Abstract
Metronidazole, which is used for the treatment of infections caused by anaerobic organisms, was evaluated in Mycobacterium tuberculosis-infected guinea pigs. M. tuberculosis can adapt to hypoxia, which is present in the primary lesions of infected guinea pigs. Metronidazole treatment (for 6 weeks at 100 mg/kg of body weight) resulted in no reduction in the bacillary burden and significantly worsened lesion inflammation.
The excellent distribution of metronidazole (MET) in all organs and its good bactericidal activity, including its activity against quiescent bacteria, make it the compound of choice for the treatment of many infections caused by anaerobic organisms. Mycobacterium tuberculosis also has the ability to adapt to gradual oxygen depletion or stationary growth. This phenomenon has been studied extensively by Wayne and Hayes and has been described as a sequential progression through two stages, defined as nonreplicating phase 1 (NRP-1) and NRP-2 (15). MET has no effect on exponentially growing bacilli or on NRP-1 bacilli, but it is bactericidal for bacilli in stationary phase and NRP-2 (6, 16).
Nonprogressive M. tuberculosis lesions in the lungs often have limited vascularization (due to necrosis and dystrophic mineralization), causing a limited O2 supply. Bacterial persistence in these hypoxic lesions has been thought to be accompanied by susceptibility to MET. In mouse models of tuberculosis (TB), MET has failed to show consistent activity (5, 6, 10). This is not surprising, as hypoxia was found to be completely absent in the M. tuberculosis lesions of infected mice (1, 11), and the progression of disease rarely reaches the stages of extensive necrosis (2, 3, 8, 12). Lung lesions in guinea pigs infected with M. tuberculosis, on the other hand, show caseous necrosis, mineralization, and hypoxia, which are also seen in natural infections in humans (13, 14). Guinea pigs develop necrotic primary lesions that differ in their morphology compared to those of the secondary lesions that result after the activation of adaptive immunity (8). In an earlier paper, we described that the persisting, acid-fast bacilli are primarily found extracellularly in a hypoxic microenvironment of primary lesion necrosis and that only few a bacilli are localized within the secondary granulomas (8). The same study also showed evidence of hypoxia in these primary lesions of guinea pigs when pimonidazole was used (8), and therefore, we chose to explore the potential bactericidal activity of MET in the guinea pig model.
To establish appropriate drug doses in guinea pigs for the anti-M. tuberculosis drugs used in this study, pharmacokinetic analysis was performed with a single dose each of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and MET. All drugs were obtained from Sigma Chemical Co. (St. Louis, MO). INH, PZA, and MET were dissolved in distilled water, while RIF was dissolved in dimethyl sulfoxide (final concentration, 0.5%) with sucrose (40%, wt/vol) to increase its palatability. The pharmacokinetic data obtained by validated high-pressure liquid chromatography assays (National Jewish Center, Denver, CO) yielded areas under the concentration-time curves from 0 to 24 h (AUC0-24s) of 18.22 mg·h/ml for INH at 30 mg/kg, 6.22 mg·h/ml for RIF at 50 mg/kg, 31.82 mg·h/ml for PZA at 40 mg/kg, and 35 mg·h/ml for MET at 100 mg/kg. To reach doses in the animals equivalent to the clinical doses (on the basis of the AUC0-24 and the maximum concentration of drug in plasma), the doses were calculated and adjusted to prevent toxicity and were as follows: 30 mg/kg for INH, 50 mg/kg for RIF, 100 mg/kg for PZA, and 100 mg/kg for MET. In the combination treatment trial, the dose of MET had to be reduced from 100 mg/kg in the first week to 50 mg/kg in the second week due to weight loss (the trial had to be terminated after 2 weeks of treatment due to severe toxicity).
To assess the efficacy of MET in guinea pigs, the protocol followed was that described before (7, 8). Briefly, female outbred Hartley guinea pigs were exposed to an aerosol of M. tuberculosis H37Rv (4). Treatment started 30 days after aerosol infection by administering the drug dose in 40% (wt/vol) sucrose. The animals were killed at intermittent time points (after 2, 4, and 6 weeks for the first experiment with single drugs and after 2 and 4 weeks for the drug combination trial). The detection limit of the plating procedure was ∼25 CFU, as described before (8). Statistical analysis of the CFU data was performed by one-way analysis of variance, followed by the Tukey test (8). Following euthanasia, the left cranial lung lobe was infused with 10% neutral-buffered formalin and embedded in paraffin, and sections were stained for histologic analysis with hematoxylin and eosin (H&E) or acid-fast stain. The H&E-stained sections were ranked in the order of severity of the lesion burden, and the individual primary and secondary lesions were scored as described earlier (9).
