and human P2X1 receptors
The values are shown as the means ± S.E., and the numbers of cells tested are shown in parentheses. Δ indicates difference in pEC50 value compared to control. NT, concentration not tested.
| Control | Suramin (10 μm) | Δ | NF 449 (3 nm) | Δ | NF449 (300 nm) | Δ | |
|---|---|---|---|---|---|---|---|
| Mouse P2X1 | |||||||
| Wild type | 6.5 ± 0.12 (66) | 6.2 ± 0.53 (40) | 0.3 | 6.1 ± 0.22 (20) | 0.4 | <4 (11) | >2.5 |
| Q111K | 5.7 ± 0.22 (22) | 5.6 ± 0.78 (14) | 0.1 | 6.2 ± 0.29 (17) | -0.5 | <<4 (14) | >>0.5 |
| Q127K | 7.1 ± 0.26 (27) | 6.3 ± 0.48 (21) | 0.8 | 6.1 ± 0.35 (16) | 1.0 | <4 (14) | >3.1 |
| E138K | 6.5 ± 0.33 (28) | 4.9 ± 0.14 (18) | 1.6a | <<4 (14) | >2a | <<4 (14) | >>2.5 |
| N148K | 6.8 ± 0.15 (25) | 6.0 ± 0.26 (20) | 0.8 | 6.0 ± 0.09 (13) | 0.8 | <4 (14) | >2.5 |
| Four-K | 6.4 ± 0.26 (33) | 4.8 ± 0.09 (19) | 1.6a | <4 (14) | >2a | <<4 (14) | >>2.5 |
| E138D | 5.7 ± 0.28 (23) | 5.6 ± 0.07 (20) | 0.1 | 6.6 ± 0.28 | -0.9 | NT | |
| Human P2X1 | |||||||
| Wild type | 6.5 ± 0.06 (57) | 5.1 ± 0.15 (28) | 1.4 | 6.2 ± 0.24 (22) | 0.3 | <4 (13) | >2.5 |
| K111Q | 6.5 ± 0.21 (23) | 5.4 ± 0.51 (15) | 1.1 | 6.4 ± 0.17 (21) | 0.1 | <4 (14) | >2.5 |
| K127Q | 6.8 ± 0.09 (21) | 5.3 ± 0.37 (15) | 1.5 | 6.4 ± 0.3 (16) | 0.4 | <4 (12) | >2.8 |
| K138E | 6.9 ± 0.18 (26) | 5.9 ± 0.18 (18) | 1.0a | 6.6 ± 0.25 (24) | 0.3 | 5.2 ± 0.19 (18) | 1.5a |
| K148N | 6.6 ± 0.22 (20) | 5.0 ± 0.17 (15) | 1.6 | 6.2 ± 0.20 (17) | 0.4 | <4 (14) | >2.6 |
| No-K | 6.4 ± 0.09 (20) | 5.4 ± 0.08 (14) | 1.0a | 6.4 ± 0.20 (19) | 0 | ≈5 (15) | ≈1.4a |
| K138R | 6.2 ± 0.23 (26) | 4.8 ± 0.14 (18) | 1.4 | 6.4 ± 0.18 (28) | 0.2 | NT | |
Largest differences from corresponding control.
Human Receptors: Effect of Suramin and NF449—Application of ATP for 1 s evoked concentration-dependent inward currents with a pEC50 of 6.5 ± 0.06 (n = 57) at human P2X1 receptors (hP2X1), which is similar to values reported previously in HEK 293 cells (12) (Fig. 4 and Table 1). Inward currents were also evoked by αβ-methylene-ATP, with a pEC50 of 5.0 ± 0.33 (n = 17). The action of ATP at hP2X1 receptors was effectively antagonized by suramin (10 μm) (Fig. 4). The concentration-response curve to ATP was shifted ∼30-fold to the right, giving a pEC50 of 5.1 ± 0.15 (n = 28) (Fig. 4B and Table 1). Mutation of each of the four lysine residues in hP2X1 receptors to the corresponding amino acid in the mouse receptor did not produce any significant change in the effectiveness of ATP, either in the single substitutions or in the quadruple mutation (hP2X1[No K]) (Table 1). Suramin was a less effective antagonist in the case of hP2X1[K138E] and hP2X1[No K]; in this case the rightward shift was ∼10-fold. On the other hand, the effectiveness of suramin (10 μm) was little different in the case of hP2X1[K127Q] and hP2X1[K148N] (where the rightward shifts were ∼38- and ∼42-fold). The case of hP2X1[K111Q] was less easy to interpret, because suramin also reduced the maximum effect of ATP by ∼30% (Fig. 4B).