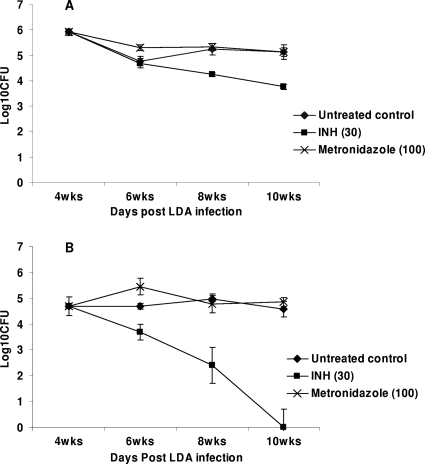
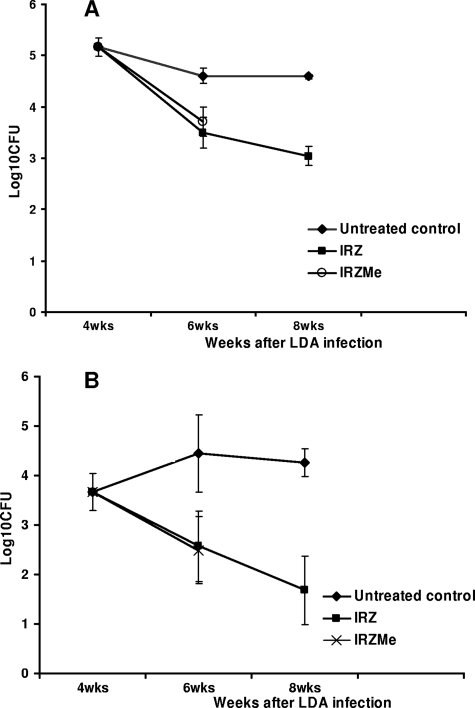
In the first study, the efficacy of MET (100 mg/kg) as well as that of INH (30 mg/kg) was evaluated when they were used as single drugs for the treatment of guinea pigs infected with M. tuberculosis. At the start of treatment, the bacterial load in the guinea pig lungs reached ∼6.0 log10 CFU. At the completion of the study, the bacterial load in the sucrose-treated control group was ∼5 log10 CFU (Fig. 1). The activities of both drugs in groups of guinea pigs killed every 2 weeks were evaluated over 6 weeks. INH significantly reduced the bacterial load in the lungs over time, with a more pronounced activity in spleens (P < 0.05) (Fig. 1). MET did not show any significant activity in the lungs and spleens compared with that in the lungs and spleens of the sucrose-treated group for all the time points tested (P > 0.05) (Fig. 1). In the second study, MET (at 50 mg/kg) was tested in combination with INH (30 mg/kg), RIF (50 mg/kg), and PZA (100 mg/kg) (IRZMe), and its activity was compared to that of the standard regimen of INH, RIF, and PZA (IRZ). At the start of treatment, the bacterial load in the guinea pig lungs reached ∼5.2 log10 CFU. After 4 weeks of treatment, the bacterial load in the sucrose-treated control group was ∼4.8 log10 CFU (Fig. 2). The animals were scheduled to be killed after 2 and 4 weeks of treatment. After 2 weeks of treatment, the bacterial numbers in the IRZMe group were significantly reduced compared with those in the untreated controls (P < 0.05) but was found to be similar to that in the IRZ control group (P > 0.05) (Fig. 2). In conclusion, no additional activity was observed for MET when it was combined with the standard regimen. In addition, MET caused severe toxicity from the second week of the combination treatment. The animals in the IRZMe group started to lose weight (more than 20% of their body weight), and four animals had to be euthanized. The remaining animals were killed at the 2-week treatment time point, and therefore, no data for the IRZMe group are available beyond this time point due to the severe toxicity (Fig. 2).
FIG. 1.
Viable bacterial numbers in whole lungs (A) and spleens (B) of M. tuberculosis-infected guinea pigs treated with 40% (wt/vol) sucrose, INH, or MET for 2, 4, and 6 weeks on 5 days per week (five animals per group). LDA, low-dose aerosol.
FIG. 2.
Viable bacterial numbers in whole lungs (A) and spleens (B) of M. tuberculosis-infected guinea pigs treated with sucrose, IRZ, or IRZMe for 2 and 4 weeks on 5 days per week (six animals per group). LDA, low-dose aerosol.
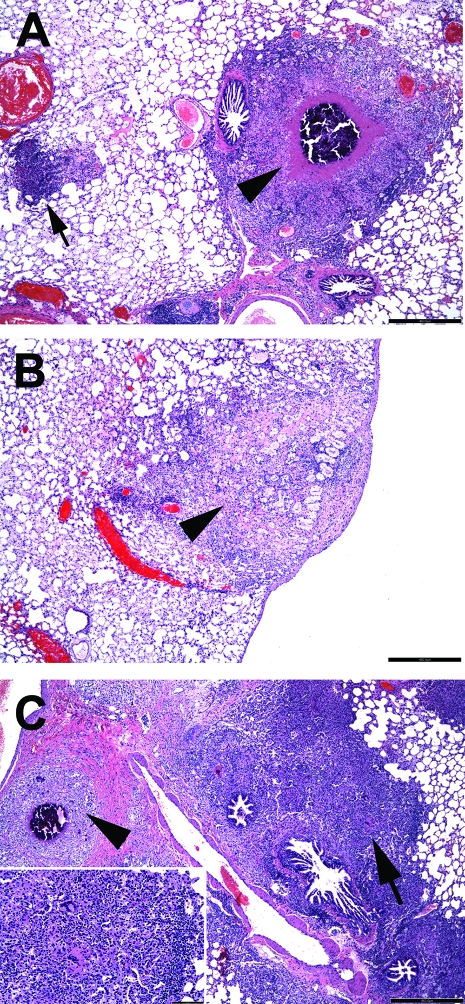
Histologic analysis of the lung samples collected throughout the first drug trial showed differences in lung pathology (Fig. 3). At the start of treatment, the lungs of the infected guinea pigs showed both primary and secondary granulomas. Untreated guinea pigs showed, as expected, an increase in inflammation of the secondary granulomas over time, while the primary granulomas largely remained unchanged. After treatment with INH or MET as single drugs, the morphology of the primary lesions stayed the same (Fig. 3A), however, treatment considerably affected the development and progression of the secondary inflammatory lesions compared to the effect in the untreated controls (Fig. 3B and C). While INH halted the progression of inflammation and prevented the development of new secondary lesions (Fig. 3B), in the MET-treated group, the inflammation and the secondary lesion involvement in the lung increased considerably compared with those in the untreated controls (Fig. 3C). The reasons for the increase in inflammation in the MET-treated group are unclear. Although the bacillary burden in the MET-treated group was not statistically different from that in the sucrose-treated control group for the time points tested, one can speculate that a slight difference in the early dissemination of the bacteria (suggested by a slightly increased bacterial load 2 weeks after MET treatment; Fig. 1) may have effects later on. Alternatively, the accelerated formation of TB lesions may be induced by the drug itself by influencing the host immune response.
FIG. 3.
Secondary granulomas in lungs of M. tuberculosis-infected guinea pigs after drug treatment. The extent and the morphology of the lesions in M. tuberculosis-infected guinea pigs either untreated or treated for 4 weeks with INH or MET were compared. The images show the lesions of one animal from each group, which represents an average animal. (A) Untreated guinea pig treated with 40% (wt/vol) sucrose for 4 weeks. A single primary granuloma (arrowhead) has central calcification and residual central lesion necrosis. A single secondary lesion is characterized by increased numbers of lymphocytes (basophilia) and is devoid of central necrosis (arrow). (B) An INH-treated guinea pig has a single primary lesion that lacks necrosis and calcification but that has fibrosis (arrowhead). No secondary granulomas are evident in the field. (C) A MET-treated guinea pig has extensive inflammation, primarily represented by secondary lesions (arrow) that coalesce in the perivascular and peribronchial pulmonary parenchyma. Among the lymphocytes and mononuclear phagocytes are occasional multinucleated giant cells (inset, white arrowhead). A single primary granuloma is evident by the central calcification (black arrowhead). H&E staining was used. Bar, 460 μm.