At wild type hP2X1 receptors, NF449 produced a concentration-dependent antagonism. At 3 nm there was a small rightward shift (pEC50 6.2 ± 0.24 (n = 22) compared with the control of 6.5 ± 0.06 (n = 57). At 300 nm there was almost complete inhibition (NF449 at 30 nm had an intermediate effect; data not shown) (Fig. 5). The major effect of removing the lysine residues from the hP2X1 receptor was to reduce the effectiveness of NF449 (300 nm) as an ATP antagonist, in the case of hP2X1[K138E] and hP2X1[No K] (Fig. 5), whereas for the substitutions K111Q, K127Q, and K148N, there was little change in the effectiveness of NF449 at 300 nm. The results with NF449 at 3 nm were consistent with the reduced antagonism in the case of hP2X1[K138E] and hP2X1[No K] (ΔpEC50 values of 0.3 and 0.0, respectively) (Table 1), but these results are complicated by the finding that 3 nm NF449 also reduced the “maximum” ATP response at hP2X1 receptors with the single lysine substitutions (Fig. 5).
Substitution of lysine by arginine at position 138 (hP2X1[K138R]) resulted in a receptor that was not different from wild type hP2X1 receptors in its sensitivity to suramin and NF449 (Table 1).
DISCUSSION
This study presents substantial differences between the sensitivity to suramin of mouse and human P2X1 receptors. We tested the hypothesis that this difference resulted from four lysine residues that are present in the human P2X1 receptor ectodomain but that are replaced by neutral or negatively charged residues in the mouse receptor (Fig. 1). Lysine residues have been shown to play a critical role in P2X receptor function. This is particularly so for positions 68 and 309 of the human P2X1 receptor (23) (and many other P2X receptors (24), where any replacement of the lysine results in a channel that is barely or not at all activated by ATP. It seems rather unlikely that these residues are in or near an ATP-binding site because they are not at all conserved among P2X subunits.
In the present work, the effectiveness of the agonist ATP was not different between the two species and was not consistently affected by any of the mutations used (Table 1). For example the substitution Q111K in the mP2X1 receptor reduced the ATP pEC50 by 0.8 log units, but the converse mutation K111Q in the human receptor had no effect (Table 1). In our discussion of the relative effects of the antagonists suramin and NF449, we have measured effects independently of these changes in the effectiveness of ATP (Table 1).
Suramin (10 μm) was a much more effective blocker at the human receptor than at the mouse receptor. It shifted the ATP concentration-response curve 3-fold in the mouse and 40-fold in the human (Table 1). Introduction of a single lysine into the mouse sequence (E138K) resulted in a receptor that was at least as sensitive to suramin as the wild type human receptor (which contains lysine at this position) (Fig. 2). This was true whether the lysine was introduced alone or in combination with three others (Q111K, Q127K, and N148K). Conversely, in the wild type human receptor suramin caused a 25-fold shift in the ATP concentration-response curve; this was only 10-fold for the human receptors in which Lys138 was replaced by glutamate, either alone or in combination with three others (K111Q, K127Q, and K148N) (Table 1) (Fig. 4).
The results with NF449 were in general agreement to those with suramin. The inhibition observed with 3 nm NF449 was rather small for the wild type mouse P2X1 receptor but became quite striking when a lysine was introduced at position 138 (mP2X1[N138K] and mP2X1[Four K]) (Fig. 3). Conversely, 300 nm NF449 strongly blocked the wild type human P2X1 receptor but had much less effect on hP2X1[K138E] or hP2X1[No-K]. Taken together these reciprocal changes in the effectiveness of suramin and NF449 indicate that the most important contributor to their blocking action is the residue at position 138. These findings were further supported by the restoration of antagonism profiles of wild type P2X1 receptors, by substituting like for like amino acids at Glu138 (mouse) and Lys138 (human), mP2X1[E138D] and hP2X1[K138R] mutants, respectively. NF449 has eight distributed sulfonate moieties, whereas suramin has six, but the results indicate that it is the same lysine residue that is mostly involved in each case. It is tempting to interpret these results in the context of an electrostatic interaction between antagonist and receptor, although the mutation could also induce a conformational change that influences antagonist binding indirectly. At this position, in contrast to the other three, the mutations cause charge reversal as compared with simple charge addition or charge removal. Indeed, the identity of the amino acid at any of the four positions studied in the present work is remarkably degenerate among seven human P2X subunits; position 111 is occupied by five different residues, position 128 is occupied by five different residues, position 138 is occupied by six different residues, and position 148 is occupied by three different residues. In contrast, the lysine residues of the P2X1 receptor that likely contribute to ATP-binding site (at positions 68, 70 and 309) are much more conserved (25–28).
Suramin blocks rat P2X2 receptors at concentrations very similar to those that were effective in the present study (12, 29). However, the rat P2X2 receptor sequence does not possess any lysine residues that correspond in position to those of the human P2X1 receptor. On the other hand, the rat P2X4 receptor is notable for its very weak sensitivity to blockade by suramin (13); it also lacks lysines at the corresponding positions. However, in the case of the P2X4 receptor, the human form is more sensitive than the rat. Soto et al. (14) showed that replacing a glutamine residue at position 78 with lysine, which is the residue at the equivalent position in the human sequence, could increase the effectiveness of suramin. This also does not align with any of the four lysines studied in the present work. Taken together with the weak sequence conservation in this part of the receptor, the results seem more consistent with the provision of a nonspecific cloud of positivity where the attachment of a suramin (or NF449) can inhibit the conformational changes required for gating.
In the present work, the agonist ATP was applied to the cells by ejection of a solution of known concentration for 1 s, from a pipette positioned ∼15 μm from the cell. It is therefore not likely that the agonist concentration was at steady state. Furthermore, in addition to reducing the peak amplitude of the response, both antagonists (but particularly NF449) consistently prolonged the decay phase of the inward current (Figs. 2, 3, 4, 5). This was observed for the wild type and all of the mutant forms examined (data not shown). P2X1 receptors undergo pronounced desensitization when ATP (or αβ-methylene-ATP) is applied in μm range (11, 30), so that under the present experimental conditions it is not possible to say whether the primary effects of the antagonists result primarily from binding to open, closed, or desensitized states.
Suramin was first reported to inhibit native P2X receptors in smooth muscle of the mouse vas deferens (7). Therefore, the comparative lack of effect on the antagonism by suramin (10 μm) in native mouse P2X1 receptors (19, 20) and confirmed in heterologous expressed recombinant P2X1 receptors in the present study was unexpected. There is thus a difference between the weak blockade by suramin of homomeric mouse P2X1 receptors (present study), mouse macrophage receptors (19), and mouse megakaryocyte receptors (20) and the stronger block observed in the mouse vas deferens (7). However, vas deferentia removed from mice bred without P2X1 receptor subunits do not respond to added ATP (31). A possible explanation for these apparently discrepant results might be that the P2X receptor of the mouse vas deferens is actually a heteromeric protein that includes a P2X1 subunit. The results certainly call into question the block by suramin as any kind of defining characteristic of nonhuman P2X1 receptors present in native cells and tissues.
Acknowledgments
We thank Kyraki Dossi and Laura Smith for tissue culture and Louise Almond for work on mutagenesis.
Author's Choice—Final version full access.
This work was supported by the Wellcome Trust. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: NF449, 4,4′,4″,4‴-(carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakis-benzene-1,3-disulfonic acid; HEK, human embryonic kidney.