The reason that treatment with MET did not result in any significant reduction in culturable bacterial numbers in M. tuberculosis-infected guinea pigs can be multifold. We showed that MET reached adequate levels in the plasma of the guinea pigs; however, data about the actual drug penetration into the necrotic core of the hypoxic granulomas are not available, as the determination of drug penetration into the necrotic core is a challenging task. In addition, MET at 16 μg/ml has been shown to be mostly effective against M. tuberculosis under completely anaerobic conditions, whereas higher drug concentrations (64 μg/ml) are required under microaerophilic conditions (6, 16). We showed earlier that guinea pigs infected with M. tuberculosis develop hypoxic lesions (8); however, it seems improbable that the inner core of this caseous necrosis is totally anoxic. To obtain activity against M. tuberculosis under these microaerophilic conditions, high drug doses are required, and these concentrations would likely result in significant toxicity. In TB patients, it is possible that certain lesion types which are not present in the guinea pig (such as solid caseous necrotic lesions or liquefactive necrotic lesions, as well as closed calcified lesions) could be entirely anoxic. Therefore, the issue of the anti-M. tuberculosis activity of MET in vivo cannot be completely closed without further trials involving animal models with these pathological features. Nevertheless, the lack of reproducible activity by MET in mouse and guinea pig models suggests that the use of MET for the treatment of TB might be limited. Clinical trials conducted to explore the efficacy and tolerability of MET in combination with the standard regimen for TB will provide definite answers to these questions and are under way.
Acknowledgments
We acknowledge the staff of the Laboratory Animal Resources (Colorado State University) for animal care.
Support was provided by National Institutes of Health (NIH) research and development contract NO1 AI-95385 and NIH grant AI-061505.
Footnotes
Published ahead of print on 11 August 2008.
REFERENCES
- 1.Aly, S., K. Wagner, C. Keller, S. Malm, A. Malzan, S. Brandau, F. C. Bange, and S. Ehlers. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol. 210:298-305. [DOI] [PubMed] [Google Scholar]
- 2.Basaraba, R. J., D. D. Dailey, C. T. McFarland, C. A. Shanley, E. E. Smith, D. N. McMurray, and I. M. Orme. 2006. Lymphadenitis as a major element of disease in the guinea pig model of tuberculosis. Tuberculosis (Edinburgh) 86:386-394. [DOI] [PubMed] [Google Scholar]
- 3.Basaraba, R. J., E. E. Smith, C. A. Shanley, and I. M. Orme. 2006. Pulmonary lymphatics are primary sites of Mycobacterium tuberculosis infection in guinea pigs infected by aerosol. Infect. Immun. 74:5397-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, J. V., S. K. Furney, and I. M. Orme. 1999. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 43:1285-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon, J., B. W. Allen, Y. M. Hu, A. R. Coates, and D. A. Mitchison. 1998. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int. J. Tuberc. Lung Dis. 2:736-742. [PubMed] [Google Scholar]
- 7.Johnson, C. M., R. Pandey, S. Sharma, G. K. Khuller, R. J. Basaraba, I. M. Orme, and A. J. Lenaerts. 2005. Oral therapy using nanoparticle encapsulated antituberculosis drugs in guinea pigs infected with Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 49:4335-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenaerts, A. J., D. Hoff, S. Aly, S. Ehlers, K. Andries, L. Cantarero, I. M. Orme, and R. J. Basaraba. 2007. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob. Agents Chemother. 51:3338-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordway, D., G. Palanisamy, M. Henao-Tamayo, E. E. Smith, C. Shanley, I. M. Orme, and R. J. Basaraba. 2007. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J. Immunol. 179:2532-2541. [DOI] [PubMed] [Google Scholar]
- 10.Paramasivan, C. N., G. Kubendiran, and D. Herbert. 1998. Action of metronidazole in combination with isoniazid & rifampin on persisting organisms in experimental murine tuberculosis. Indian J. Med. Res. 108:115-119. [PubMed] [Google Scholar]
- 11.Tsai, M. C., S. Chakravarty, G. Zhu, J. Xu, K. Tanaka, C. Koch, J. Tufariello, J. Flynn, and J. Chan. 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 8:218-232. [DOI] [PubMed] [Google Scholar]
- 12.Turner, O. C., R. J. Basaraba, and I. M. Orme. 2003. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect. Immun. 71:864-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrichs, T., and S. H. Kaufmann. 2006. New insights into the function of granulomas in human tuberculosis. J. Pathol. 208:261-269. [DOI] [PubMed] [Google Scholar]
- 14.Ulrichs, T., M. Lefmann, M. Reich, L. Morawietz, A. Roth, V. Brinkmann, G. A. Kosmiadi, P. Seiler, P. Aichele, H. Hahn, V. Krenn, U. B. Gobel, and S. H. Kaufmann. 2005. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J. Pathol. 205:633-640. [DOI] [PubMed] [Google Scholar]
- 15.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayne, L. G., and H. A. Sramek. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]