References
- 1.Town, B. W., Wills, E. D., and Wormal, A. (1949) Nature 163 735. [PubMed] [Google Scholar]
- 2.Town, B. W., Wills, E. D., Wilson, E. J., and Wormal, A. (1950) Biochem. J. 47 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomominski, I., and Gray, S. (1961) Nature 192 683. [DOI] [PubMed] [Google Scholar]
- 4.Layton, D., and Azzi, A. (1974) Biochem. Biophys. Res. Commun. 59 322–325 [DOI] [PubMed] [Google Scholar]
- 5.Smolen, J. E., and Weissmann, G. (1978) Biochim. Biophys. Acta 512 525–538 [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E. (1979) Cancer Lett. 8 9–22 [DOI] [PubMed] [Google Scholar]
- 7.Dunn, P. M., and Blakeley, A. G. H. (1988) Br. J. Pharmacol. 93 243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneddon, P. (1992) Br. J. Pharmacol. 107 101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock, G., and Kennedy, C. (1985) Gen. Pharmacol. 16 433–440 [DOI] [PubMed] [Google Scholar]
- 10.North, R. A. (2002) Physiol. Rev. 82 1013–1067 [DOI] [PubMed] [Google Scholar]
- 11.Valera, S., Hussy, N., Evans, R. J., Adami, N., North, R. A., Surprenant, A., and Buell, G. (1994) Nature 371 516–519 [DOI] [PubMed] [Google Scholar]
- 12.Evans, R. J., Lewis, C., Buell, G., Valera, S., North, R. A., and Surprenant, A. (1995) Mol. Pharmacol. 48 17–29 [PubMed] [Google Scholar]
- 13.Buell, G., Lewis, C., Collo, G., North, R. A., and Surprenant, A. (1996) EMBO J. 15 55–62 [PMC free article] [PubMed] [Google Scholar]
- 14.Soto, F., Garcia-Guzman, M., Gomez-Hernandez, J. M., Hollmann, M., Karschin, C., and Stühmer, W. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 3684–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahaut-Smith, M. P., Tolhurst, G., and Evans, R. J. (2004) Platelets 15 131–144 [DOI] [PubMed] [Google Scholar]
- 16.Fung, C. Y., Cendana, C., Farndale, R. W., and Mahaut-Smith, M. P. (2007) J. Thromb. Haemostasis 5 910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun, K., Rettinger, J., Ganso, M., Kassack, M., Hildebrandt, C., Ullmann, H., Nickel, P., Schmalzing, G., and Lambrecht, G. (2001) Naunyn-Schmiedeberg's Arch. Pharmacol. 364 285–290 [DOI] [PubMed] [Google Scholar]
- 18.Hülsmann, M., Nickel, P., Kassack, M., Schmalzing, G., Lambrecht, G., and Markwardt, F. (2003) Eur. J. Pharmacol. 470 1–7 [DOI] [PubMed] [Google Scholar]
- 19.Sim, J. A., Park, C. K., Oh, S. B., Evans, R. J., and North, R. A. (2007) Br. J. Pharmacol. 152 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda, M. (2007) Thromb. Res. 119 343–353 [DOI] [PubMed] [Google Scholar]
- 21.Surprenant, A., Schneider, D. A., Wilson, H. L., Galligan, J. J., and North R. A. (2000) J. Auton. Nerv. Syst. 81 249–263 [DOI] [PubMed] [Google Scholar]
- 22.Lê, K. T., Boué-Grabot, E., Archambault, V., and Séguéla, P. (1999) J. Biol. Chem. 274 15415–15419 [DOI] [PubMed] [Google Scholar]
- 23.Ennion, S., Hagan, S., and Evans, R. J. (2000) J. Biol. Chem. 275 29361–29367 [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson, W. J., Jiang, L. H., Surprenant, A., and North, R. A. (2006) Mol. Pharmacol. 70 1159–1163 [DOI] [PubMed] [Google Scholar]
- 25.Marquez-Klaka, B., Rettinger, J., Bhargava, Y., Eisele, T., and Nicke, A. (2007) J. Neurosci. 27 1456–14566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts, J. A., Vial, C., Digby, H. R., Agboh, K. C., Wen, H., Atterbury-Thomas, A., and Evans, R. J. (2006) Pflugers Arch. Eur. J. Pharm. 452 486–500 [DOI] [PubMed] [Google Scholar]
- 27.Roberts, J. A., and Evans, R. J. (2007) J. Neurosci. 27 4072–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts, J. A., and Evans, R. J. (2004) J. Biol. Chem. 279 9043–9055 [DOI] [PubMed] [Google Scholar]
- 29.North, R. A., Surprenant, A. (2000) Annu. Rev. Pharmacol. Toxicol. 40 563–580 [DOI] [PubMed] [Google Scholar]
- 30.Rettinger, J., and Schmalzing, G. (2003) J. Gen. Physiol. 121 451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulryan, K., Gitterman, D. P., Lewis, C. J., Vial, C., Leckie, B. J., Cobb, A. L., Brown, J. E., Conley, E. C., Buell, G., Pritchard, C. A., and Evans, R. J. (2000) Nature 403 86–89 [DOI] [PubMed] [Google Scholar]